Abstract
Background
The prevalence of cognitive impairment and dementia are projected to rise dramatically over the next 40 years, and strategies for maintaining cognitive function with age are critically needed. Physical or mental activity alone result in relatively small, domain-specific improvements in cognitive function in older adults; combined interventions may have more global effects.
Methods
We performed a randomized, controlled trial to examine the combined effects of physical plus mental activity on cognitive function in 126 community-residing older adults with cognitive complaints. All subjects engaged in home-based mental activity (1 hour/day, 3 days/week) plus class-based physical activity (1 hour/day, 3 days/week) for 12 weeks and were randomized to either mental activity intervention (MA-I, intensive computer) or mental activity control (MA-C, educational DVDs) plus exercise intervention (EX-I, aerobic) or exercise control (EX-C, stretching/toning) using a 2×2 factorial design so that there were 4 groups: MA-I/EX-I, MA-I/EX-C, MA-C/EX-I, MA-C/EX-C. The primary outcome was global cognitive change based on a comprehensive neuropsychological test battery. Analyses were intent-to-treat using repeated measures random effects regression.
Results
Subjects had a mean age of 73 years; 65% were women and 35% Hispanic or non-white. There were no significant differences between the groups at baseline. Global cognitive scores improved significantly over time (mean 0.16 standard deviations [SD]; p<0.001) but did not differ between groups when comparing MA-I to MA-C (ignoring exercise, p=0.17), EX-I to EX-C (ignoring mental activity, p=0.74), or across all four randomization groups (p=0.26).
Conclusions
In inactive older adults with cognitive complaints, 12 weeks of physical plus mental activity was associated with significant improvements in global cognitive function with no evidence of difference between intervention and active control groups. These findings may reflect practice effects or may suggest that the amount of activity is more important than the type in this subject population.
Background
Over the next 40 years, it is anticipated that there will be an epidemic of dementia worldwide, with a three- to four-fold increase in the number of prevalent cases due to longer life expectancies and demographic changes.1 Current treatments provide some symptomatic relief but do not alter the course of the disease,2 and several therapies that initially appeared promising have recently failed in Phase III clinical trials.3-5 Attention is turning toward identification of preclinical disease and development of treatments to prevent or delay dementia onset;6 however, there is also concern about potential side effects of pharmacological treatment in individuals who may never become symptomatic. Behavioral interventions offer a potential strategy to prevent or delay dementia onset with minimal side effects in asymptomatic individuals.
There is growing evidence that both physical and mental activity can improve cognitive function in the short term and may lower the risk of developing dementia over the long term. Numerous observational studies7-17 and several systematic reviews18-20 have found that older adults who engage in mental or physical activity are less likely to experience cognitive decline or develop dementia, and several have suggested that physical and mental activity may have independent or additive effects.7, 11 However, randomized, controlled trials (RCTs) are needed to establish a clear causal relationship between engaging in physical or mental activity and cognitive benefits.
To date, RCTs of physical and mental activity interventions have had mixed results.21 Mental activity interventions in both healthy elders22, 23 and individuals with mild cognitive impairment (MCI)24, 25 have typically found domain-specific improvements, in which benefits are observed in the specific cognitive activities trained with little evidence of generalization to other activities or domains. Exercise interventions in both healthy elders26, 27 and individuals with MCI28-30 have found that both aerobic exercise and resistance training are associated with small to moderate improvements in cognitive function, particularly measures of attention, processing speed and executive function.31-33
Taken together, these studies suggest that behavioral interventions that combine physical and mental activity may have more global effects than either physical or mental activity alone. However, few RCTs have studied the effects of physical and mental activity together.34 Therefore, we performed an RCT with a 2×2 factorial design to compare the effects of different physical and mental activity combinations on cognitive function in community-residing older adults with self-reported cognitive complaints.
Methods
Participants
Study participants were recruited primarily through direct mailing to the neighborhoods adjacent to the intervention site (Stonestown YMCA, San Francisco, CA), as well as advertisements, fliers, physician and friend referrals, and recruitment databases. Inclusion criteria were age ≥ 65 years, cognitive complaint (defined as answering ‘yes’ to the question “Do you feel that your memory or thinking skills have gotten worse recently?”), English language fluency, not currently engaging in aerobic exercise or intensive computer training (≤2 days/week, ≤30 minutes/session in the past 3 months) and not planning to travel > 1 week during the study period. Exclusion criteria were dementia (based on self-report, physician diagnosis or scoring ≤ 18 on the modified Telephone Interview for Cognitive Status35), other neurological or major psychiatric disorder, significant heart or lung disease, limited life expectancy, or other factors that could potentially limit ability to participate fully in the intervention.
All study procedures were approved by the Committee on Human Research at the University of California, San Francisco, and the Research and Development Committee at the San Francisco Veterans Affairs Medical Center. All subjects provided written informed consent and received physician approval to participate.
Interventions
Study participants were randomized to both a home-based mental activity intervention (MA-I) or mental activity control (MA-C) group plus a class-based exercise intervention (EX-I) or exercise control (EX-C) group using a 2×2 factorial design (Figure 1). Active control groups were utilized to account for the effects of factors such as interaction with a computer and social interaction during a group exercise class.
Figure 1.
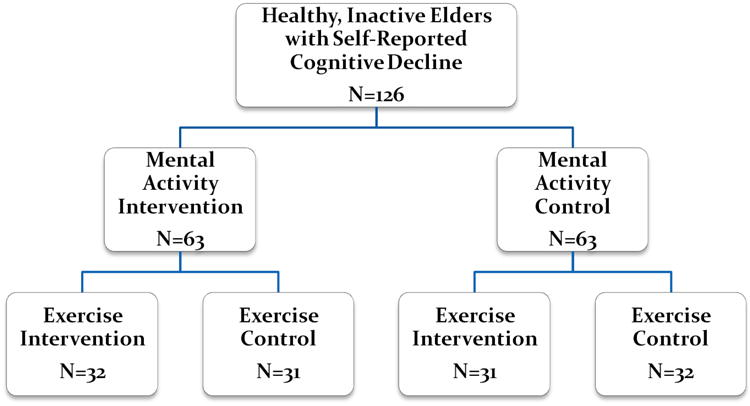
Mental Activity and eXercise (MAX) Trial Design.
The MAX Trial used a 2×2 factorial design in which subjects were first randomized to either the mental activity intervention (MA-I: intensive computer training) or mental activity control (MA-C: educational DVD) group and then to the exercise intervention (EX-I: aerobic) or exercise control (EX-C: stretching/toning) group. This design enables comparisons to be made between the mental activity groups (ignoring exercise) and between the exercise groups (ignoring mental activity) and also to assess for evidence of interaction between mental activity and exercise.
Mental Activity
All participants were provided with detailed written and in-person verbal instructions regarding their assigned mental activities, which were performed independently at home on a computer for 60 minutes/day, 3 days/week for 12 weeks. The MA-I group performed games designed to enhance the speed and accuracy of visual and auditory processing (Posit Science Corporation, San Francisco, CA). For the first 6 weeks, games focused on visual tasks including determining the direction of visual ‘sweeps’, identifying bird pairs, tracking the location of moving ‘gems’ and identifying targets in peripheral vision. For the second 6 weeks, games focused on auditory tasks including determining the direction of auditory ‘sweeps’, distinguishing between similar sounds, matching sound pairs, recalling a series of sounds, following verbal instructions and answering questions about verbal stories. Program difficulty adjusted automatically and continuously based on each participant's level of performance to maintain a ‘correct’ response level of approximately 85%.
The MA-C group watched educational lectures on art, history and science on DVDs. After each session, participants answered approximately six paper-based, multiple choice or short answer lecture-specific questions. Participants watched the DVDs on a computer for 60 minutes/day, 3 days/week for 12 weeks to match the conditions of the MA-I group.
Exercise
All participants attended study-specific group exercise classes at a local YMCA for 60 minutes/day, 3 days/week for 12 weeks. The EX-I class consisted of 10 minutes warm-up, 30 minutes aerobic exercise (traditional dance-based aerobics format), 5 minutes cool-down, 10 minutes strength training, and 5 minutes stretching/relaxation. Heart rate was monitored by having participants check their wrist or neck pulse for 10 seconds at the beginning, peak and end of class and record the values in an exercise journal, with a target peak heart rate of 60-75% of maximum for age.
The EX-C class consisted of 10 minutes warm-up, 30 minutes stretching and toning, 10 minutes strength training, and 10 minutes relaxation. Heart rate was monitored in the same manner, with a goal of not raising heart rate above resting levels. All classes were taught by a single, certified exercise instructor with experience conducting classes in the elderly with a maximum of 12 subjects per class at any given time.
Compliance and adverse events were monitored using weekly journals and bi-weekly telephone check-ins, and motivational counseling was provided if compliance fell below 80%. Daily attendance was also recorded for the exercise classes.
Randomization and Blinding
Subjects were randomized in blocks of four. The randomization sequence was prepared in advance using a random number generator on a computer. Research staff involved with enrollment and outcome assessment were unaware of the randomization sequence and blinded to group assignment. Study participants were unaware of study hypotheses and were told that the goal of the study was to compare the effects of different physical and mental activity programs.
Outcomes
Primary outcome
The primary outcome of the study was 12-week change in cognitive function based on a composite score from a comprehensive neuropsychological test battery. Specific tests were selected because they have good validity and reliability and are sensitive to cognitive decline in older adults and included measures of verbal learning and memory (Rey Auditory Verbal Learning Test [RAVLT]),36 verbal fluency (letter and category),37 processing speed (Digit Symbol Substitution Test [DSST]),38 executive function/mental flexibility (Trail-Making Test, Parts A & B),39 executive function/inhibition (Eriksen Flanker Test [EFT] congruent and incongruent reaction times),40 and visuospatial function (Useful Field of View [UFOV] processing speed, divided attention and selective attention).41
The Modified Mini-Mental State Examination (3MS)42 was performed at baseline only to assess global cognitive status (range: 0-100).
A composite cognitive score was created by converting all individual cognitive scores to standardized z-scores by subtracting the baseline group mean and dividing by the baseline group standard deviation (SD) and averaging them. For tests with more than one component (e.g., Trails A & B), the component scores were averaged prior to calculating the composite score so that all tests were weighted equally. In subjects who were missing data for three or fewer cognitive tests, the composite score was based on non-missing tests.
Other measures
Demographic measures included age, sex, race/ethnicity and income. Comborbid medical conditions were determined based on self-report of prior physician diagnosis. Physical performance was assessed with the Senior Fitness Test,43 which includes standard measures of chair stand, arm curl, 2-minute step test, sit-and-reach, back scratch, and 8-foot up-&-go.
Power
Our study had 80% power to detect an effect size of approximately 0.3 SDs when examining the main effects of MA-I vs. MA-C (ignoring exercise) and EX-I vs. EX-C (ignoring mental activity) (n=63/group) and an effect size of 0.45 SDs when comparing across the 4 randomization groups (n=31/group), assuming 80% correlation between measures within subjects and using two-sided alpha=0.05.
Analyses
All analyses were intent-to-treat. Baseline characteristics were compared between groups using Chi-square for categorical variables and analysis of variance (ANOVA) for continuous variables. Mixed effects linear regression models were utilized to examine change in cognitive scores as a function of randomization group, time (baseline or post-intervention), and the group by time interaction.44 Random intercepts for each subject were utilized to model correlation between subjects over time. Robust estimation of the variance/covariance structure was used to account for the possibility of unequal variance at the two time points. Due to the factorial design, analyses were performed to compare MA-I vs. MA-C (ignoring exercise) and EX-I vs. EX-C (ignoring mental activity) as well as all four randomization groups. Analyses were performed using the xtreg command in Stata 12 (StataCorp LP, College Station, TX).
Secondary analyses examined the impact of the interventions on individual cognitive tests. Based on prior studies,45, 46 we hypothesized a priori that the effects of EX-I would be greatest for measures of executive function, especially the Eriksen Flanker incongruent task, and that the effects of the MA-I would be greatest for the Useful Field of View test, which is similar to one of the games in the training program.
To help interpret the main results of the study, we also performed two post-hoc sub-group analyses to assess whether associations differed as a function of low memory (defined as delayed recall ≤5 words [≥1 SD below the mean] on the RAVLT) or poor physical function (defined as <65 full steps on the two-minute step test) at baseline by including 3-way interaction terms for these variables, randomization group and time in the random effects regression models.
All results were similar when analyses were restricted to subjects who completed the study; therefore, only intent-to-treat results are shown.
Results
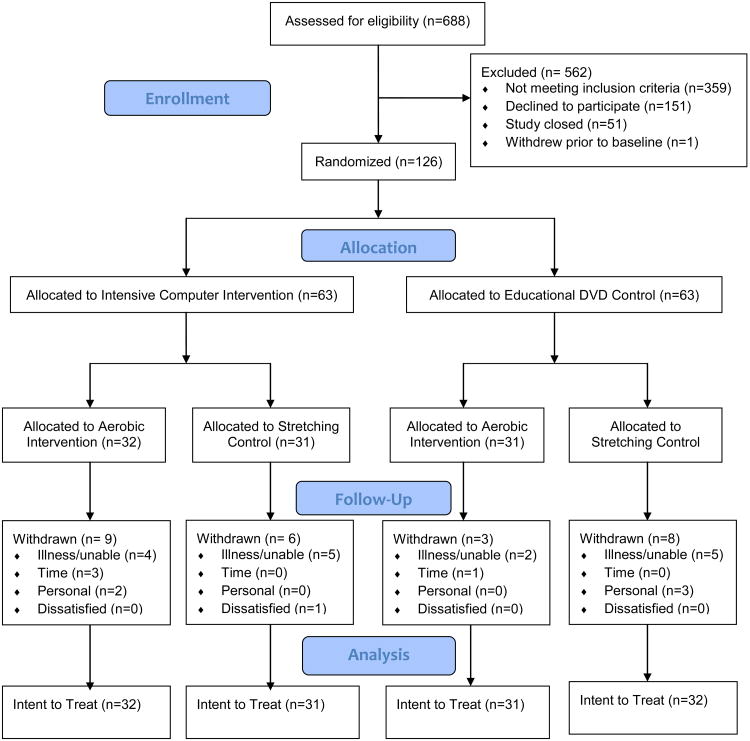
The flow of participants through the study is shown in Figure 2. Subjects were enrolled from January 2008 to September 2009, and data collection was completed in December 2009. A total of 688 individuals contacted us for more information and were assessed for eligibility. Of these, 562 were excluded (359 did not meet eligibility criteria, primarily due to high levels of current physical activity; 151 declined to participate, primarily due to lack of interest or time; 51 contacted us after the study had been closed; and 1 withdrew prior to baseline) and 126 were enrolled. Thirty-two participants were randomized to the MA-I/EX-I group (intensive computer/aerobic exercise), 31 to the MA-I/EX-C group (intensive computer/stretching&toning), 31 to the MA-C/EX-I group (DVDs/aerobic) and 32 to the MA-C/EX-C group (DVDs/stretching).
Figure 2.
Flow Chart
A total of 26 (21%) subjects withdrew during the study: 8 developed an illness, 5 were physically unable to perform the study activities, 3 were not approved by their physician, 4 cited time constraints, 5 cited personal reasons such as a family member's illness, and 1 was dissatisfied. Withdrawals did not differ significantly between the MA-I and MA-C groups, the EX-I and EX-C groups, or all four randomization groups.
In addition, 9 (7%) of subjects experienced an adverse event that was considered possibly or probably study related (4 pain, 2 falls, 2 dizziness, 1 hospitalization for pulmonary edema). All subjects recovered without residual problems.
Baseline characteristics of study participants by randomization group are shown in Table 1. Overall, participants had a mean age of 73 years and 16 years of education; 63% were women and 35% were Hispanic or non-white. Participants had relatively high global cognitive function (mean 3MS, 94.4). Fifty-six percent had hypertension, 13% had diabetes mellitus, 9% had previously had a myocardial infarction; and 52% were current or former smokers. There were no significant differences in baseline characteristics between the MA-I or MA-C groups, the EX-I or EX-C groups, or all four randomization groups.
Table 1. Baseline Characteristics of 126 Study Participants by Randomization Group*.
| Mental Activity Control | Mental Activity Intervention | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Characteristic | Exercise Control (n=32) | Exercise Intervention (n=31) | Exercise Control (n=31) | Exercise Intervention (n=32) | p-value |
| Age, years | 73.9 (6.3) | 71.1 (5.5) | 73.8 (5.7) | 74.8 (6.1) | 0.08 |
| Gender, female | 20 (62.5) | 21 (67.7) | 18 (58.1) | 20 (62.5) | 0.89 |
| Education, years | 16.3 (2.1) | 15.6 (2.8) | 16.8 (2.3) | 16.7 (2.2) | 0.18 |
| Race/ethnicity, non-Hispanic white | 22 (68.8) | 17 (54.8) | 22 (71.0) | 21 (65.6) | 0.55 |
| Global cognition (3MS) | 94.8 (4.7) | 94.6 (5.6) | 94.4 (3.9) | 94.0 (5.2) | 0.92 |
| Hypertension | 17 (53.1) | 20 (64.5) | 14 (45.2) | 19 (59.4) | 0.45 |
| Diabetes | 5 (15.6) | 5 (16.1) | 4 (12.9) | 3 (9.4) | 0.85 |
| Myocardial infarction | 4 (12.5) | 2 (6.5) | 3 (9.7) | 2 (6.3) | 0.79 |
| Current/former smoker | 16 (53.3) | 18 (58.1) | 12 (40.0) | 16 (51.6) | 0.54 |
Values reflect mean (standard deviation) or number (percent). P-values based on analysis of variance or Chi-square test across the four groups. Data missing as follows: education (n=3), race/ethnicity (n=1), smoking status (n=4). 3MS, Modified Mini-Mental State Examination. No significant differences between the mental activity intervention and mental activity control groups (ignoring exercise), exercise intervention and exercise control groups (ignoring mental activity), or all four randomization groups.
Baseline cognitive test scores by randomization group are shown in Table 2. On average, study participants had moderate to high levels of cognitive function at baseline, consistent with their age and education levels. There were no significant differences in baseline cognitive test scores between the MA-I or MA-C groups, the EX-I or EX-C groups, or all four randomization groups.
Table 2. Baseline Cognitive Scores by Randomization Group.
| Mental Activity Control | Mental Activity Intervention | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Cognitive Test* | Exercise Control (n=32) | Exercise Intervention (n=31) | Exercise Control (n=31) | Exercise Intervention (n=32) | p-value |
| Verbal learning & memory (RAVLT) | |||||
| No. words learned | 41.0 (8.9) | 41.5 (9.0) | 40.0 (9.9) | 41.2 (10.2) | 0.93 |
| No. words recalled | 7.3 (2.6) | 8.3 (2.8) | 7.2 (4.1) | 7.1 (2.9) | 0.47 |
| Verbal fluency | |||||
| No. words, letter | 12.8 (3.7) | 12.4 (5.3) | 12.6 (4.0) | 12.8 (4.6) | 0.98 |
| No. words, category | 18.9 (5.1) | 18.3 (5.1) | 17.2 (5.4) | 18.6 (4.4) | 0.55 |
| Processing speed (DSST) | |||||
| No. correct | 55.7 (13.9) | 58.1 (13.9) | 54.8 (14.6) | 56.6 (11.0) | 0.80 |
| Executive function/mental flexibility | |||||
| Trails A, seconds | 41.6 (15.3) | 37.2 (14.5) | 36.5 (17.1) | 44.4 (14.8) | 0.16 |
| Trails B, seconds | 100.9 (47.6) | 102.9 (56.9) | 94.7 (46.4) | 87.6 (31.1) | 0.58 |
| Executive function/inhibition (EFT) | |||||
| Congruent reaction time, milliseconds | 622.2 (113.0) | 600.8 (153.7) | 612.8 (116.1) | 659.2 (136.5) | 0.34 |
| Incongruent reaction time, milliseconds | 691.1 (135.8) | 685.2 (115.7) | 700.4 (151.0) | 732.6 (147.7) | 0.55 |
| Visuospatial function (UFOV) | |||||
| Processing speed, milliseconds | 33.7 (39.7) | 36.5 (49.3) | 34.9 (33.3) | 34.6 (35.6) | 0.99 |
| Divided attention, milliseconds | 115.5 (116.1) | 129.3 (137.1) | 118.4 (116.0) | 117.0 (124.3) | 0.97 |
| Selective attention, milliseconds | 238.9 (107.8) | 217.3 (99.6) | 228.8 (116.8) | 234.3 (107.5) | 0.89 |
RAVLT, Rey Auditory Verbal Learning Test; DSST, Digit Symbol Substitution Test; EFT, Eriksen Flanker Test; UFOV, Useful Field of View. No significant differences between the mental activity intervention and mental activity control groups (ignoring exercise), exercise intervention and exercise control groups (ignoring mental activity), or all four randomization groups.
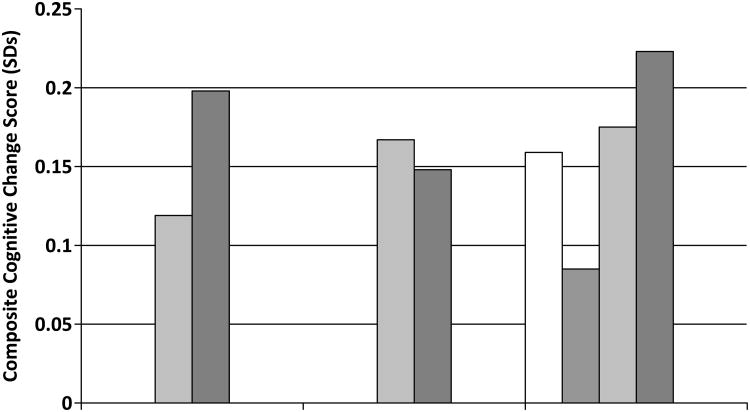
The impact of the interventions on our primary outcome of cognitive change in the composite score is shown in Figure 3. There was a significant main effect of time indicating significant improvement over all of the groups (mean, 0.16 SDs; p<0.001). However, there were no significant differences when we compared MA-I vs. MA-C (p=0.17), EX-I vs. EX-C (p=0.74), or all four randomization groups (p=0.26).
Figure 3. Impact of Intervention on Composite Cognitive Score.
For the primary outcome of change on the composite cognitive score, scores improved significantly over time but did not differ between the MA-I and MA-C groups, EX-I and EX-C groups or all 4 randomization groups.
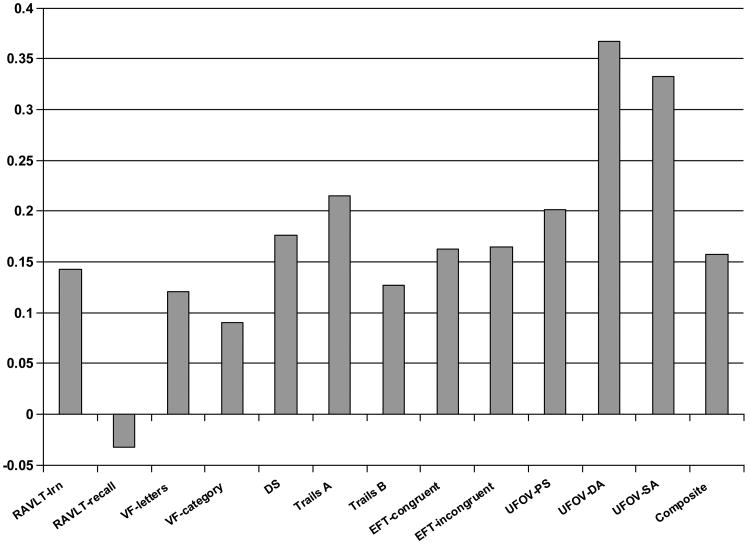
When we examined individual cognitive tests, there were significant main effects for time for the DSST, Trails A, EFT congruent and incongruent, and UFOV divided and selective attention, with trends toward improvement on most other tests except delayed recall on the RAVLT (Figure 4). The only significant differences between the groups were observed for the UFOV divided and selective attention tasks, which improved more in the MA-I than the MA-C group (Table 3).
Figure 4. Change in Individual Cognitive Tests.
Cognitive function improved significantly (p<0.05) for the Digit Symbol Substitution Test (DSST), Trails A, Eriksen Flanker Test (EFT) congruent and incongruent tests, and Useful Field of View divided attention (UFOV-DA) and selective attention (UFOV-SA), and composite score. Improvements were of borderline statistical signficance (p<.10) for Rey Auditory Verbal Learning Test total words learned (RAVLT-lrn), Trails B, and UFOV processing speed (UFOV-PS).
Table 3. Standardized Mean Change in Cognitive Scores by Randomization Group.
| Mental Activity Control | Mental Activity Intervention | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Cognitive Test* | Exercise Control (n=32) | Exercise Intervention (n=31) | Exercise Control (n=31) | Exercise Intervention (n=32) | p-value |
| Composite score | .16 (.05, .26) | .08 (-.004, .17) | .17 (.03, .31) | .22 (.12, .33) | .26 |
|
| |||||
| Verbal learning & memory (RAVLT) | |||||
| No. words learned | .33 (.09, .58) | .14 (-.14, .43) | .13 (-.11, .37) | -.04 (-.42, .33) | .38 |
| No. words recalled | .01 (-.32, .33) | .02 (-.28, .32) | -.10 (-.36, .16) | -.07 (-.46, .32) | .93 |
| Verbal fluency | |||||
| No. words, letter | -.05 (-.33, .24) | .08 (-.21, .37) | .24 (-.11, .58) | .22 (-.15, .58) | .57 |
| No. words, category | .06 (-.26, .38) | -.07 (-.41, .26) | .22 (-.06, .50) | .18 (-.24, .60) | .59 |
| Processing speed (DSST) | |||||
| No. correct | .15 (-.02, .32) | .19 (.04, .33) | .27 (.03, .51) | .08 (-.13, .30) | .71 |
| Executive function/mental flexibility** | |||||
| Trails A, seconds | -.36 (-.58, -.15) | -.12 (-.32, .07) | -.03 (-.50, .44) | -.36 (-.63, -.08) | .24 |
| Trails B, seconds | -.22 (.45, .002) | -.18 (-.49, .13) | .13 (-.21, .48) | -.25 (-.51, .01) | .31 |
| Executive function/inhibition (EFT)** | |||||
| Congruent reaction time, milliseconds | -.17 (-.51, .16) | .01 (-.42, .43) | -.17 (-.41, .06) | -.33 (-.55, -.11) | .51 |
| Incongruent reaction time, milliseconds | -.12 (-.36, .12) | -.07 (-.48, .33) | -.15 (-.52, .22) | -.33 (-.58, -.08) | .60 |
| Visuospatial function (UFOV)** | |||||
| Processing speed, milliseconds | -.23 (-.66, .19) | -.04 (-.43, .34) | -.17 (-.61, .26) | -.39 (-.77, -.02) | .65 |
| Divided attention, milliseconds | -.13 (-.48, .22) | -.17 (-.39, .05) | -.60 (-.99, -.22) | -.62 (-.97, -.26) | .05 |
| Selective attention, milliseconds | -.18 (-.59, .22) | -.14 (-.43, .16) | -.34 (-.58, -.11) | -.71 (-.96, -.46) | .02 |
RAVLT, Rey Auditory Verbal Learning Test; DSST, Digit Symbol Substitution Test; EFT, Eriksen Flanker Test; UFOV, Useful Field of View. Significant differences between mental activity intervention and mental activity control groups for UFOV-divided attention and UFOV-selective attention. No other significant differences between mental activity intervention and mental activity control groups (ignoring exercise), exercise intervention and exercise control groups (ignoring mental activity), or all four randomization groups.
For timed tests, negative change reflects improvement (i.e., faster performance).
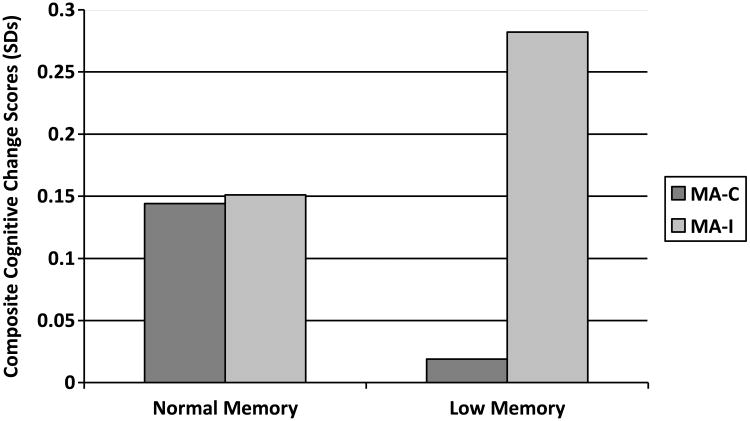
Approximately 27% (n=34) of subjects had low memory scores at baseline. The 3-way interaction between baseline memory, mental activity group and time was of borderline statistical significance (p=0.054) and, in subjects with low memory, the difference in cognitive change between the MA-I and MA-C groups was also of borderline statistical significance (p=0.08) (Figure 5). There was no evidence of interaction between baseline physical function, randomization group and time (data not shown).
Figure 5. Comparison of Mental Activity Intervention (MA-I) versus Mental Activity Control (MA-C) Groups in Subjects With Normal Memory and Low Memory.
Figure 5 show the three-way interaction between baseline memory, mental activity group and time and raises the possibility that the mental activity intervention (MA-I) training may have led to greater cognitive improvements than the mental activity control (MA-C) training in subjects with low memory at baseline, although the interaction and the between-group effects were both of borderline statistical significance.
Comment
In this 2×2 factorial RCT examining the effects of different physical and mental activity interventions on cognitive function in non-demented, inactive elders with cognitive complaints, we found that cognitive scores improved significantly over 12 weeks, but there were no significant differences between the intervention and active control groups. These results may suggest that, in this study population, the amount of activity is more important than the type of activity, since all groups participated in both mental activity and exercise for 60 minutes/day, 3 days/week for 12 weeks. Alternatively, the cognitive improvements observed may be due to practice effects.
To assess the possibility that our results were due to practice, we recruited an additional 12 subjects to participate in a no-contact control study in which all study procedures were identical except that there was no intervention. Composite cognitive test scores in these subjects improved 0.08 SDs, compared with 0.16 SDs in the main study, suggesting that some, but not all, of the improvements observed may have been due to repeated testing.
Our findings differ from prior RCTs, which have found that a similar intensive computer training program improved cognitive function more than educational DVDs in healthy elders.46, 47 It is possible that this is attributable to differences in the outcome measures used or the manner in which the intervention was implemented. Consistent with prior studies, we found that the MA-I training yielded significantly greater improvements in those cognitive tests that were similar to the training program.22, 23
We did not observe any differences between the EX-I (aerobic) and EX-C (stretching/toning) groups for either global cognitive function or individual cognitive tests. This differs from prior RCTs in healthy elders, which have found that aerobic exercise improves cognitive function and increases hippocampal volume when compared with stretching/toning.26, 48, 49 It is possible that our 12-week intervention was not long enough or intense enough to achieve a substantially greater aerobic response in the intervention group, and that a difference between the groups would have emerged in a longer study. It also is possible that subjects in the stretching/toning group engaged in more aerobic activities outside of the study as a function of their participation and readiness to exercise. Alternatively, it is possible that, in our study population of older adults with cognitive complaints, both aerobic exercise and stretching/toning resulted in physical changes that were equally beneficial. This hypothesis is supported by other recent RCTs that have found resistance training alone is associated with improvements in executive function.27, 29
Strengths and limitations
Our study had several key strengths, including the 2×2 factorial design and the use of active control conditions, which enabled us to control for factors such as social interaction during the group exercise class and mental stimulation associated with using a computer. However, there is growing evidence that even these less intensive interventions may have cognitive and physical benefits in older adults.50,51 Future studies should consider inclusion of both active and no-contact control conditions.
Our study also has several potential limitations. Although more than one-third of participants were Hispanic or non-white, most were highly educated, raising concern about the generalizabililty of the findings. In addition, our study did not include objective measures of aerobic fitness such as peak oxygen consumption; therefore, we cannot be sure that subjects in the EX-I group achieved improvements in aerobic fitness. Our cognitive test battery also did not include the Digit Span test, which was found in a recent review to be most consistently affected by exercise.33 Finally, we did not perform a full clinical evaluation on study participants.
Conclusion
In inactive elders with cognitive complaints, 12 weeks of a combined mental activity and exercise program was associated with significant improvements in global cognitive function with no evidence of difference between intervention and active control groups. These findings may reflect practice effects or may suggest that the amount of activity is more important than the type in this subject population.
Acknowledgments
Financial disclosures: This study was funded through a Career Development Award from the National Institute on Aging (K01-AG024069), the Alzheimer's Association (IIRG-06-27306) and the University of California School of Medicine. This work also was supported by National Institutes of Health/National Center for Research Resources/University of California, San Francisco – Clinical and Translational Science Institute (KL2 RR024130). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Computers, software and DVDs were donated by Posit Science (San Francisco, CA). Exercise space and equipment was donated by the Stonestown YMCA (San Francisco, CA).
Dr. Barnes had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Registered at ClinicalTrials.gov: #NCT00522899.
http://clinicaltrials.gov/ct2/show/NCT00522899.
Full trial protocol available upon request from Dr. Barnes.
Footnotes
Prior presentation: Findings from this study were presented at the International Conference on Alzheimer's Disease in Honolulu, HI, on July 14, 2010.
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007 Jul;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer's Association. Treatment horizon. [Accessed August 29, 2012]; http://www.alz.org/research/science/alzheimers_treatment_horizon.asp.
- 3.Bezprozvanny I. The rise and fall of Dimebon. Drug News Perspect. 2010 Oct;23(8):518–523. doi: 10.1358/dnp.2010.23.8.1500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green RC, Schneider LS, Amato DA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. Jama. 2009 Dec 16;302(23):2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzheimer Research Forum. Clinical trials of intravenous Bapineuzumab halted. [Accessed August 28, 2012]; http://www.alzforum.org/new/detail.asp?id=3234.
- 6.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011 May;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002 Jun 15;155(12):1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 8.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003 Jun 19;348(25):2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002 Dec 24;59(12):1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Jama. 2002 Feb 13;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 11.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001 Dec 26;57(12):2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006 Jan 17;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 13.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005 Apr 1;161(7):639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 14.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005 Nov;4(11):705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 15.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. Jama. 2004 Sep 22;292(12):1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 16.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001 Mar;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 17.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995 Jun;45(6):1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 18.Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: a non-parametric systematic review. Psychol Med. 2006 Aug;36(8):1065–1073. doi: 10.1017/S0033291706007744. [DOI] [PubMed] [Google Scholar]
- 19.Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006 Apr;36(4):441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- 20.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009 Jan;39(1):3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 21.Daviglus ML, Plassman BL, Pirzada A, et al. Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol. 2011 Sep;68(9):1185–1190. doi: 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]
- 22.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. Jama. 2002 Nov 13;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. Jama. 2006 Dec 20;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: A systematic review. Ageing Res Rev. 2012 Jul 25; doi: 10.1016/j.arr.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Barnes DE, Yaffe K, Belfor N, et al. Computer-based cognitive training for mild cognitive impairment: Results from a pilot randomized, controlled trial. Alzheimer's Disease and Associated Disorders. 2009;23:205–210. doi: 10.1097/WAD.0b013e31819c6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer AF, Hahn S, Cohen NJ, et al. Ageing, fitness and neurocognitive function. Nature. 1999 Jul 29;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 27.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010 Jan 25;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. Jama. 2008 Sep 3;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 29.Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012 Apr 23;172(8):666–668. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010 Jan;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003 Mar;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 32.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;(3):CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 33.Hogervorst E, Clifford A, Stock J, Xin X, Bandelow S. Exercise to prevent cognitive decline and Alzheimer's disease: For whom, when, what and (most importantly) how much? J Alzheimers Dis Parkinsonism. 2012;2:e117. [Google Scholar]
- 34.Fabre C, Chamari K, Mucci P, Masse-Biron J, Prefaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int J Sports Med. 2002 Aug;23(6):415–421. doi: 10.1055/s-2002-33735. [DOI] [PubMed] [Google Scholar]
- 35.Lines CR, McCarroll KA, Lipton RB, Block GA Prevention of Alzheimer's in Society's Elderly Study Group. Telephone screening for amnestic mild cognitive impairment. Neurology. 2003;60(2):261–266. doi: 10.1212/01.wnl.0000042481.34899.13. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt M. Rey Auditory-Verbal Learning Test. Lutz, FL: PAR Inc; 1996. [Google Scholar]
- 37.Fine EM, Kramer JH, Lui LY, Yaffe K Study Of Osteoporotic Fractures Sof Research G. Normative data in women aged 85 and older: verbal fluency, digit span, and the CVLT-II short form. Clin Neuropsychol. 2012 Jan;26(1):18–30. doi: 10.1080/13854046.2011.639310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 39.Reitan RM. The relation of the Trail Making Test to organic brain damage. Journal of Consulting Psychology. 1955;19:393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 40.Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- 41.Ball K, Owsley C. The Useful Field of View Test: A new technique for evaluating age-related declines in visual function. Journal of the American Optometric Association. 1993;64:71–79. [PubMed] [Google Scholar]
- 42.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987 Aug;48(8):314–318. [PubMed] [Google Scholar]
- 43.Rikli R, Jones CJ. Senior Fitness Test Manual. Champaign, IL: Human Kinetics; 2001. [Google Scholar]
- 44.Stanek EJ. Choosing a pretest-posttest analysis. American Statistician. 1988;42(3):178–183. [Google Scholar]
- 45.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith GE, Housen P, Yaffe K, et al. A Cognitive Training Program Based on Principles of Brain Plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) Study. J Am Geriatr Soc. 2009 Feb 7; doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahncke HW, Connor BB, Appelman J, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A. 2006 Aug 15;103(33):12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006 Nov;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 49.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlson MC, Erickson KI, Kramer AF, et al. Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. J Gerontol A Biol Sci Med Sci. 2009 Dec;64(12):1275–1282. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mortimer JA, Ding D, Borenstein AR, et al. Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community-based sample of non-demented Chinese elders. J Alzheimers Dis. 2012;30(4):757–766. doi: 10.3233/JAD-2012-120079. [DOI] [PMC free article] [PubMed] [Google Scholar]