Abstract
Objectives
Abdominal obesity is linked with a higher risk of developing ovarian cancer. However, the link between abdominal obesity and survival after diagnosis of ovarian cancer is unknown. The purpose of this study was to determine the impact of abdominal obesity on progression-free survival in patients with ovarian cancer.
Methods
Among 258 patients, visceral and subcutaneous adipose tissue volume, along with perirenal adipose tissue thickness (a visceral adiposity proxy measure) was retrospectively measured from abdominal computed tomography (CT) scans obtained within 6 months of ovarian cancer diagnosis. Progression-free survival was computed using the Kaplan-Meier method and log-rank tests. Univariate and multivariate Cox proportional hazards analysis was used to determine relationships between measures of abdominal obesity and clinical variables in relation to progression-free survival.
Results
Patients with perirenal adipose tissue thickness greater than 5 mm(median) had lower rates of progression-free survival at 5 years compared with patients with perirenal adipose tissue thickness less than 5 mm (45.6% vs 53.8%, respectively). Perirenal adipose tissue thickness less than 5 mm was associated with lower rates of progression-free survival on multivariate analysis (hazard ratio = 1.37; 95% confidence interval, 1.03–1.82). There was no correlation with other metrics of abdominal adiposity on progression-free survival in univariate or multivariate analysis.
Conclusions
Our data suggest that perirenal adipose, but not body mass index, visceral, or subcutaneous fat volume that were measured within 6 months from diagnosis, is associated with lower rates of progression-free survival in ovarian cancer.
Keywords: Ovarian cancer, Perirenal adipose tissue thickness, Visceral adipose tissue, Subcutaneous adipose tissue, Body mass index
According to the World Health Organization’s International Agency for Research on Cancer, obesity is linked with a higher risk of many types of malignancy.1 Specifically, abdominal obesity increases the risk of metabolic dysfunction and intraabdominal cancers, including ovarian cancer.2–6 Abdominal obesity is composed of fat deposits within the subcutaneous, visceral, or perirenal regions. In excessive amounts, both visceral adipose tissue (VAT) and perirenal adipose tissue, which are defined as the fat compartments between the kidney and abdominal wall, are considered markers of metabolic dysfunction.3,4,7 Moreover, the thickness of the perirenal fat depot is highly correlated with visceral adiposity and has been proposed as a proxy measure for visceral fat.8,9 Overall, visceral and perirenal fat compartments within abdominal obesity may serve as significant contributors to increasing risk of intraabdominal cancers.
Among the types of intraabdominal cancers, in ovarian cancer, VAT may be particularly relevant. This is because omentum, which is composed of VAT, is a frequent distant metastatic site of ovarian cancer.10 Furthermore, adipocytes can directly stimulate the adhesion, migration, and invasion of ovarian cancer cells,11,12 and multipotent mesenchymal stromal cells located in VAT, adipose stromal cells, promote tumor growth more potently than adipose stromal cells from subcutaneous adipose tissue (SAT).13 Therefore, the purpose of this study was to assess the impact of different measures of abdominal adiposity on progression-free survival in patients with ovarian cancer.
METHODS
Design
This was a retrospective analysis comprising patients with stage I to IV ovarian or primary peritoneal cancer who were included in a previously established tumor banking protocol and underwent initial surgery between January 1, 2001 and December 31, 2009 with an evaluable computed tomography (CT) scan obtained within 6 months of diagnosis: 94% of patients had CT scans performed 6months before surgery. The study was approved by the institutional review board at The University of Texas MD Anderson Cancer Center, Houston, Tex.
Patient and Disease Characteristics
Patient characteristics and disease history were obtained from the medical record. Age and body mass index (BMI) used in the present investigation corresponded with the date of the CT scan (see further section for details). Variables related to disease history included CA125 levels before and after treatment, treatment type, cancer stage, histology, and presence and degree of residual disease. Time to recurrence or death was computed from the date of diagnosis.
Visceral Adiposity, Subcutaneous Adiposity, Perirenal Fat Thickness
All participants underwent a CT scan of the abdomen and pelvis as part of their routine care. The General Electric CT scanner (GE Medical Systems, Milwaukee, Wis) was used to perform these scans, and images were saved as digital imaging and communications in medicine files for analysis. Standard procedures were followed, using 120 kV, 5 mm thickness, and a field of view of 50 cm.
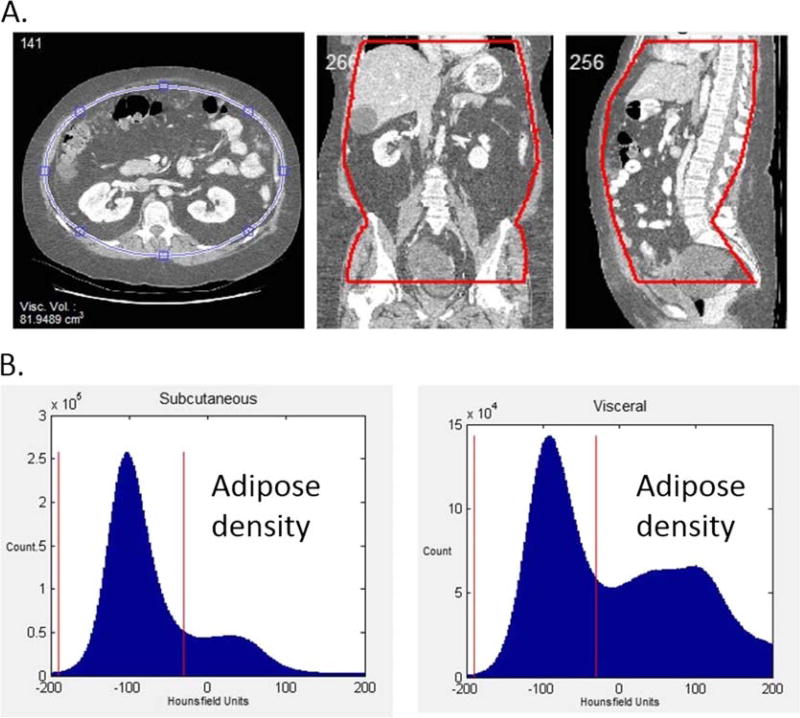
For the present investigation, visceral and subcutaneous fat volume was measured from these CT scans using in-house software, the volumetric visceral adipose quantitation using CT. Digital imaging and communications in medicine images were imported into the new software program to calculate volume of visceral and abdominal subcutaneous fat. The region of interest was defined from the dome of the liver to the tip of the femoral heads. The abdominal contents were then defined with a series of ellipses, which defined the intraabdominal contents. The slice thickness and pixel number were used to determine the volume of interest. The volume of fat within the abdominal contents was then quantified by counting the number of voxels corresponding to adipose tissue within the 2 regions and multiplying by the volume of each voxel. Adipose tissue consisted of tissues between −190 and −30 Hounsfield Units (HU) (Fig. 1). The volume calculation has been validated using a phantom, and the mean intraobserver and interobserver coefficient of variance was 5.9% and 8.5%, respectively.
FIGURE 1.
Visceral adipose tissue volume measured by CT imaging. A, Axial, coronal, and sagittal views of a CT abdominal series of an obese patient. Visceral and subcutaneous tissues are separated by defining a region of interest through ellipse interpolation. Visceral tissues are contained within the blue ellipse shown on the axial image, whereas subcutaneous tissues are located outside the ellipse. The region of interest is also shown on coronal and sagittal images. B, The distributions of HU values corresponding to subcutaneous and visceral abdominal tissues in an obese patient as detected by CT. In obese patients, both subcutaneous and visceral abdominal tissue distributions show a large peak from −190 to −30 HU (denoted by the red vertical lines) corresponding to abdominal adipose.
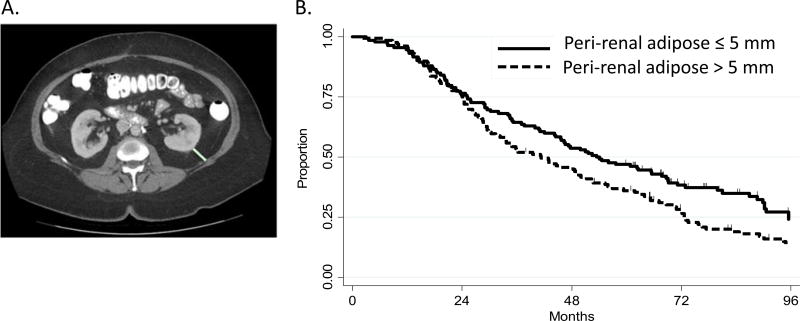
Furthermore, from this method, percent visceral fat and subcutaneous fat from the abdominal cavity were calculated along with ratio of visceral fat volume to subcutaneous fat volume. Perirenal fat thickness (PRF) was measured at the level of the renal vein. The distance between the posterior renal capsule and abdominal wall was used to determine fat thickness (Fig. 2A).
FIGURE 2.
Perirenal adipose measurement. A, Perirenal adipose was measured as the distance from the kidney to the abdominal wall at the level of the renal vein as a direct line posteriorly from the renal capsule to the posterior abdominal wall (green line). B, Impact of PRF on progression-free survival rates over time.
Statistical Analyses
All data were analyzed using Stata/MP 13.1 for Windows (StataCorp 2013, Stata: Release 13, Statistical Software, College Station, Tex). Descriptive statistics and frequencies were used to determine patient characteristics as appropriate. Pearson product-moment correlation coefficients were derived for BMI and measures of central adiposity (subcutaneous adipose volume, visceral adipose volume, percent of VAT, and PRF). Cox proportional hazard regression models were used to perform univariate and multivariate linear regression analyses to determine the prognostic significance of visceral and subcutaneous fat volume, PRF, BMI, participant age, disease stage, change in CA125 level pretreatment and posttreatment, and treatment type. Residual analyses were performed to verify whether the assumptions of the regression models were satisfied. Progression-free survival probabilities were estimated using the Kaplan-Meier product limit method. No correction was performed for multiple testing. Our significance level was set at alpha 0.05, and the power of the analyses was calculated at 0.80.
RESULTS
Patient Characteristics
Patient characteristics are listed in Table 1. A total of 258 patients with ovarian cancer were treated at MD Anderson including definitive surgery. On average, patients were aged 62 years (median: 61 years, range: 26–82 years), classified as overweight per BMI (mean: 27.47 kg/m2, median: 26.5 kg/m2, range: 15.04–66.80 kg/m2), and had a pretreatment CA125 level of 1684.21 IU/mL (median: 601.4 IU/mL, range: 5.5–32,570.5 IU/mL). The majority of patients were diagnosed with stage III and IV ovarian cancer. Ninety-three patients received neoadjuvant chemotherapy, with the remainder receiving adjuvant chemotherapy after surgery. Eight-five patients had residual disease after surgery. Thirty-six of these patients received neoadjuvant chemotherapy and 49 did not. A total of 160 patients had serous ovarian cancer and 60 had mixed histologic type. More than 74% of patients had carboplatin/paclitaxel chemotherapy, and close to 6% of patients had cisplatin or carboplatin treatment combined with other chemodrugs. The rest of the patients had docetaxel, taxotere, or any other treatment.
TABLE 1.
Patients’ clinical characteristics
| Characteristics | Values |
|---|---|
| Age (y) | |
| Mean | 62 |
| Median | 61 |
| Range | 26–82 |
| Cancer Stage | |
| ≤II | 23 |
| III | 149 |
| IV | 74 |
| Unknown | 12 |
| BMI (kg/m2) | |
| Mean | 27.5 |
| Median | 26.5 |
| Range | 15.04–66.80 |
| Pretreatment CA125 (UI/mL) | |
| Mean | 1,684.21 |
| Median | 601.4 |
| Range | 5.5–32,570.5 |
| Postsurgical residual disease | |
| No gross residual | 85 |
| <2cm | 98 |
| >2cm | 52 |
| Unknown | 23 |
| Histology group | |
| Serous | 160 |
| Mixed | 60 |
| Other | 38 |
| Chemotherapy regimens | |
| Carboplatin/taxol | 191 |
| Other platinum combination* cisplatin or carboplatin | 15 |
| Doxil | 1 |
| Taxotere | 8 |
| Other | 43 |
any other chemotherapy drug with cisplatin or carboplatin.
Correlation Between BMI and Measures of Abdominal Obesity
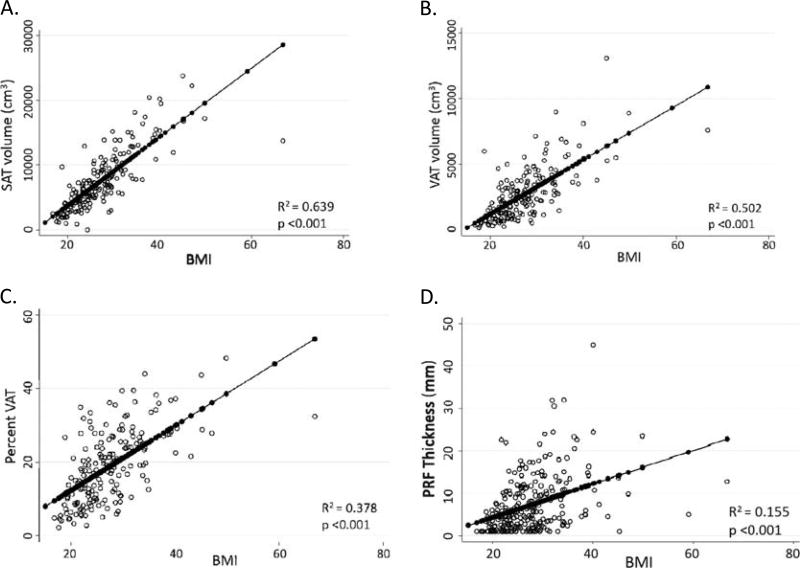
The BMI correlated most closely with subcutaneous fat volume (r = 0.799, r2 = 0.639) followed by visceral fat volume (r = 0.709, r2 = 0.502), percent of VAT within the abdominal cavity (r = 0.615, r2 = 0.378), and PRF (r = 0.394, r2 = 0.155) (Fig. 3).
FIGURE 3.
The BMI and measures of central adiposity. A, Subcutaneous adipose tissue volume versus BMI. B, Visceral adipose tissue volume versus BMI. C, Percent VAT versus BMI. D, Perirenal fat thickness versus BMI.
Prognostic Significance of Clinical Variables and Abdominal Obesity Measures in Relation to Progression-Free Survival
Table 2 provides detail regarding univariate analyses assessing the relationship between clinical variables and progression-free survival. Univariate analyses demonstrated lower progression-free survival for patients with higher posttreatment CA125 levels (2.69, 95% confidence interval [CI] 1.82–3.99), higher stage (III: 2.21, 95%CI 1.12–4.35; IV: 4.83, 95% CI 2.41–9.68; V: 2.72, 95% CI 1.05–7.06), measurable residual disease postsurgery (<2 cm: 1.60, 95% CI 1.14–2.25; >2 cm: 2.64,95%CI 1.79–3.9; unknown: 1.89, 95%CI 1.14–3.13), and evidence of disease postchemotherapy (2.42, 95%CI 1.79–3.26). Progression-free survival was higher in patients with nonserous histology (0.59, 95% CI 0.38–0.92), after optimal debulking during surgery (0.57, 95%CI 0.41–0.78), and treatment without neoadjuvant chemotherapy (0.56, 95% CI 0.43–0.74).
TABLE 2.
Clinical variables and progression-free survival (univariate analyses)
| Progression-Free Survival | ||||
|---|---|---|---|---|
|
|
||||
| Variables | HR | 95% CI | P | Reference |
| Pretreatment CA125 level continuous | 1.00 | 1.00–1.00 | 0.128 | Continuous |
| Posttreatment CA125 level continuous | 1.00 | 1.00–1.00 | 0.009 | Continuous |
| Posttreatment CA125 level | ||||
| Positive | 2.69 | 1.82–3.99 | <0.001 | Negative |
| Unknown | 1.23 | 0.86–1.75 | 0.251 | |
| Age | 1.01 | 0.99–1.02 | 0.243 | Continuous |
| Ethnic Group – not white | 0.87 | 0.62–1.23 | 0.436 | White |
| Disease site | 0.93 | 0.68–1.28 | 0.667 | Ovary |
| Histology group | ||||
| Papillary serous | 0.75 | 0.51–1.10 | 0.14 | Serous |
| Mixed | 0.75 | 0.53–1.06 | 0.102 | |
| Other | 0.59 | 0.38–0.92 | 0.019 | |
| FIGO stage | ||||
| III | 2.21 | 1.12–4.35 | 0.022 | ≤II |
| IV | 4.83 | 2.41–9.68 | <0.001 | |
| Unknown | 2.72 | 1.05–7.06 | 0.04 | |
| Residual disease | ||||
| <2 cm | 1.60 | 1.14–2.25 | 0.006 | No residual |
| >2 cm | 2.64 | 1.79–3.9 | <0.001 | |
| Unknown | 1.89 | 1.14–3.13 | 0.013 | |
| Optimal debulking (operation note) | ||||
| Yes | 0.57 | 0.41–0.78 | <0.001 | No |
| Unknown | 0.60 | 0.24–1.5 | 0.272 | |
| Neoadjuvant chemotherapy | ||||
| No | 0.56 | 0.43–0.74 | <0.001 | Yes |
| Unknown | 1.02 | 0.32–3.24 | 0.968 | |
| Status postchemotherapy | ||||
| Disease present | 2.42 | 1.79–3.26 | <0.001 | No evidence of disease |
| Unknown | 1.52 | 0.99–2.34 | 0.056 | |
Table 3 provides details regarding measures of abdominal obesity with the median values and correlation with progression-free survival. There was no significant difference in risk of progression-free survival as a function of BMI, visceral or subcutaneous adipose volume, or percent visceral adipose volume. Increased thickness of perirenal adipose (greater than the median of 5 mm) was associated with a lower progression-free survival (1.30, 95% CI 0.98–1.70). In actuarial analysis, patients with PRF greater than 5 mm had lower rates of progression-free survival, 45.6%, compared with patients with PRF less than or equal to 5 mm, 53.8% after 5 years (P = 0.05; Fig. 2B).
TABLE 3.
Measures of abdominal obesity and progression-free survival (univariate analyses)
| Progression-Free Survival | ||||
|---|---|---|---|---|
|
|
||||
| Variables | HR | 95% CI | P | Reference |
| BMI > 27 kg/m2 | 1.11 | 0.84–1.46 | 0.467 | ≤27 |
| SAT volume > 6600 cm3 | 0.79 | 0.57–1.08 | 0.142 | <6600 |
| VAT volume > 2320 cm3 | 0.94 | 0.68–1.29 | 0.693 | ≤2320 |
| Percent VAT > 18% | 0.78 | 0.56–1.07 | 0.122 | ≤18 |
| PRF > 5 mm | 1.30 | 0.98–1.70 | 0.064 | ≤5 mm |
Multivariate analysis was performed to determine if measures of central adiposity were independently predictive of progression-free survival (Table 4). Perirenal fat thickness of greater than 5 mm was significantly associated with lower progression-free survival (1.37, 95% CI 1.03–1.82). Among other clinical variables, a positive posttreatment CA125 level (1.85, 95% CI 1.18–2.89), Federation of Gynecology and Obstetrics (FIGO) stage greater than II (IV: 2.83, 95% CI 1.29–6.22; other: 2.41, 95% CI 0.85–6.82) measurable residual disease postsurgery (<2 cm: 1.98, 95% CI 1.35–2.91; >2 cm: 2.99, 95% CI 1.82–4.93; unknown: 2.78, 95% CI 1.58–4.89), and evidence of the disease postchemotherapy (1.53, 95% CI 1.04–2.25) all remained significantly associated with lower progression-free survival. Treatment without neoadjuvant chemotherapy was still significantly associated with improved progression-free survival (0.61, 95% CI 0.43–0.86). Although progression-free survival was higher in patients with nonpapillary serous or nonmixed histology group by univariate analysis in Table 2, we don’t see histology type as significantly associated with progression-free survival in multivariate analysis.
TABLE 4.
Predictors of progression-free survival (multivariate analyses)
| Progression-Free Survival | ||||
|---|---|---|---|---|
|
|
||||
| Variables | HR | 95% CI | P | Reference |
| PRF > 5 mm | 1.37 | 1.03–1.82 | 0.031 | ≤5 mm |
| Posttreatment CA125 level | ||||
| Positive | 1.85 | 1.18–2.89 | 0.007 | Negative |
| Unknown | 1.57 | 0.90–2.75 | 0.112 | |
| FIGO stage | ≤II | |||
| III | 1.47 | 0.70–3.09 | 0.315 | |
| IV | 2.83 | 1.29–6.22 | 0.009 | |
| V | 2.41 | 0.85–6.82 | 0.096 | |
| Residual disease | ||||
| <2 cm | 1.98 | 1.35–2.91 | <0.001 | No residual |
| >2 cm | 2.99 | 1.82–4.93 | <0.001 | |
| Unknown | 2.78 | 1.58–4.89 | <0.001 | |
| Neoadjuvant chemotherapy | ||||
| No | 0.61 | 0.43–0.86 | 0.005 | Yes |
| Unknown | 0.44 | 0.12–1.71 | 0.237 | |
| Status postchemotherapy | ||||
| Disease present | 1.53 | 1.04–2.25 | 0.031 | No evidence of disease |
| Unknown | 1.26 | 0.65–2.46 | 0.495 | |
| Histology group | ||||
| Pilillary serous | 0.70 | 0.47–1.03 | 0.067 | Serous |
| Mixed | 1.03 | 0.72–1.5 | 0.856 | |
| Other | 0.65 | 0.41–1.03 | 0.069 | |
DISCUSSION
The aim of this study was to determine the impact of different measures of abdominal obesity on progression-free survival in women with ovarian cancer. We assessed abdominal obesity by measuring subcutaneous and VAT volume and PRF. In addition, we evaluated and controlled for the effects of established clinical variables including BMI, cancer stage, measurable disease after staging surgery, histology, treatment with neoadjuvant chemotherapy, and CA125 levels.
Among all of the assessed measures of abdominal obesity, PRF, but not other measures, exhibited a significant association with progression-free survival, such that lower rates of progression-free survival for women with increased PRF was observed in both univariate and multivariate analyses. Previous studies have reported strong correlations between increased perirenal fat, higher morbidity risk, and poorer health outcomes in patients with colorectal cancer.8 Although the biological mechanisms of perirenal fat in relation to cancer are still not well known, a potential explanation for these findings may be attributed to recent findings that suggest the presence of brown adipose tissue within the perirenal fat depot.14–16
Perirenal adipose tissue is enriched with brown adipose tissue.14–16 Brown adipose tissue has the unique capacity to dissipate thermogenic energy, relying on the function of uncoupling protein 1 (UCP1). The UCP1, localized to the inner mitochondrial transmembrane, allows protons from the intermembrane of the mitochondria to leak or reenter into the mitochondrial matrix without generating adenosine triphosphate, resulting in heat dissipation.17 Increased cytokine production associated with cancer has been proposed to increase UCP1 activity in brown adipose tissue, which leads to increased resting energy expenditure secondary to thermogenesis.18 This increase in resting energy expenditure has been proposed to contribute to cachexia.19 Perirenal adipose tissue is more likely to develop features of brown adipose tissue for women compared with men.20 These findings suggest that accumulation of perirenal adipose tissue may be either a marker for adverse cancer biology or may contribute to a cancer microenvironment that supports progression of ovarian cancers.
In addition to measures of abdominal obesity, we found that BMI was most strongly correlated with SAT volume and most weakly correlated with PRF, suggesting that perirenal adipose is a unique anthropomorphic metric. We also found that BMI did not correlate with progression-free survival in this series, consistent with previous reports in ovarian cancer.21,22 Our results also exhibited lower progression-free survival when CA125 levels were elevated posttreatment; these findings are consistent with previous research.23
This is the first study, to our knowledge, to assess measures of abdominal obesity along with traditional clinical variables in relation to progression-free survival in ovarian cancer. We measured adiposity using a volumetric measure of the true volume of adipose tissue within the visceral and subcutaneous adipose compartments. Other approaches to measuring intraabdominal fat rely on estimates or sampling to approximate the volume of adipose tissue. Limitations of the present investigation include use of a smaller sample size and inclusion of some unknown data points (ie, unknown residual disease). However, strengths of the present investigation include the use of highly quantitative methods to measure visceral and SAT volume.
In conclusion, we found that PRF is a unique anthropomorphic metric that correlates with progression-free survival for patients with ovarian cancer. Future studies will be needed to validate this observation and to determine if strategies to reduce perirenal adiposity can reduce the risk of ovarian cancer recurrence.
Acknowledgments
This study was supported by Ovarian Cancer Research fund (LT/MDACC/01.2011, by Dr Ann Klopp), American Cancer Society (RSG-14-159-01 by Dr Ann Klopp), and Cancer Prevention & Research Institute of Texas (CPRIT, RP140609, by Dr Ann Klopp).
This study was also supported by the Center for Energy Balance in Cancer Prevention and Survivorship, which is supported by the Duncan Family Institute for Cancer Prevention and Risk Assessment. In addition, research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number R25CA057730 (PI: Shine Chang, PhD).
Footnotes
The authors declare no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attie AD, Scherer PE. Adipocyte metabolism and obesity. J Lipid Res. 2009;50:S395–S399. doi: 10.1194/jlr.R800057-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britton KA, Massaro JM, Murabito JM, et al. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr. 2011;3:12. doi: 10.1186/1758-5996-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenquist KJ, Massaro JM, Pedley A, et al. Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality. J Clin Endocrinol Metab. 2015;100:227–234. doi: 10.1210/jc.2013-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delort L, Kwiatkowski F, Chalabi N, et al. Central adiposity as a major risk factor of ovarian cancer. Anticancer Res. 2009;29:5229–5234. [PubMed] [Google Scholar]
- 7.Roever L, Resende ES, Veloso FC, et al. Perirenal fat and association with metabolic risk factors: the Uberlândia heart study. Medicine (Baltimore) 2015;94:e1105. doi: 10.1097/MD.0000000000001105. [DOI] [PubMed] [Google Scholar]
- 8.Jung M, Volonté F, Buchs NC, et al. Perirenal fat surface area as a risk factor for morbidity after elective colorectal surgery. Dis Colon Rectum. 2014;57:201–209. doi: 10.1097/DCR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki S, Aoki K, Hasegawa O, et al. Sonographic evaluation of visceral fat by measuring para- and perirenal fat. J Clin Ultrasound. 2008;36:129–133. doi: 10.1002/jcu.20426. [DOI] [PubMed] [Google Scholar]
- 10.Halkia E, Spiliotis J, Sugarbaker P. Diagnosis and management of peritoneal metastases from ovarian cancer. Gastroenterol Res Pract. 2012;2012:541842. doi: 10.1155/2012/541842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieman KM, Romero IL, Van Houten B, et al. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim B, Kim HS, Kim S, et al. Adipose stromal cells from visceral and subcutaneous fat facilitate migration of ovarian cancer cells via IL-6/JAK2/STAT3 pathway. Cancer Res Treat. 2016;49:338–349. doi: 10.4143/crt.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klopp AH, Zhang Y, Solley T, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res. 2012;18:771–782. doi: 10.1158/1078-0432.CCR-11-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakrabarty K, Chaudhuri B, Jeffay H. Glycerokinase activity in human brown adipose tissue. J Lipid Res. 1983;24:381–390. [PubMed] [Google Scholar]
- 15.Tanuma Y, Tamamoto M, Ito T, et al. The occurrence of brown adipose tissue in perirenal fat in Japanese. Arch Histol Jpn. 1975;38:43–70. doi: 10.1679/aohc1950.38.43. [DOI] [PubMed] [Google Scholar]
- 16.Svensson PA, Lindberg K, Hoffmann JM, et al. Characterization of brown adipose tissue in the human perirenal depot. Obesity (Silver Spring) 2014;22:1830–1837. doi: 10.1002/oby.20765. [DOI] [PubMed] [Google Scholar]
- 17.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–481. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vijgen G, van Marken Lichtenbelt W. Brown adipose tissue: clinical impact of a re-discovered thermogenic organ. Front Biosci (Elite Ed) 2013;5:823–833. doi: 10.2741/e663. [DOI] [PubMed] [Google Scholar]
- 19.Argilés JM, Busquets S, Stemmler B, et al. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754–762. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 20.van den Beukel JC, Grefhorst A, Hoogduijn MJ, et al. Women have more potential to induce browning of perirenal adipose tissue than men. Obesity (Silver Spring) 2015;23:1671–1679. doi: 10.1002/oby.21166. [DOI] [PubMed] [Google Scholar]
- 21.Mao JJ, Armstrong K, Bowman MA, et al. Symptom burden among cancer survivors: impact of age and comorbidity. J Am Board Fam Med. 2007;20:434–443. doi: 10.3122/jabfm.2007.05.060225. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Chlebowski R, LaMonte MJ, et al. Body mass index, physical activity, and mortality in women diagnosed with ovarian cancer: results from the Women’s Health Initiative. Gynecol Oncol. 2014;133:4–10. doi: 10.1016/j.ygyno.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez RD, To A, Boots LR, et al. CA125 as a serum marker for poor prognosis in ovarian malignancies. Gynecol Oncol. 1987;26:284–289. doi: 10.1016/0090-8258(87)90019-9. [DOI] [PubMed] [Google Scholar]