Abstract
The nuclear factor E2-related factor 2 (Nrf2) is known as the chief regulator of cellular antioxidant defenses as well as a suppressor of inflammation. Macrophages act as major players in inflammatory responses. Because oxidative stress and inflammation are two intertwined processes, the anti-inflammatory activity of Nrf2 signaling is believed to result from its upregulation of cellular antioxidant defenses via the antioxidant response element-driven transcription. In a recent article published in Nature Communications (May 23, 2016; doi: 10.1038/ncomms11624), Kobayashi et al. reported that Nrf2 suppresses transcriptional upregulation of pro-inflammatory cytokines independent of its role in regulating cellular antioxidants and redox status. This study by Kobayashi et al. provides novel insights into the molecular basis of Nrf2 acting as a suppressor of inflammation.
Keywords: Inflammation, Macrophages, Nrf2, Redox signaling
1. ANTI-INFLAMMATORY FUNCTION OF NRF2: REDOX-DEPENDENT PATHWAY
Inflammation and oxidative stress are closely intertwined. On the one hand, activation of inflammatory cells, especially phagocytic cells results in the production of large amounts of reactive oxygen and nitrogen species (ROS/RNS) causing oxidative stress and tissue injury, as well as cancer [1, 2]. Such an oxidative tissue injury represents a major pathophysiological process of inflammatory disorders. On the other hand, ROS, due to their redox properties, activate transcription factors and intracellular signaling cascades, especially nuclear factor kappa B (NF-κB) and mitogen-activated protein kinases, leading to the increased transcription of pro-inflammatory cytokines and the consequent perpetuation of inflammatory responses [3, 4].
Because of the above relationship, it is understandable that antioxidants possess anti-inflammatory activities, at least, to some extent. It is also for the same rationale that the nuclear factor E2-related factor 2 (Nrf2), a central regulator of antioxidant genes, is widely viewed as an anti-inflammatory molecule. Indeed, Nrf2 deficiency aggravates the pathophysiology of inflammatory disorders [5], and on the contrary, Nrf2 activation frequently results in the suppression of inflammatory stress and the consequent tissue injury [6].
2. ANTI-INFLAMMATORY FUNCTION OF NRF2: REDOX-INDEPENDENT PATHWAY
Nrf2 plays a central role in regulating cellular antioxidant and other cytoprotective genes, in cells including macrophages [7, 8]. Numerous studies also demonstrate that this transcription factor appears to also modulate the expression of genes whose products have little or nothing to do with oxidative stress [9, 10]. It is not surprising for a particular transcription factor to be involved in the regulation of distinct classes of genes. In this context, a fundamental question about Nrf2 is that can its anti-inflammatory function be independent of its role in directly upregulating antioxidant genes?
In a recent study appeared in Nature Communications, Kobayashi et al. reported that Nrf2 suppresses macrophage inflammatory responses through blocking proinflammatory cytokine transcription [11]. Using bone marrow-derived macrophages, these authors first demonstrated that Nrf2 inhibited the induction by lipopolysaccharide and interferon-gamma of a sunset of proinflammatory genes [11]. With Nrf2 ChIP-seq and ChIP-qPCR analyses, Kobayashi et al. then showed the binding of Nrf2 to the proximity of proinflammatory genes of interleukin-6 (IL-6) and interleukin-1alpha and beta. They went on to further demonstrate that such Nrf2 binding directly inhibits the induced expression of the above cytokine genes [11]. Notably, the Nrf2-mediated inhibition of the proinflammatory cytokine genes is independent of the cellular redox status, further supporting the notion that the transcriptional inhibition of the cytokine genes is a primary action of Nrf2, rather than a secondary effect caused by the elimination of ROS through the production of Nrf2-regulated antioxidant enzymes and proteins [11].
To decipher the underlying molecular mechanism, Kobayashi et al. elegantly demonstrated that Nrf2 inhibits the recruitment of RNA polymerase II onto the proinflammatory cytokine gene loci without affecting the NF-κB p65 recruitment, and the inhibition occurs at the transcription initiation step [11]. NF-κB is a critical transcriptional regulator of many proinflammatory cytokine genes [12]. The no effect on NF-κB p65 recruitment suggests the involvement of other machineries in the Nrf2-mediated transcriptional suppression. Additional experiments by Kobayashi et al. also showed Nrf2-mediated inhibition of proinflammatory cytokine genes is independent of the antioxidant response element in upstream regulatory region [11].
To determine the in vivo significance, Kobayashi et al. employed the whole mount in vivo monitoring system with human IL-6-luciferase reporter to successfully verify the Nrf2-mediated inhibition of IL-6 gene induction as well as inflammatory symptoms in live mice [11].
3. CONCLUSION AND PERSPECTIVES
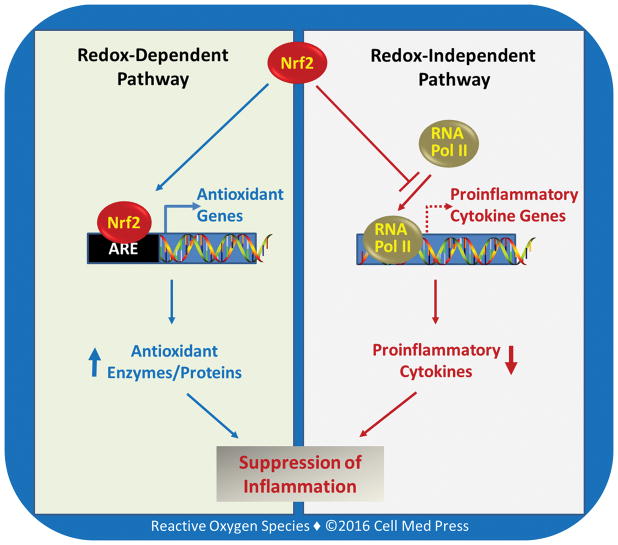
Although the detailed molecular machineries remain to be delineated, the study by Kobayashi et al. identifies Nrf2 as a direct suppressor of transcriptional induction of certain proinflammatory cytokine genes. This novel finding revealed a distinct mechanism by which Nrf2 signaling acts to inhibit inflammation and inflammatory tissue injury. It thus appears that Nrf2 may control inflammation through both antioxidant (redox)-dependent and -independent mechanisms (Figure 1). The differential contribution of these two distinct mechanisms to the anti-inflammatory function may vary with different cell types or inflammatory stimuli. Nevertheless, identification of the transcriptional suppression of certain inflammatory cytokine genes by Nrf2 in macrophages would certainly prompt more research efforts into deciphering the molecular basis of Nrf2 function in cell physiology and pathophysiology. Future work is warranted to determine the transcriptional suppression by Nrf2 signaling of other genes whose products are involved in inflammatory disorders as well as other pathophysiological conditions.
FIGURE 1. The two faces of Nrf2 in suppressing inflammation.
As illustrated, Nrf2 signaling may suppress inflammation via two pathways: redox-dependent and -independent pathways. RNA pol II denotes RNA polymerase II; ARE denoted antioxidant response element. This scheme is based on Ref. [9].
Acknowledgments
The authors of this article were supported in part by a grant from the United States National Institutes of Health/National Cancer Institute (CA192936).
ABBREVIATIONS
- Nrf2
nuclear factor E2-related factor 2
- NF-κB
nuclear factor kappa B
- ROS
reactive oxygen species
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Zhu H, Kuppusamy P, Zweier JL, Trush MA. Mitochondrial electron transport chain-derived superoxide exits macrophages: implications for mononuclear cell-mediated pathophysiological processes. Reactive Oxygen Species. 2016;1(1):81–98. doi: 10.20455/ros.2016.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38(2):225–36. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YR, Jia Z, Trush MA. Defining ROS in biology and medicine. Reactive Oxygen Species. 2016;1(1):9–21. doi: 10.20455/ros.2016.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66(24):11580–4. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 6.Keleku-Lukwete N, Suzuki M, Otsuki A, Tsuchida K, Katayama S, Hayashi M, et al. Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation. Proc Natl Acad Sci USA. 2015;112(39):12169–74. doi: 10.1073/pnas.1509158112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu H, Jia Z, Zhang L, Yamamoto M, Misra HP, Trush MA, et al. Antioxidants and phase 2 enzymes in macrophages: regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Exp Biol Med (Maywood) 2008;233(4):463–74. doi: 10.3181/0711-RM-304. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H, Zhang L, Itoh K, Yamamoto M, Ross D, Trush MA, et al. Nrf2 controls bone marrow stromal cell susceptibility to oxidative and electrophilic stress. Free Radic Biol Med. 2006;41(1):132–43. doi: 10.1016/j.freeradbiomed.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, et al. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40(15):7416–29. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway: identification of novel gene clusters for cell survival. J Biol Chem. 2003;278(10):8135–45. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87(1):13–20. doi: 10.1016/S0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]