Abstract
Objectives
Ultraviolet radiation (UVR), with UVB and UVA as the relevant components, is a risk factor for melanoma. Complete ascertainment and registration of melanoma in Iran was conducted in five provinces (Ardabil, Golestan, Mazandaran, Gilan and Kerman) during 1996–2000. The aim of our study was to compare population-based incidence data from these provinces with rates in the United States (US) while standardizing ambient UVR.
Methods
Population-based rates representing all incident cases of melanoma (1996–2000) across the five Iranian provinces were compared to rates of melanoma among white non-Hispanics in the US. Overall age-standardized rates (ASR) for Iran and the US (per 100,000 person-years adjusted to 2000 world population) and standardized rate ratios (SRR) were calculated.
We measured erythemally-weighted average solar UVR exposures (with contributions from both UVB and UVA range) of the five Iranian provinces using data from NASA's Total Ozone Mapping Spectrometer and selected five US states (Kentucky, Utah, Texas, Oklahoma, and Hawaii) with matching UVR exposure to each province. Incidence rates of melanoma during 1996–2000 in each Iranian province were compared to rates among white non-Hispanics in its UVR-matched US state.
Results
The overall male and female ASRs of melanoma were 0.60 (95%CI: 0.56–0.64) and 0.46 (95%CI: 0.42–0.49), respectively, for Iran and 22.78 (95%CI: 22.42–23.14) and 16.61 (95%CI: 16.30–16.92) for the US. SRRs of melanoma comparing US to Iran were 37.97 (95%CI: 35.78–40.29) for males and 36.11 (95%CI: 33.69–38.70) for females, indicating significantly higher incidence in the US. ASRs and age-specific rates of melanoma for both genders were significantly lower in each Iranian province compared to its UVR-matched US state.
Conclusion
The markedly lower incidence rates of melanoma in Iranian provinces with similar UVR exposures to US states underscore the need for additional comparative studies to decipher the influence of other extrinsic and intrinsic factors on the risk of this malignancy.
Keywords: Melanoma, Incidence rate, Ultraviolet radiation, Iran, NASA total ozone mapping spectrometer (TOMS)
1. Background
Incidence of malignant melanoma has been rising worldwide in the past several decades with consistent and dramatic increases noted for developed countries in Europe (Grange, 2005) and North America (Ward et al., 2006) since the 1950's. Recent trends indicate a global increase of 56% in melanoma incidence from 2005 to 2015 (Fitzmaurice et al., 2016). In the United States (US), melanoma is the fifth most common cancer among men and the seventh most common among women. There will be an estimated 52,170 new cases of melanoma among men and 34,940 cases among women in the US in 2017 (ACS, 2017). The sharp increase in melanoma incidence in the US has been reported for children as well as adults 50 and older (Jemal et al., 2011) with projected lifetime risk of developing melanoma for American men and women approaching 1 in 33 and 1 in 52, respectively (ACS, 2017). Although five-year relative survival is high at 91%, an estimated 65% of all skin cancer deaths are attributed to melanoma (Cummins et al., 2006). There will be an estimated 9730 deaths from melanoma among both sexes in the US in 2017 (ACS, 2017).
Reliable data on incidence rates of melanoma in developing countries are scarce. Studies on limited hospital and regional data from countries in Asia and Africa have suggested lower incidence but higher case fatality rates for melanoma in comparison to European and North American countries (Stubblefield and Kelly, 2014). The underlying causes of observed trends in melanoma in most countries are not known, although a number of host and environmental agents have been implicated as contributing factors (Ward et al., 2006; Rastrelli et al., 2014a; Liu et al., 2015; Cho et al., 2005). Exposure to ultraviolet radiation (UVR) from sunlight and other sources is considered an established risk factor for all major skin malignancies including melanoma (ACS, 2017; Elwood and Jopson, 1997; El Ghissassi et al., 2009). Solar UVR that reaches the surface of the earth is composed of UVB (with wavelength of 315–280 nm) and UVA (with wavelength of 400–315 nm) (Lucas et al., 2006, 2008). UVB is considered the carcinogenic component of UVR based on animal and laboratory studies (Lazovich et al., 2004; Horneck, 2000) that suggest effect on the DNA; UVA is also believed to be relevant based on its deep penetration of human skin (El Ghissassi et al., 2009; Wang et al., 2001).
Similar to other countries in the Middle East and to developing countries elsewhere, Iran does not have a comprehensive and/or centralized population-based cancer registration program. As such, regional cancer registries in existence in some provinces can provide incidence data for some cancers. During 1996–2000, the most complete and centrally-administered ascertainment and registration of melanoma in Iran was conducted for cases diagnosed in five provinces (Ardabil, Golestan, Mazandaran, Gilan and Kerman) (Fallah, 2007; Sadjadi et al., 2007). Therefore, we conducted a study to compare melanoma incidence rates from those provinces during that time period with rates in the United States (US) while standardizing ambient UVR. Ecologic studies describe patterns of disease and exposure at the population level and may be hypothesis-refining or -generating. As such, our ecologic study using perspectives from an understudied population may provide clues with respect to other influences on melanoma risk in the context of standardized UVR.
2. Methods
All cases of melanoma diagnosed in five provinces (Ardabil, Golestan, Mazandaran, Gilan and Kerman) during 1996–2000 were actively ascertained by Iran's Cancer Registry Unit at the Digestive Disease Research Center (DDRC) of the School of Medical Sciences at the University of Tehran as previously reported (Fallah, 2007; Sadjadi et al., 2007). As described in their publications, survey teams composed of one medical doctor and two medical students in each province, specifically trained for the task by the DDRC, actively collected data on cancer cases diagnosed in 1996–2000 from all hospitals, oncologists' offices, pathology laboratories, radiology clinics, and central death registry offices in each province. A copy of the pathology reports on each diagnosed case was sent to the Cancer Registry Unit where extensive quality control measures were employed prior to registration. These measures included careful review of all records to ensure inclusion of malignant primaries only (i.e., all benign and metastatic cases as well as cases with unknown origin were excluded) and deletion of all duplicates from the system (Fallah, 2007; Sadjadi et al., 2007). Thus, all cases of melanoma diagnosed for the first time between 1996 and 2000 among residents of the five provinces were registered. We obtained published (Fallah, 2007; Sadjadi et al., 2007) and updated incidence data from the DDRC for our study.
Population-based rates representing all incident cases of melanoma (1996–2000) across the five Iranian provinces were compared to rates of melanoma among white non-Hispanics in the US during 1996–2000 by calculating age-standardized rates (ASR) per 100,000 person-years adjusted using the 2000 world population. The overall rates for the US were calculated across the 13 areas of the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program (SEER), which include San Francisco-Oakland, Connecticut, Metropolitan Detroit, Hawaii, Iowa, New Mexico, Seattle-Puget Sound, Utah, Metropolitan Atlanta, San Jose-Monterey, Los Angeles, Alaska, and Rural Georgia. SEER rates are representative of cancer incidence in the total US population. SEER*Stat (Surveillance Research Program) and International Classification of Diseases for Oncology (ICD-O-3), Third Edition, site recode 25010 for melanoma of the skin (Fritz et al., 2000) were used for the analyses.
US rates for white non-Hispanics were used for comparison based on similarities in ancestry and pigmentation. Individuals from Iran are classified as white non-Hispanic in the census and in cancer registries in the US. There is no Spanish or Latino heritage or admixture among the Iranian population. In addition to exhibiting close genetic distance with European Caucasian populations (Cavalli-Sforza et al., 1994), Iranians are classified as “lightly pigmented” (i.e., skin types I to IV from north to south, respectively, on the Fitzpatrick pigmentation scale (Fitzpatrick, 1988)) similar to white non-Hispanics in the US and to European Caucasians (Lucas et al., 2006).
Standardized rate ratios (SRR), estimating the relative risks of melanoma in the US compared to Iran, were calculated by taking the ratios of the overall ASRs and calculating 95% confidence intervals (95% CI) using standard formulas (Cancer Registration, 1991); comparison was considered significant if the interval did not include 1.
In order to select US states to be matched to Iranian provinces on ambient UVR, the geographic coordinates of each province and their distances from the equator were obtained from the World Atlas (The World Atlas). US states were chosen based on similarity in latitude, altitude, and ambient erythemally-weighted average solar UVR to the Iranian provinces as well as availability of age-specific cancer incidence data in the North American Association of Central Cancer Registries (NAACCR) for the entire period 1996–2000. Grid points corresponding to geographic resolution of one degree were assigned to each province and state and were used for analysis of ambient UVR exposures. Erythemally-weighted average solar UVR exposure was measured using data from the National Aeronautics and Space Administration (NASA) Total Ozone Mapping Spectrometer (TOMS) (NASA; NASA, 2005). Data consisted of an ongoing time series of erythemally-weighted UVR exposure values (mW/m2) for the entire globe, derived from directly-measured noon irradiance values which took into account length of day, cloud conditions, and ozone column. The erythemally-weighted average exposure was the combination of wavelengths from 280 to 400 nm and therefore included contributions from both UVA (starting at 315 nm) and UVB (starting at 280); this average best describes the susceptibility of Caucasian skin to sunburn.
Erythemally-weighted average solar UVR exposure values were based on measurements between January 1,1997 and December 31, 2000 at a geographic resolution of one degree. Data for 1996 were recorded but excluded from analysis due to incompleteness. A degree was about 111 kilometers north to south and between 75 and 101 kilometers east to west in the continental US and between 85 and 100 kilometers east to west in Iran, depending on latitude. Each location had measurements for 88%–97% of the days, with most of the missing values due to the orbital path of the satellite. Erythemally-weighted average UVR exposures for each state and province were calculated by averaging the values of the grid points within each state and province.
The five state-province combinations matched based on ambient erythemally-weighted average solar UVR and used for comparison of incidence data were as follows: Ardabil-Kentucky, Gilan-Oklahoma, Golestan-Utah, Kerman-Hawaii, and Mazandaran-Texas. Data on the population of each US state was obtained from Census Bureau Data 2000 for white non-Hispanic population. Data on the population of each Iranian province from 1995 Census Data and projected to year 2000 based on annual health surveys was obtained from the DDRC. Incidence rates of melanoma among white non-Hispanic individuals in the five US States (Kentucky, Utah, Texas, Oklahoma, and Hawaii) were obtained from the NAACCR for 1996–2000 (NAACCR, 2008). Age-specific rates (intervals: 0–14, 15–24, 25–34, 35–44, 45–54, 55–64, and ≥65 years) and ASR (per 100,000 person-years adjusted using 2000 standard populations) were compared between each province and its UVR-matched US state.
3. Results
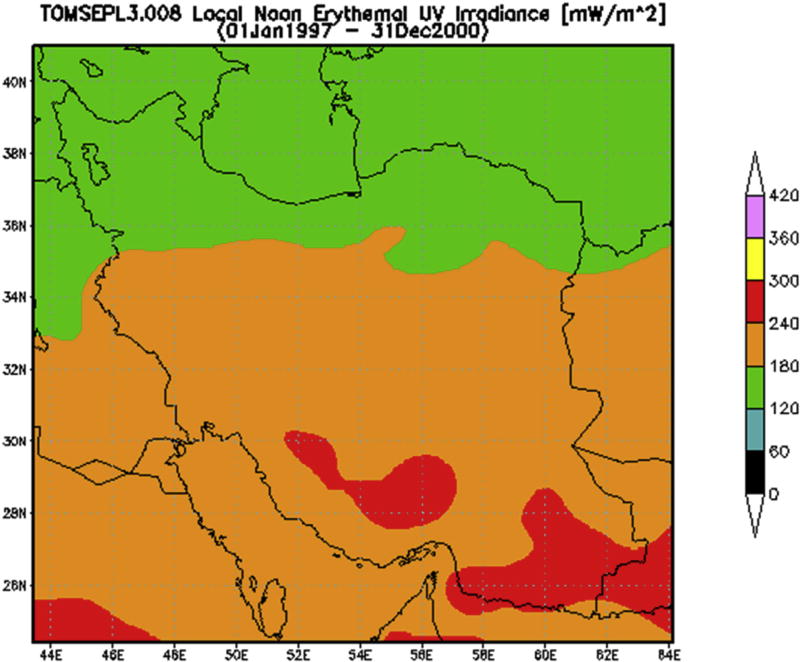
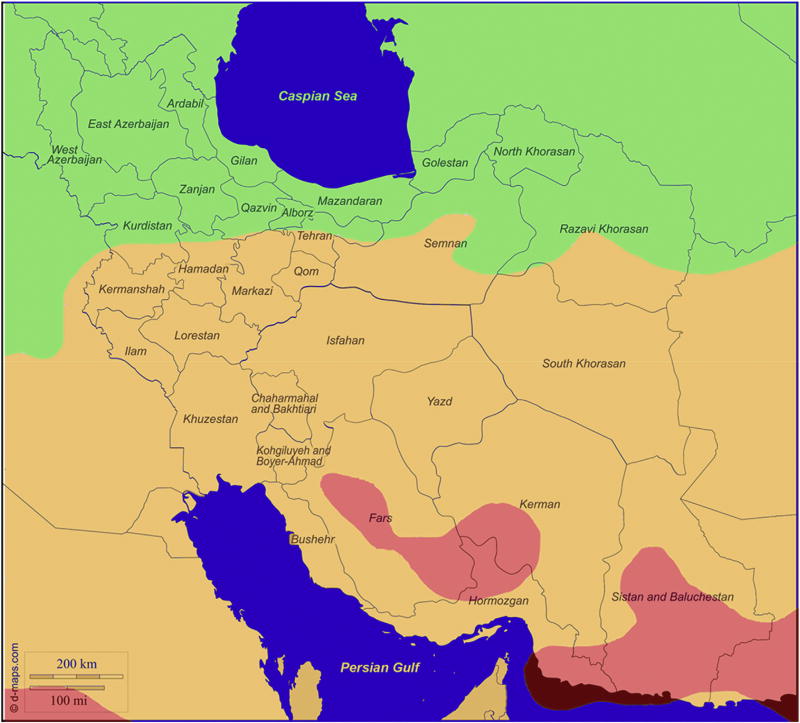
The location of Iran and its provinces are indicated in Fig. 1A. All five provinces had nearly equal proportions of males and females (Table 1) as well as similar ratio of rural to urban populations (data not shown). The 8,677,475 inhabitants of the five Iranian provinces represented ~13.0% of the total 68 million Iranian population during 1996–2000. There were nearly equal proportions of males and females residing in the five US States during the same time period (Table 1). The Annual erythemally-weighted average solar UVR exposure for the period 1997–2000 ranged from 140.3 mW/m2 in Ardabil to 230.5 mW/m2 in Kerman (Fig. 1A–C, Table 2). Fig. 1B is a map generated by NASA TOMS depicting the ambient erythemally-weighted average solar UV noon-time irradiance for all regions in Iran. Fig. 1C is a similar depiction obtained by overlaying the NASA TOMS-generated UV map for Iran (Fig.1B) on the geographic map of Iran (Fig. 1A). The exposures of the corresponding US states ranged from 127.9 mW/m2 in Kentucky to 248.4 mW/m2 in Hawaii during the same period (Table 2).
Fig. 1. Map of Iranian Provinces and Ultraviolet Radiation Exposure Patterns.
Panel A. Map of Iran created using d maps.com. The four provinces of Golestan, Mazandaran, Gilan and Ardabil in the north and the province of Kerman in the south east of the country are shown in green.
Panel B. Map of Erythemally-weighted average solar UV radiation (UVR) exposure values for Iranian provinces. Erythemally-weighted solar UV (with contributions from both UVB and UVA) was measured using data from National Aeronautics and Space Administration (NASA) Total Ozone Mapping Spectrometer (TOMS) (NASA; NASA, 2005). The color-coded scale on the right depicts the value of UV irradiance in mW/m2 for each province, showing a general increase in UVR exposure values from north to the south of the country.
Panel C. Panel B (Erythemally-weighted average Solar UV irradiance values depicted by color codes) superimposed on Panel A (geographic map of Iran).
Table 1.
| Mazandaran | Gilan | Golestan | Kerman | Ardabil | |
|
| |||||
| Male | 863,075 | 1,115,391 | 774,772 | 1,018,201 | 589,946 |
| Female | 877,697 | 1,126,505 | 747,696 | 986,127 | 578,065 |
| Total | 1,740,772 | 2,241,896 | 1,522,468 | 2,004,328 | 1,168,011 |
| Grid Points | 6 | 4 | 7 | 20 | 6 |
|
| |||||
| Texas | Oklahoma | Utah | Hawaii | Kentucky | |
|
| |||||
| Male | 5,377,619 | 1,248,024 | 946,784 | 149,079 | 1,757,620 |
| Female | 5,555,694 | 1,308,344 | 957,481 | 128,012 | 1,850,393 |
| Total | 10,933,313 | 2,556,368 | 1,904,265 | 277,091 | 3,608,013 |
| Grid Points | 69 | 22 | 23 | 10 | 16 |
Population data for Iranian provinces from Census Data 1995 and projected to year 2000 based on annual health surveys [obtained from Digestive Disease Research Center (DDRC)]; population data for American states obtained from Census Bureau Data 2000 for white non-Hispanic population.
Grid point represents geographic resolution of one-degree and was used for analysis of erythemally-weighted average solar ultraviolet radiation (UVR) exposure of each province and state.
Table 2.
Ambient erythemally-weighted average solar ultraviolet radiation exposurea for Iranian provinces and matching American states.
| 1996b | 1997 | 1998 | 1999 | 2000 | 1997–2000c | |
|---|---|---|---|---|---|---|
| Mazandaran (Texas) | 162.6 (158.6) | 170.6 (176.6) | 170.9 (181.7) | 176.7 (182.0) | 175.2 (176.3) | 173.3 (179.1) |
| Gilan (Oklahoma) | 134.9 (130.1) | 143.6 (150.1) | 146.1(155.8) | 148.6 (154.2) | 145.0 (149.7) | 145.8 (152.4) |
| Golestan (Utah) | 149.9 (140.4) | 159.5 (157.2) | 159 (155.7) | 161.7 (157.8) | 162.3 (159.8) | 160.6 (157.6) |
| Kerman (Hawaii) | 215.2 (237.9) | 228 (253.8) | 231.8 (252.2) | 233 (244.2) | 229.2 (243.6) | 230.5 (248.4) |
| Ardabil (Kentucky) | 131.2 (113.2) | 138 (126.3) | 141.3 (125.9) | 143.5 (133.2) | 138.6 (126.1) | 140.3 (127.9) |
Ultraviolet Radiation (UVR) exposure (with contributions from UVB and UVA) in mW/m2 obtained from NASA's Total Ozone Mapping Spectrometer (TOMS) [http://toms.gsfc.nasa.gov].
1996 UVR data were only available for period of July 22–December 31.
UVR averages were calculated for 1997–2000 period due to incomplete data for 1996.
During 1996–2000, the overall ASR of melanoma incidence for Iran was estimated at 0.60 (95%CI: 0.56–0.64) and 0.46 (95%CI: 0.42–0.49) per 100,000 person-years among males and females, respectively (Table 3). During the same time period, the overall incidence rate of melanoma in the US (among white non-Hispanics) was 22.78 (95%CI: 22.42–23.14) and 16.61 (95%CI: 16.30–16.92) per 100,000 person-years among males and females, respectively (Table 3). The SRR of melanoma comparing the overall ASR in the US to Iran was 37.97 (95%CI: 35.78–40.29) for males and 36.11 (95% CI: 33.69–38.70) for females, indicating statistically significantly higher incidence of melanoma in the US.
Table 3.
Incidence ratesa of melanoma in Iran and the United States (US) for 1996–2000.
| 1996 | 1997 | 1998 | 1999 | 2000 | 1996–2000b | SRR (95%CI)c | |
|---|---|---|---|---|---|---|---|
| Male, Iran | 0.48 | 0.76 | 0.59 | 0.60 | 0.50 | 0.60, 95%CI: 0.56–0.64 | |
| Male, US | 21.97 | 21.87 | 22.50 | 23.07 | 24.44 | 22.78, 95%CI: 22.42–23.14 | 37.97 (35.78–40.29) |
| Female, Iran | 0.42 | 0.22 | 0.58 | 0.51 | 0.55 | 0.46, 95%CI: 0.42–0.49 | |
| Female, US | 15.63 | 16.23 | 16.52 | 17.23 | 17.43 | 16.61, 95%CI: 16.30–16.92 | 36.11 (33.69–38.70) |
Age-standardized rates (ASR) per 100,000 adjusted to 2000 world (WHO, 2000–2025) population.
Population-based incidence rates and 95% Confidence Intervals (95%CI) for Iran from the cancer registries of the provinces of Mazandaran, Gilan, Golestan, Kerman and Ardabil [obtained from Digestive Disease Research Center (DDRC) (Fallah, 2007). Rates for the United States were obtained from population-based incidence data reported for white non-Hispanics in 13 Surveillance, Epidemiology and End Results (SEER) areas, which include San Francisco-Oakland, Connecticut, Metropolitan Detroit, Hawaii, Iowa, New Mexico, Seattle-Puget Sound, Utah, Metropolitan Atlanta, San Jose-Monterey, Los Angeles, Alaska, and Rural Georgia.
Standardized rate ratio (SRR): the ratio of ASR in the US compared to Iran, representing the relative risk of melanoma. 95% confidence interval (95% CI) of SRR calculated using standard formulas (Cancer Registration, 1991).
Age-specific rates and ASR of melanoma for both genders were significantly higher in each of the UVR-matched US states than the corresponding Iranian provinces (Table 4). Incidence rates among males in the five Iranian provinces in comparison to their UVR-matched US states were as follows: 0.30 (22.6) for Ardabil (Kentucky), 1.20 (26.6) for Golestan (Utah), 0.73 (18.1) for Mazandaran (Texas), 0.17 (15.8) for Gilan (Oklahoma), and 0.90 (75.3) for Kerman (Hawaii). Incidence rates of melanoma among females were as follows: 0.20 (16.0) for Ardabil (Kentucky), 0.60 (17.5) for Golestan (Utah), 0.47 (10.6) for Mazandaran (Texas), 0.17 (8.9) for Gilan (Oklahoma), and 0.90 (36.5) for Kerman (Hawaii) (Table 4).
Table 4.
Age-specific and standardized incidence ratesa of melanoma in five Iranian provinces and American states matched based on ambient erythemally-weighted average solar ultraviolet radiation exposure (1996–2000).
| Number of Cases | 0–14 | 15–24 | 25–34 | 35–44 | 45–54 | 55–64 | ≥65 | ASR | |
|---|---|---|---|---|---|---|---|---|---|
| Males | |||||||||
| Mazandaran (Texas) | 26 (4720) | 0.06 (0.2) | 0.00 (2.1) | 0.46 (5.9) | 1.08 (12.2) | 1.08 (22.0) | 0.84 (36.5) | 4.74 (69.8) | 0.73 (18.1) |
| Gilan (Oklahoma) | 7 (829) | 0.00 (0.0) | 0.00 (1.6) | 0.13 (4.9) | 0.17 (8.9) | 0.00 (20.8) | 1.28 (31.6) | 0.30 (62.7) | 0.17 (15.8) |
| Golestan (Utah) | 26 (921) | 0.00 (0.3) | 0.40 (2.7) | 0.40 (11.1) | 0.50 (18.3) | 2.80 (37.6) | 4.70 (52.4) | 4.20 (94.9) | 1.20 (26.6) |
| Kerman (Hawaii) | 38 (546) | 0.10 (1.0) | 0.20 (6.1) | 0.20 (30.1) | 0.60 (51.4) | 0.40 (107.9) | 4.40 (141.8) | 5.20 (276.4) | 0.90 (75.3) |
| Ardabil (Kentucky) | 4 (1867) | 0.00 (0.1) | 0.00 (2.5) | 0.00 (7.5) | 0.00 (14.6) | 0.00 (30.1) | 1.80 (48.6) | 1.80 (83.3) | 0.30 (22.6) |
| Females | |||||||||
| Mazandaran (Texas) | 20 (3257) | 0.00 (0.1) | 0.00 (3.2) | 0.44 (7.8) | 0.61 (12.6) | 0.00 (15.0) | 1.31 (19.3) | 3.3 (26.6) | 0.47 (10.6) |
| Gilan (Oklahoma) | 7 (561) | 0.00 (0.3) | 0.00 (1.6) | 0.13 (4.7) | 0.17 (11.3) | 0.28 (11.2) | 0.34 (15.7) | 1.05 (26.1) | 0.17 (8.9) |
| Golestan (Utah) | 13 (717) | 0.00 (0.2) | 0.10 (4.9) | 0.20 (15.8) | 0.30 (20.2) | 2.20 (21.6) | 1.70 (31.5) | 2.40 (45.1) | 0.60 (17.5) |
| Kerman (Hawaii) | 26 (260) | 0.10 (0.0) | 0.00 (8.5) | 0.00 (29.4) | 0.60 (49.3) | 0.40 (58.4) | 4.50 (47.1) | 5.10 (89.6) | 0.90 (36.5) |
| Ardabil (Kentucky) | 4 (1564) | 0.00 (0.1) | 2.00 (5.0) | 0.30 (13.3) | 0.00 (19.7) | 0.00 (25.4) | 2.10 (29.2) | 0.00 (33.7) | 0.20 (16.0) |
Incidence rates for Iran were obtained from the Digestive Disease Research Center (DDRC) and age-adjusted to the 2000 Iran population. Incidence rates among white non-Hispanics for the five US States obtained from the North American Association of Central Cancer Registries (NAACCR); Cancer in North America (CiNA) Analytic File 1995–2005 for NAACCR Hispanic/Latino Identification Algorithm (NHIAv.2) Origin, Standard File, Springfield, IL: NAACCR 2008 and adjusted to the 2000 US standard population (19 age groups - Census P25-1130).
Among the Iranian provinces, Golestan had the highest ASR of melanoma incidence for men followed by Kerman with second highest incidence among men and highest rate for women. Gilan had the lowest rates for both genders (Table 4). Among the US states, Hawaii had the highest ASR of melanoma for both men and women, and Oklahoma had the lowest rates (Table 4). While analysis of melanoma rates by age and gender in the five Iranian provinces did not reveal a consistent pattern with respect to age (probably due to small number of cases in some of the age groups) (Table 4), melanoma rates in the US increased steadily with age until the 7th or 8th decades of life for both genders (Table 4). Incidence rates were either similar for both genders or lower among women in the Iranian provinces; lower rates were noted among women in the US states examined (Table 4).
4. Discussion
We have conducted an ecologic study of melanoma, comparing overall incidence rates in Iran and the US as well as incidence rates in five Iranian provinces and five US states matched based on ambient erythemally-weighted average solar UVR. To our knowledge, a direct comparison of incidence rates of melanoma while standardizing UVR exposure, such as ours, has not been previously reported. Our analysis revealed significantly lower incidence rates of melanoma in Iran compared to the US. Additionally, incidence of melanoma was lower in each of the five provinces compared to each UVR-matched US state for both genders across all ages and years during the study period.
We chose incidence during 1996–2000 in five provinces for our comparison since the most complete and centrally-administered ascertainment and registration of melanoma in Iran was conducted for cases diagnosed in those provinces during that time period (Fallah, 2007; Sadjadi et al., 2007). Using incidence rates based on complete ascertainment was important given that melanoma incidence measures are particularly vulnerable to under-reporting, even in established and mandated cancer registries in developed countries (Cockburn et al., 2008; Koh et al., 1992). Therefore, underreporting was not a major contributing factor to the lower rates in Iranian provinces in this particular study. Under-diagnosis is also unlikely to explain the significantly lower rates in Iran as there have been no reports of problems with under-diagnosis of melanoma in any country including Iran. Although, it is possible that cases among the elderly and among those living in rural areas could have been missed in terms of diagnosis, it is important to note that our rates for Iran are consistent with the most recent GLOBOCAN (GLOBOCAN; Ferlay et al., 2013) estimates of ASR of melanoma for Iran males (0.9 per 100,000) and females (0.6 per 100,000). GLOBOCAN estimates for most developing countries are obtained from limited hospital- and regional-based data from the country itself (if available) or its neighbors. Reliable cancer incidence data from countries neighboring Iran are generally not available. A recent report of ASRs (per 100,000 person-years during 2003–2007) of melanoma across four regions (Antalya, Edrine, Izmir, and Trabzon) in Turkey, a country that borders Iran on the West, revealed a similar pattern of low melanoma incidence among both males (1.7–2.1) and females (1.1–1.9) (Cancer Incidence in Five Continents, 2014).
Our intra-country comparisons revealed a general but inconsistent trend of increasing melanoma rates with increasing ambient erythemally-weighted average solar UVR exposure. Among the US States, Hawaii had the highest UVR exposure values and the highest melanoma incidence rates for both males and females. Kentucky had the lowest UVR exposure values but the lowest melanoma incidence among both genders was seen in Oklahoma. Higher rates of melanoma in Utah compared to Texas despite lower UVR exposure values cannot be readily explained. Similarly, higher rates of melanoma in Kentucky compared to Texas despite lower UVR exposure values does not have an immediate explanation. Among the Iranian provinces, Kerman had the highest UVR exposure value and the highest rate of melanoma among females only. Among males, rates were highest in Golestan despite lower UVR exposure in that province compared to Kerman and Mazandaran. Ardabil with lowest UVR exposure estimates among all Iranian provinces had higher rates of melanoma compared to Gilan, which had slightly higher UVR exposure than Ardabil but the lowest rates of melanoma for both genders.
Provinces of Mazandaran, Gilan, and Golestan are located in the north and harbor the moderate climate of the Caspian seashore. The majority of the populations of these three provinces are Persians (of ancient Arya or Caucasian ancestry). The province of Ardabil, located in the north west mountainous region, experiences cool summers and cold winters. A large proportion of inhabitants of Ardabil are Azeri (also of Caucasian ancestry). The average skin pigmentation of people living in these provinces is light, showing affinities with less pigmented populations residing in neighboring countries to the West of Iran (Mehrai and Sunderland, 1990). The province of Kerman is located in southeastern Iran with dry moderate climate in the north of the province and warm and humid in the south. The population of Kerman are also majority Persians but with deeper skin pigmentation.
Most reports of cancer incidence in Iran do not report rates for melanoma separately, but instead focus on rates for either non-melanoma skin cancer or all skin cancers combined. Two previous studies, which analyzed melanoma rates using data from a single regional registry, found incidence rates compatible with ours. One study analyzed 20 years of data collected from the cancer registry of the province of Yazd in south central Iran during 1988–2008 and found the mean crude incidence rates of 0.40 per 100,000 person-years for males and 0.27 for females, with relatively fixed temporal trends (Noorbala et al., 2013). Yazd, a province with one million inhabitants, is located in the hot and dry desert area of the country with bright sunlight during most seasons. Based on the UVR exposure map (Fig. 1A–C), Kerman (the southern province analyzed in our study) has higher erythemally-weighted average solar UVR exposure than Yazd, which may explain the higher rates of melanoma for Kerman in our study compared to those reported for Yazd in the cited reference (Noorbala et al., 2013). Another report from the population-based registry in the southern province of Fars (Fig.1A–C) found rates comparable to our study and increasing trends of melanoma for both genders. Among men, the ASR (per 100,000) increased from 0.8 in the previous survey (1988–1992) to 3.8 in the current registry survey (1998–2002), and in women, this increase was from 0.7 to 2.5 per 100,000 (Masoompour et al., 2011). A study of the overall rate of skin cancer in all 30 provinces in Iran reported a male to female ratio of ~1.6–~1.7 for all skin cancers combined, but did not analyze rates separately for melanoma (Razi et al., 2015). Our comparisons of sex-specific incidence rates also depict a pattern of lower overall rates of melanoma among women in Iran.
Despite decades of debate about the relationship between UVR and melanoma (Moan et al., 2008; Rastrelli et al., 2014b), higher rates of this malignancy among Caucasians compared to racial/ethnic groups with deeper skin pigmentation (Moan et al., 2008) and among patients with xeroderma pigmentosum (XP) (Lehmann, 2003), a rare disorder of the nucleotide excision repair (NER) pathway (i.e., cellular pathway which repairs damage to DNA caused by UVR), have supported the evidence for UVR as a major risk factor for melanoma. It has been suggested that incidence of melanoma is determined as much by the pattern of sun exposure as by its total accumulated dose. The intermittent exposure hypothesis suggests that frequent exposure of untanned skin to intense sunlight is particularly effective in increasing risk of melanoma (Elwood and Jopson, 1997). The current belief is that UVR is responsible for up to 90% of melanoma incidence in the US, with up to 8% attributed to indoor tanning (AACR, 2016). Among sun safe practices recommended to reduce melanoma risk are use of hats and clothing that cover arms and legs (AACR, 2016). Given similarities in skin pigmentation between the Iranian and European American populations analyzed in our study, lower melanoma rates in Iran in the context of standardized UVR may be partly explained by differences in clothing and coverage in the two populations.
For both women and men in Iran, there is a dress code in effect, which constitutes outfits that cover nearly the entire arms and legs. The dress code for women also involves covering their hair to various degrees with scarves or hats. Men may also wear hats, particularly those involved in farming or other outdoor occupations. In the northern provinces of Mazandaran, Gilan and Golestan, it is not uncommon for women to work alongside men in outdoor occupations related to rice agriculture; the location of these provinces also allows inhabitants unique access to the Caspian Sea year round for water-related activities.
Other environmental/lifestyle factors such as diet and nutrition may also have a main or modifying effect on the risk of melanoma; these potential extrinsic factors remain to be identified and their roles remain to be elucidated. There are suggestions that intrinsic factors such as individual DNA repair capacity and genetic profiles may confound or modify the UVR-melanoma association (Bishop et al., 2009; Mocellin et al., 2009; Hsu et al., 2013), but the findings remain to be validated. Epigenetic profiles may also be relevant and remain to be investigated in well-designed studies of the role of intrinsic factors in melanoma etiology.
Ecologic studies such as ours, which describe patterns of disease and exposure at the population level, are particularly well-suited to investigations of exposures such as UVR. Reliable individual-level information on exposure to UVR is difficult to obtain in observational studies. We were able to utilize our expertise in geographic information systems to modify our ecologic study to further standardize the exposure variable for a more effective comparison of disease rates in the two populations. Alongside these strengths are certain limitations of our study. US states are on average bigger than Iranian provinces and may have a wider range of UVR exposure values influencing the calculated averages. The ecologic design of the study also makes it difficult to determine the underlying risk or causative factors for the reported differences. Other exposures, such as diet/nutrition or genetic/epigenetic profiles may exhibit wide variations within the population and would be better studied at the individual level using other observational study designs such as cohort or case-control as opposed to ecologic. Future studies will also benefit from inclusion of information on histopathologic features of the disease (such as site, stage, and thickness of melanoma), which were unavailable to us for this study.
5. Conclusions
In summary, using an ecologic study design modified to allow for standardization of the exposure, we found markedly lower incidence rates of cutaneous malignant melanoma in five Iranian provinces (with complete case ascertainment) compared to five US states with similar ambient erythemally-weighted average solar UVR exposure patterns. Our findings underscore the need for additional comparative studies to decipher the influence of other extrinsic and intrinsic factors on the risk of this potentially preventable malignancy.
Acknowledgments
The authors are grateful to Dr. Paul H. Levine at the University of Nebraska Medical Center, to Dr. Amiran Dzutsev at the National Cancer Institute of the National Institutes of Health, and to the staff at the Digestive Disease Research Center of the School of Medical Sciences at the University of Tehran for their support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- ASR
Age Standardized Rate
- DDRC
Digestive Disease Research Center
- NAACCR
North American Association of Central Cancer Registries
- NASA
National Aeronautics and Space Administration
- SEER
Surveillance, Epidemiology and Ends Results
- TOMS
Total Ozone Mapping Spectrometer
- US
United States
- UVR
Ultraviolet Radiation
- UVB
Ultraviolet radiation with wavelengths of 315–280 nm
- UVA
Ultraviolet radiation with wavelengths of 400–315 nm
Footnotes
This paper has been recommended for acceptance by David Carpenter.
Authors' contributions
RM contributed to the inception of the study and the study design/methodology, obtained the Iran incidence data, analyzed the US SEER incidence data, guided the statistical comparisons of the overall incidence rates, guided the interpretations of study findings, and drafted the manuscript. NZ helped with the analysis of the NASA TOMS UVR exposure data, statistical comparisons of the overall incidence rates, and interpretations of findings. FPB contributed to the study design/methodology, analyzed the US NAACCR incidence data, guided the analyses of NASA TOMS UVR exposure data, and helped with interpretations.
Authors' information
RM is a genetic epidemiologist with expertise in genomic and epidemiologic studies of cancer and cancer precursors, who is engaged in research and teaching as an Associate Professor at University at Albany. NZ obtained her doctoral degree in epidemiology at University at Albany under RM's mentorship and is now a post-doctoral fellow at Columbia University. FPB is a cancer epidemiologist at the New York State (NYS) Cancer Registry at the NYS Department of Health and a research professor at University at Albany.
References
- American Association for Cancer Research. AACR cancer progress report 2016. Clin. Cancer Res. 2016;22(Suppl. 1):S1–S137. doi: 10.1158/1078-0432.CCR-16-1993. [DOI] [PubMed] [Google Scholar]
- American Cancer Society Cancer Facts & Figures 2017. Atlanta: American Cancer Society; 2017. [Google Scholar]
- Bishop DT, Demenais F, Iles MM, Harland M, Taylor JC, Corda E, Randerson-Moor J, Aitken JF, Avril MF, Azizi E, et al. Genome-wide association study identifies three loci associated with melanoma risk. Nat. Genet. 2009;41(8):920–925. doi: 10.1038/ng.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Incidence in Five Continents. IARC Scientific Publication No. 164. X. Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- Jensen OM, Parkin DM, MacLennan R, Muir CS, Skeet RG, editors. International Agency for Cancer Research Publication No. 95. 1991. Cancer Registration: Principles and Methods. [Google Scholar]
- Cavalli-Sforza LL, Menozzi P, Piazza A. The History and Geography of Human Genes. Princeton University Press; Princeton, N.J.: 1994. [Google Scholar]
- Cho E, Rosner BA, Colditz GA. Risk factors for melanoma by body site. Cancer Epidemiol. Biomarkers Prev. 2005;14(5):1241–1244. doi: 10.1158/1055-9965.EPI-04-0632. [DOI] [PubMed] [Google Scholar]
- Cockburn M, Swetter SM, Peng D, Keegan TH, Deapen D, Clarke CA. Melanoma underreporting: why does it happen, how big is the problem, and how do we fix it? J. Am. Acad. Dermatol. 2008;59(6):1081–1085. doi: 10.1016/j.jaad.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins DL, Cummins JM, Pantle H, Silverman MA, Leonard AL, Chanmugam A. Cutaneous malignant melanoma. Mayo Clin. Proc. 2006;81(4):500–507. doi: 10.4065/81.4.500. [DOI] [PubMed] [Google Scholar]
- El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, et al. A review of human carcinogens–part D: radiation. Lancet Oncol. 2009;10(8):751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int. J. Cancer. 1997;73(2):198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Fallah M. Cancer Incidence in Five Provinces of Iran University of Tampere 2007 [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. International Agency for Research on Cancer. Lyon, France: 2013. [accessed on day/month/year. 2013]. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. [Internet] Available from: http://globocan.iarc.fr. [Google Scholar]
- Global Burden of Disease Cancer C. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al. Global, regional, and national cancer incidence, Mortality, Years of life Lost, Years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2016;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988;124(6):869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM. International Classification of Diseases for Oncology. third. World Health Organization; Geneva (Switzerland): 2000. p. 61. [Google Scholar]
- Grange F. Epidemiology of cutaneous melanoma: descriptive data in France and Europe. Ann. Dermatol Venereol. 2005;132(12 Pt 1):975–982. doi: 10.1016/s0151-9638(05)79560-0. [DOI] [PubMed] [Google Scholar]
- Horneck G. Quantification of biologically effective environmental UV irradiance. Adv. Space Res. 2000;26(12):1983–1994. doi: 10.1016/s0273-1177(00)00172-1. [DOI] [PubMed] [Google Scholar]
- Hsu I, Chen R, Ramesh A, Corona E, Kang HP, Ruau D, Butte AJ. Systematic identification of DNA variants associated with ultraviolet radiation using a novel Geographic-Wide Association Study (GeoWAS) BMC Med. Genet. 2013;14:62. doi: 10.1186/1471-2350-14-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J, Wiggins CL, Wingo PA. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J. Am. Acad. Dermatol. 2011;65(5 Suppl. 1):S17–S25. e11–13. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Koh HK, Geller A, Miller DR, Clapp RW, Lew RA. Underreporting of cutaneous melanoma in cancer registries nationwide. J. Am. Acad. Dermatol. 1992;27(6 Pt 1):1035–1036. doi: 10.1016/s0190-9622(08)80285-x. [DOI] [PubMed] [Google Scholar]
- Lazovich D, Sweeney C, Weinstock MA, Berwick M. Re: a prospective study of pigmentation, sun exposure, and risk of cutaneous malignant melanoma in women. J. Natl. Cancer Inst. 2004;96(4):335. doi: 10.1093/jnci/djh054. author reply 337–338. [DOI] [PubMed] [Google Scholar]
- Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85(11):1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang W, Gao T, Li C. Is UV an etiological factor of acral melanoma? J. Expo. Sci. Environ. Epidemiol. 2015;26(6):539–545. doi: 10.1038/jes.2015.60. [DOI] [PubMed] [Google Scholar]
- Lucas R, McMichael T, Smith W, Armstrong B. Solar Ultraviolet Radiation, Global Burden of Disease from Solar Ultraviolet Radiation. World Health Organization. Environmental Burden of Disease Series, No 13 2006 [Google Scholar]
- Lucas RM, McMichael AJ, Armstrong BK, Smith WT. Estimating the global disease burden due to ultraviolet radiation exposure. Int. J. Epidemiol. 2008;37(3):654–667. doi: 10.1093/ije/dyn017. [DOI] [PubMed] [Google Scholar]
- Masoompour SM, Yarmohammadi H, Rezaianzadeh A, Lankarani KB. Cancer incidence in southern Iran, 1998–2002: results of population-based cancer registry. Cancer Epidemiol. 2011;35(5):e42–47. doi: 10.1016/j.canep.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Mehrai H, Sunderland E. Skin colour data from Nowshahr City, northern Iran. Ann. Hum. Biol. 1990;17(2):115–120. doi: 10.1080/03014469000000862. [DOI] [PubMed] [Google Scholar]
- Moan J, Porojnicu AC, Dahlback A. Ultraviolet radiation and malignant melanoma. Adv. Exp. Med. Biol. 2008;624:104–116. doi: 10.1007/978-0-387-77574-6_9. [DOI] [PubMed] [Google Scholar]
- Mocellin S, Verdi D, Nitti D. DNA repair gene polymorphisms and risk of cutaneous melanoma: a systematic review and meta-analysis. Carcinogenesis. 2009;30(10):1735–1743. doi: 10.1093/carcin/bgp207. [DOI] [PubMed] [Google Scholar]
- NASA Goddard Space Flight Center Total Ozone Mapping Spectrometer Data Product: Erythemal UV Exposure. 2005 http://toms.gsfc.nasa.gov/ery_uv/euv_v8.thm.
- Noorbala MT, Mohammadi S, Noorbala M. Cutaneous malignant melanoma in central Iran: a 20-year study. Iran. Red. Crescent Med. J. 2013;15(8):690–694. doi: 10.5812/ircmj.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastrelli M, Tropea S, Pigozzo J, Bezzon E, Campana LG, Stramare R, Alaibac M, Rossi CR. Melanoma m1: diagnosis and therapy. In Vivo. 2014;28(3):273–285. [PubMed] [Google Scholar]
- Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28(6):1005–1011. [PubMed] [Google Scholar]
- Razi S, Enayatrad M, Mohammadian-Hafshejani A, Salehiniya H, Fathali-Loy-Dizaji M, Soltani S. The epidemiology of skin cancer and its trend in Iran. Int. J. Prev. Med. 2015;6:64. doi: 10.4103/2008-7802.161074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadjadi AZ, Darvish MJ, Moghadam S, Nouraei M, Alimohammadian M, Ghorbani A, Bahmanyar S, Mohagheghi MA, Malekzadeh R. The first population-based cancer survey in kerman province of Iran. Iran. J. Public Health. 2007;36(4):26–34. [Google Scholar]
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg. Clin. North Am. 2014;94(5):1115–1126. doi: 10.1016/j.suc.2014.07.008. (ix) [DOI] [PubMed] [Google Scholar]
- Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, Bart RS. Ultraviolet A and melanoma: a review. J. Am. Acad. Dermatol. 2001;44(5):837–846. doi: 10.1067/mjd.2001.114594. [DOI] [PubMed] [Google Scholar]
- Ward EM, Thun MJ, Hannan LM, Jemal A. Interpreting cancer trends. Ann. N. Y. Acad. Sci. 2006;1076:29–53. doi: 10.1196/annals.1371.048. [DOI] [PubMed] [Google Scholar]
- The World Atlas. [accessed 06/09/2016]; www.worldatlas.com)
- National Aeronautics and Space Administration (NASA) Total Ozone Mapping Spectrometer (TOMS) [accessed 06/09/2016]; ( http://ozoneaq.gsfc.nasa.gov/)
- North American Association of Central Cancer Registries (NAACCR) Cancer in North America (CiNA) Analytic File 1995–2005 for NAACCR Hispanic/Latino Identification Algorithm (NHIAv.2) Origin, Standard File. Springfield, IL: NAACCR; 2008. [accessed 06/09/2016]. ( http://www.naaccr.org/) [Google Scholar]
- SEER Surveillance Epidemiology and End Results (SEER) Program. National Cancer Institute (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 13 Regs Research Data. Nov 2014 Sub (1996–2000)<Katrina/Rita Population Adjustment> - Linked to County Attributes – Total U.S., 1969–2013 Counties. [accessed 03/15/2016];National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. released April 2015, based on the Nov. 2014 submission.
- Surveillance Research Program Surveillance Research Program, National Cancer Institute SEER*Stat software (www.seer.cancer.gov/seerstat) [accessed 03/15/2016];version 8.2.1.
- GLOBOCAN. GLOBOCAN 2012, V1.0. Cancer Incidence and Mortality Worldwide: IARC; [accessed 06/09/2016]. http://globocan.iarc.fr. [Google Scholar]