Abstract
To explore the role of auxin-binding protein (ABP1) in planta, a number of transgenic tobacco (Nicotiana tabacum) lines were generated. The wild-type KDEL endoplasmic reticulum targeting signal was mutated to HDEL, another common retention sequence in plants, and to KEQL or KDELGL to compromise its activity. The auxin-binding kinetics of these forms of ABP1 were found to be similar to those of ABP1 purified from maize (Zea mays). To test for a physiological response mediated by auxin, intact guard cells of the transgenic plants were impaled with double-barreled microelectrodes, and auxin-dependent changes in K+ currents were recorded under voltage clamp. Exogenous auxin affected inwardly and outwardly rectifying K+ currents in a dose-dependent manner. Auxin sensitivity was markedly enhanced in all plants overexpressing ABP1, irrespective of the form present. Immunogold electron microscopy was used to investigate the localization of ABP1 in the transgenic plants. All forms were detected in the endoplasmic reticulum and the KEQL and KDELGL forms passed further across the Golgi stacks than KDEL and HDEL forms. However, neither electron microscopy nor silver-enhanced immunogold epipolarization microscopy revealed differences in cell surface ABP1 abundance for any of the plants, including control plants, which indicated that overexpression of ABP1 alone was sufficient to confer increased sensitivity to added auxin. Jones et al. ([1998] Science 282: 1114–1117) found increased cell expansion in transgenic plants overexpressing wild-type ABP1. Single cell recordings extend this observation, with the demonstration that the auxin sensitivity of guard cell K+ currents is mediated, at least in part, by ABP1.
Auxin is ubiquitous in the regulation of plant growth and development, having been implicated in responses as diverse as embryogenesis, vascular differentiation, guard cell movements, and elongation growth (Davies, 1995). Putative components of auxin signal transduction pathways have been suggested (for review, see Macdonald, 1997) and early events in auxin action such as proton extrusion, plasma membrane hyperpolarization, and ion currents in guard cells and coleoptile cells have been characterized (Rayle and Cleland, 1992; Blatt and Thiel, 1994; Barbier-Brygoo et al., 1996; Claussen et al., 1997). In recent years, a number of auxin-regulated genes have been described (Guilfoyle et al., 1998; Abel and Theologis, 1996; Sitbon and Perrot-Rechenmann, 1997), including some that appear to encode transcription factors (Kim et al., 1997; Rouse et al., 1998). Some of the events contributing to auxin-induced gene expression have been linked in a mechanistic model, which helps tie diverse observations together (Walker and Estelle, 1998). However, a coherent picture of auxin signaling remains elusive. In particular, the mechanism by which auxin is perceived by its receptor(s) is poorly understood. Much of the effort addressing this problem has focused on auxin-binding protein (ABP1), a putative auxin receptor (Hertel, 1995; Jones, 1994; Venis, 1995).
Originally identified in maize (Zea mays), ABP1 is found in a large number of plant species and remains the best-characterized candidate for an auxin receptor to date (Venis and Napier, 1995; Macdonald, 1997). Lacking any obvious transmembrane spanning domain, ABP1 is a soluble, reticuloplasmin protein primarily confined to the lumen of the endoplasmic reticulum (ER) by virtue of a carboxy-terminal KDEL (Lys, Asp, Glu, and Leu) ER retention motif (Pelham, 1990). As such it bears no resemblance to well-known hormone receptors from animal systems. However, there are data that implicate ABP1 in auxin-mediated electrophysiological responses at the plasma membrane of tobacco (Nicotiana tabacum) and maize protoplasts and it is possible that the amount of ABP1 on the outer surface of the plasma membrane mediates the sensitivity of a protoplast's response to added auxin (Barbier-Brygoo et al., 1989; Barbier-Brygoo et al., 1991; Rück et al., 1993; Leblanc et al., 1999). ABP1 also affects cytosolic pH and modulates K+ currents in guard cells of broad bean and the orchid Paphiopedilum tonsum (Thiel et al., 1993; Gehring et al., 1998). Furthermore, in transgenic tobacco plants expressing ABP1 under the control of a tetracyclin-inducible promoter, specific growth responses were modulated in an auxin-dependent manner (Jones et al., 1998). Taken together, all of these reports are consistent with the notion that ABP1 functions as an auxin receptor. They also link the activity of ABP1 to events likely to be connected with cell expansion.
Several techniques have identified ABP1 at the plasma membrane, although estimates of the amount vary (Jones and Herman, 1993; Diekmann et al., 1995). It is certain that ABP1 is targeted to the ER in the first instance and that only a small fraction, estimated to be less than 2% of the total (Henderson et al., 1997), may reach the cell surface. The endogenous mechanism for releasing ABP1 from the ER is not known, but for experimental purposes it has been reasoned that mutations of the KDEL motif should give rise to an increase in flux of the protein out of the ER to reach the cell surface via the secretory pathway. We report on the characterization of tobacco plants overexpressing such mutant forms of ABP1.
Single cell recordings of K+ currents have been used previously to help describe the actions of auxin on guard cells (Blatt and Thiel, 1994) and have been employed here to evaluate the consequences of ABP1 overexpression. We show that retargeting ABP1 has little effect, but overexpression generates a marked change in auxin responsiveness.
RESULTS
Transformation and Screening of Primary Transformants
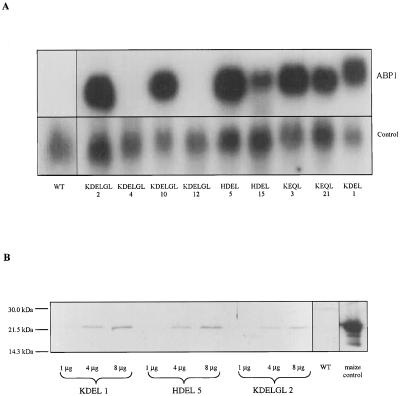
PCR-based mutagenesis was used to change the C-terminal ER-retention motif of maize ABP1 from KDEL to KEQL, KDELGL, or HDEL. The wild-type and mutated coding regions were placed under the control of a cauliflower mosaic virus 35S promoter and transferred to transgenic tobacco plants. Control plants transformed with the empty expression cassette were also constructed. Primary transformants were screened for transgene expression by northern hybridization (Fig. 1A). Hybridization of the maize ABP1 cDNA probe with endogenous tobacco transcripts could not be detected and plants accumulating high levels of maize ABP1 mRNA were selected for further characterization.
Figure 1.
Expression of the ABP1 transgenes. A, Northern blot showing maize ABP1 mRNA accumulation in primary transformants. Blots were hybridized sequentially to a maize ABP1 probe (ABP1) and the constitutively expressed pCNT 6 (Memelink et al., 1987; control). The larger size of KDEL-ABP1 mRNA compared with the mutated ABP1 mRNAs is due to the (intentional) loss of some 3′-untranslated region during the in vitro mutagenesis schedule. The maize ABP1 probe did not detect wild-type tobacco ABP1 in untransformed plants (WT). B, Representative immunoblot showing expression of ABP1 in selected transgenic plants. Total microsomal protein loading ranged from 1 to 8 μg, as indicated, for the transgenic lines and 20 μg for microsomal protein from a wild-type (untransformed) tobacco plant (WT). ABP1 (250 ng) from maize microsomes was used as a positive control (maize control).
Accumulation of ABP1 protein in the transgenic plants was detected in microsomal extracts by SDS-PAGE and immunoblotting (Fig. 1B). Although our antibody has been shown previously to cross-react with partially purified tobacco ABP1 (Venis et al., 1992), native tobacco ABP1 was undetectable in these preparations. This suggests that maize ABP1 was overexpressed in the transgenic tobacco compared with the endogenous protein, although differences in the relative affinity of the antibody for the different homologs cannot be ruled out.
Auxin-Binding Activity of Mutated Forms of ABP1
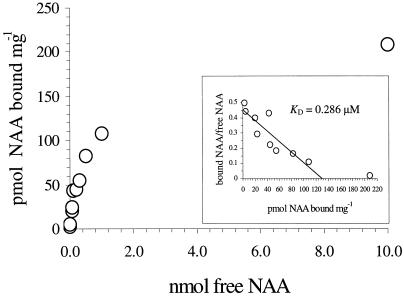
Despite a high level of transcript accumulation (Fig. 1A), levels of expression of ABP1 in the transgenic plants were at least 100-fold lower on a fresh weight basis than we could obtain from ABP1 expression in the baculovirus system (Macdonald et al., 1994). In consequence, baculovirus constructs for the various forms of ABP1 were generated for expression in insect cells to facilitate their purification in relatively large quantities for auxin-binding assays (Fig. 2). The values for the KD of the mutated forms of ABP1 ranged between 0.12 to 0.48 μm 1- naphthylacetic acid. This compares well with those measured for ABP1 from maize (the most plentiful source of ABP1 in plants) in our laboratory (0.15–0.28 μm; Macdonald et al., 1994) and in a number of other laboratories (0.05–0.7 μm; Batt and Venis, 1976; Ray et al., 1977a, 1977b; Cross and Briggs, 1978; Murphy, 1980; Löbler and Klämbt, 1985; Shimomura et al., 1986; Hesse et al., 1989).
Figure 2.
Auxin-binding activity. All forms of maize ABP1 displayed similar binding kinetics regardless of the C-terminal sequence. A representative plot for the KDELGL form, adjusted for non-saturable background, is shown. Inset, Estimates of KD were made by Scatchard analysis.
Expression of ABP1 and Guard Cell K+ Currents
Blatt and Thiel (1994) reported auxin-dependent K+ currents in voltage-clamped broad bean guard cells. The same technique was applied to each transgenic tobacco line to test for changes in sensitivity to applied auxin. Measurements were carried out on intact guard cells in epidermal peels using a two-electrode voltage clamp. As was previously observed for guard cells of broad bean (Blatt and Thiel, 1994), auxin affected both the inwardly (IK,in) and the outwardly rectifying (IK,out) K+ currents (Fig. 3).
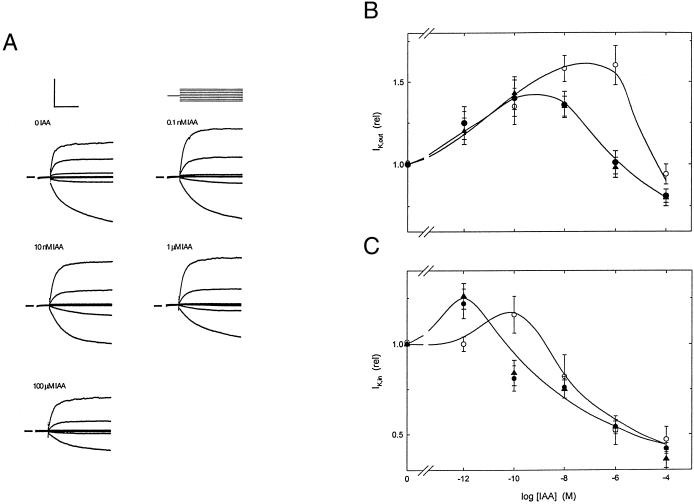
Figure 3.
Potassium currents from voltage-clamped guard cells. A, K+ current from one guard cell of a tobacco plant carrying the KDEL form of the ABP1 transgene. Data recorded in 5 mm Ca2+-MES, pH 6.1, with 10 mm KCl and IAA added at the concentrations indicated. All measurements shown were carried out after current responses reached steady state with respect to auxin. Clamp cycle (above, right): −100 mV conditioning voltage followed by steps (eight cycles) to voltages of +30 to −200 mV. Scale (above, left): vertical 100 μA cm−2 or 600 mV; horizontal, 2 s. Current zero indicated on left by each set of traces. B, Response of IK,in and IK,out (C) currents to IAA concentrations between 1 pM and 100 μm. Data were pooled as indicated in the text for wild-type plus vector control (o), and ABP1 transgenics. (●), KDEL/HDEL ABP1; (▴), KEQL/KDELGL ABP1. Points represent means from 12 to 15 independent experiments, in each case normalized to their respective controls prior to auxin addition. IK,out values were taken from currents recorded at +20 mV. IK,in values were taken from currents recorded at −200 mV.
A series of voltage clamps obtained from one guard cell of a plant overexpressing KDEL-ABP1 is shown in Figure 3A. In this experiment, clamp steps of a 5-s duration were run at voltages from +30 to −200 mV following a 1-s conditioning step to −100 mV. Activation of IK,out was evident as the outward current rising (upward, positive-going) during the first second of steps to voltages positive of −50 mV. In all the plants this current was increased between 40% and 50% in the presence of 0.1 to 10 nm indole-3-acetic acid (IAA), the enhancement being lost at higher concentrations. Activation of IK, in was evident as the inward current rising (downward, negative-going) over 2- to 4-s of steps to voltages negative of −150 mV.
Data from such experiments were collated to produce full comparisons of IK,out and IK,in as a function of IAA concentration (Fig. 3, B and C). The curves shown are empirical, although the data are based on the broad bean model (Blatt and Thiel, 1994). There was no marked difference between wild-type plants and plants carrying the empty vector and these data have been pooled (white symbols). Likewise data from all the plants overexpressing ABP1 gave similar results, regardless of the type of ABP1 expressed, and these data too have been pooled (black symbols). Thus each data point represents the mean of 12 to 15 experiments. Comparison of those plants expressing ABP1 constructs with the control plants shows a measurable difference in auxin sensitivity for the outwardly and inwardly rectifying currents (Fig. 3, B and C, respectively).
For IK,out, increasing auxin concentrations promoted the current at all but the highest concentration (Fig. 3B). The effect of the transgene was to displace the point of maximum stimulation by roughly 100-fold to lower concentrations and to reduce the magnitude of the maximum. For IK,in, all auxin concentrations above 0.1 nm produced a fall in current. In plants expressing the transgenes, the response curve was displaced to lower concentrations, again suggesting an altered auxin sensitivity.
Intracellular Localization of ABP1
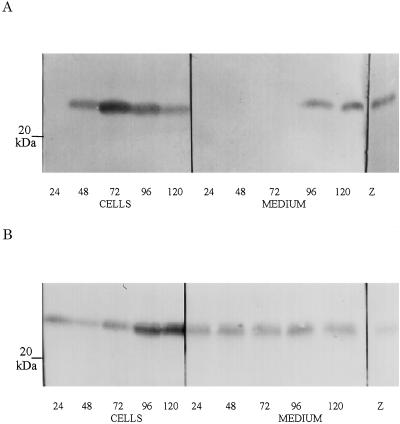
Having found no difference in auxin responses between the different transgenic lines overexpressing ABP1, we examined the retention and secretion of each mutated form of ABP1, initially using the baculovirus system. The KDEL and HDEL forms of ABP1 were efficiently retained by baculovirus-infected insect cells, until cell lysis (Fig. 4A). In a converse manner, the KEQL and KDELGL forms of ABP1 were secreted into the cell culture medium (Fig. 4B). Given that insect cells have a KDEL/HDEL ER retention mechanism homologous to that in plants and animals (Vuori et al., 1992; Henderson et al., 1996), these results are consistent with the predicted effects of these C-terminal ABP1 mutations.
Figure 4.
Time course of expression of ABP1 in Spodoptera frugiperda cells. A, Representative immunoblot for expression of KDEL-ABP1 up to 120 h postinfection. KDEL and HDEL forms of ABP1 were efficiently retained until cell lysis, which occurs between 72 and 96 h postinfection; thereafter, some ABP1 reaches the medium. B, Representative immunoblot for the expression of KEQL-ABP1. The KEQL and KDELGL forms of ABP1 were constitutively secreted. CELLS, Protein from cell lysates; MEDIUM, protein from cell culture medium.
It was more difficult to demonstrate the localization of the mutated forms in planta. Leaf cell wall washes using vacuum infiltration and Suc density gradient fractionation of microsomal membranes were inconclusive and failed to demonstrate a convincing rise in the secretion of KEQL and KDELGL forms of ABP1 (not shown). In consequence, immunogold electron microscopy was used to visualize directly the intracellular distribution of ABP1 in the transgenic plants (Fig. 5).
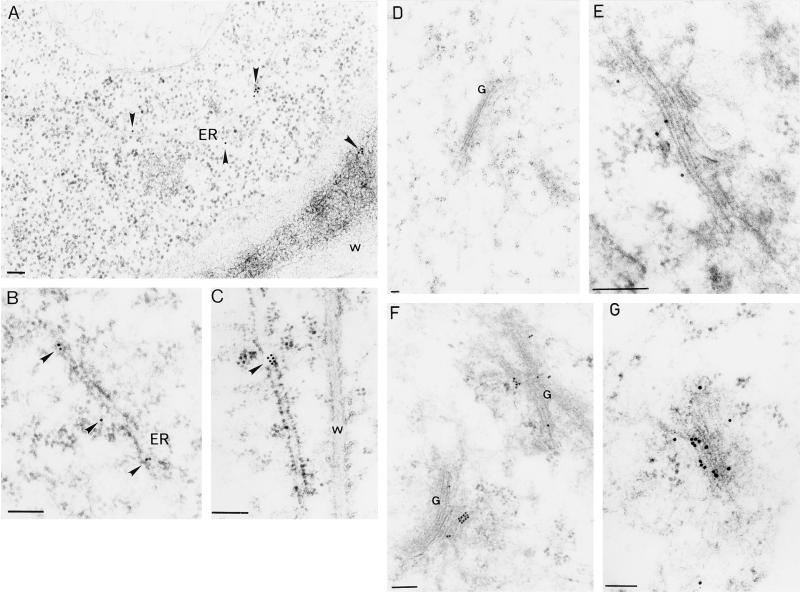
Figure 5.
Immunolocalization of ABP1 in transgenic tobacco. A through C, Labeling of the luminal ER was observed for all forms of ABP1. Sections from plants expressing KEQL (A) and KDEL (B and C) forms of ABP1 are shown. D, Control, the primary antibody was pre-incubated with purified ABP1; E, KDEL (and HDEL, not shown). ABP1 is confined to one side of the Golgi stack. F and G, KDELGL (and KEQL, not shown). ABP1 labeling in the Golgi extends from the cis to the trans side. W, Cell wall; ER, endoplasmic reticulum; G, Golgi stack. Scale bar = 100 nm. In each case representative micrographs are shown.
Ultrathin sections were prepared from hydroponically grown cuttings and ABP1 was immunolocalized using Fab fragments of a polyclonal antibody raised against maize ABP1. Visualization of ABP1 in leaf cell sections was not feasible because of high background staining and poor structural preservation in the fixation conditions necessary to maintain high antigenicity (not shown). In consequence, root tips were chosen for electron microscopy since they are less vacuolate, allowing relatively low concentrations of fixative to be used. In wild-type and transformed plants, ABP1 signal was associated with the ER (a total of 108 gold particles associated with the ER in 11 sections showing stretches of ER; Fig. 5, A–C), a staining pattern characteristic of other ER luminal proteins (Napier et al., 1992; Shorrosh, 1993; Robinson et al., 1995). The specificity of the staining was confirmed by elimination of the signal following pre-incubation of the antibody with purified maize ABP1 (Fig. 5D). Although staining at the cell wall was exceptionally rare (a total of eight gold particles were detected at the cell wall in 16 sections showing stretches of cell wall; Fig. 5A), the Golgi apparatus was relatively frequently labeled (a total of 144 gold particles in 21 Golgi stacks; Fig. 5, E–G). The pattern of labeling in the Golgi did, however, vary according to the ABP1 genotype. In the cases of KDEL and HDEL forms of ABP1, labeling was confined strictly to one side of the Golgi stacks (a total of 33 gold particles in 9 Golgi stacks; Fig. 5E), a staining pattern analogous to that obtained in animal Hep-2 cells for a chimeric horseradish peroxidase protein tagged with a KDEL signal (Stinchcombe et al., 1995). In contrast, the Golgi appeared to be more heavily labeled in the case of KEQL and KDELGL forms and labeling was found on both sides of the Golgi stacks (a total of 115 gold particles in 12 Golgi stacks; Fig. 5, F and G). These staining patterns are consistent with the view that salvage of KDEL and HDEL proteins occurs from the cis Golgi in plants as it does in animals and yeast (Andres et al., 1991; Denecke et al., 1992; Sönnichsen et al., 1994; Bassham and Raikhel, 1996). The presence of KEQL and KDELGL forms of ABP1 in the trans Golgi indicated that these mutations were having the anticipated effect, enabling ABP1 to bypass KDEL salvage in the cis Golgi. However, these observations were not matched by a rise in abundance of ABP1 at the cell surface (see above). In a similar manner, we were unable to detect an increase in labeling when visualizing ABP1 at the plasma membrane of leaf mesophyll protoplasts using silver-enhanced immunogold epipolarization microscopy (SEIG EPOM; Fig. 6).
Figure 6.
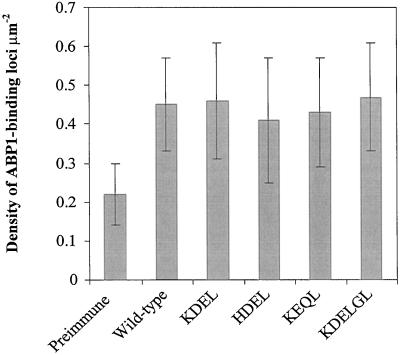
Measurements of ABP1 at the surface of tobacco protoplasts by SEIG EPOM. ABP1 was assayed by point light sources at the protoplast surface and expressed as density of ABP1 loci μm−2. Preimmune, Background density of point light sources on protoplasts from wild-type untransformed plants. For each genotype, 50 protoplasts were examined. Error bars show sd.
DISCUSSION
Overexpression of ABP1 led to changes in K+ fluxes in intact guard cells (Fig. 3). These observations complement those of Jones et al. (1998) who reported that ABP1 overexpression gave rise to an auxin-dependent elevation of cell expansion in transgenic tobacco leaf epidermal cells and those of Claussen et al. (1997), who showed that auxin-induced growth is dependent on K+ influx. Changes in K+ current underly cell expansion and stomatal opening. The results are also consistent with the responses of tobacco and orchid guard cells to ABP1 antibodies and peptides (Thiel et al., 1993; Gehring et al., 1998).
Both inwardly and outwardly rectifying K+ currents were auxin sensitive, as in broad bean (Blatt and Thiel, 1994). However, both currents displayed dose-response relationships over very wide concentration ranges and it seems likely that several response systems are involved. For IK,out (Fig. 3B), there was a clear sensitivity shift, confined to the upper range of auxin concentrations, the range appropriate for the KD of ABP1 (Fig. 2; see also Löbler and Klämbt, 1985). At much lower concentrations of auxin, the response of IK,out was similar between control plants and those overexpressing ABP1. For IK,in (Fig. 3C), plants overexpressing ABP1 also exhibited a sensitivity shift although it is unlikely that the responses of either current to concentrations of auxin below 0.1 nm were directly linked to ABP1. At auxin concentrations above 10−10 M, these plants showed a greater reduction in the amplitude of IK,in than control plants. The apparent one-half maximal inhibition lay between concentrations of 10 and 100 nm, again appropriate for the KD of ABP1.
The most striking feature of our data was that ABP1 overexpression alone was sufficient to alter auxin sensitivity. It has been shown that the mutations introduced into ABP1 did not affect binding kinetics (Fig. 2), but some effect of redirecting ABP1 had been anticipated. Nevertheless, responses from plants expressing mutated forms of ABP1 were indistinguishable from those overexpressing the wild type, KDEL form of the protein. This parallels the phenotypes observed by Jones et al. (1998), which were from plants overexpressing wild-type (KDEL) ABP1 in a tetracycline-inducible system. In consequence, redirection of ABP1 to escape ER retention would appear to be unnecessary for altering the activity of ABP1 in guard cells and other cells of the leaf epidermis.
Much previous work has suggested that ABP1 is active at the cell surface (Barbier-Brygoo et al., 1989; Venis et al., 1992; Rück et al., 1993; Thiel et al., 1993; Gehring et al., 1998; Leblanc et al., 1999) and that auxin sensitivity can be manipulated through several orders of magnitude by the addition of exogenous ABP1 (Barbier-Brygoo et al., 1991). However, our data show a marked increase in auxin responsiveness in the absence of a detectable rise in ABP1 at the cell surface. Our staining pattern for ABP1 in tobacco root cap cells is in broad agreement with that reported by Bronsema et al. (1998) for maize coleoptiles and embryos. The HDEL and KDEL forms of ABP1 never passed further than the cis Golgi, from where it is likely they are salvaged back to the ER, as suggested by the oligo-Man structure of the N-linked glycan (Henderson et al., 1997). The KEQL and KDELGL forms of ABP1 were detected in the trans Golgi, suggesting that they were in the process of being secreted, as in insect cells (Fig. 4). However, it was not possible to measure a rise in ABP1 at the plant cell surface. Virtually no ABP1 was detected in the cell walls (Fig. 5) and using SEIG EPOM, which reports only on antigen on the cell surface, no more ABP1 was detected on the plasma membrane surface in plants overexpressing ABP1 than in the control plants (Fig. 6). Finally, we did not detect ABP1 in or around vacuolar compartments. Collectively these results differ from the outcome of overexpression in insect cells (Fig. 4). It is possible that in intact plants the protein is broken down rapidly in the apoplast (Henderson et al., 1997) or lost by diffusion, but after measurements with a range of techniques we remain unable to measure elevated ABP1 levels at the cell surface. In consequence it is difficult to draw firm conclusions on the localization of ABP1 with respect to the cell surface. Nevertheless, although we cannot exclude the possibility that small changes in plasma membrane ABP1 abundance occur in planta, it seems unlikely that any such changes are substantial.
If the site of action of ABP1 is at the cell surface as suggested by previous reports (for review, see Jones, 1994; Napier and Venis, 1995), then it would seem that a relatively small increase in the pool of ABP1 at the plasma membrane (an increase below the limits of detection by the methods used here and which can be provided by overexpression of the KDEL or HDEL forms of ABP1 alone) is sufficient to confer a marked shift in auxin sensitivity. Another interpretation is also possible. The apparent lack of difference in the levels of ABP1 at the cell surface in plants overexpressing ABP1 (regardless of the C terminus) correlates with the fact that they all showed a similar sensitivity to auxin. However, there was also no marked change in the cell surface abundance of ABP1 in these transgenic plants compared with control and wild-type plants, groups between which there was a marked change in sensitivity to auxin. This opens up again the question of whether ABP1 might also be active inside the cell.
In conclusion, it appears that overexpression of ABP1 is sufficient to confer increased sensitivity to auxin. This is consistent with the report of Jones et al. (1998) in which overexpression of wild-type (KDEL) ABP1 was found to induce increased leaf epidermal cell sizes and to raise the capacity of epidermal cells to expand in the presence of exogenous auxin. Our data suggest that part of the mechanism through which these responses are mediated might be through the modulation of K+ currents. Influx of K+ has been shown to play a role in auxin signaling and auxin-induced cell expansion (Claussen et al., 1998) and auxin-induced K+ channel expression has been found to be an essential step in coleoptile growth and gravitropism (Philippar et al., 1999). Single cell recordings (Fig. 3) complement and extend all these reports by illustrating an ABP1-dependent shift in auxin sensitivity for K+ currents in guard cells.
MATERIALS AND METHODS
In Vitro Mutagenesis of ABP1 and Construction of Vectors
The plasmid pAux3 (Macdonald et al., 1994) contains a maize (Zea mays) wild-type ABP1 cDNA (Lazarus et al., 1991) flanked by BamHI (5′) and KpnI (3′) restriction sites. The PCR was used to introduce changes into a subcloned EcoRI fragment that extended from within the coding region to a downstream linker sequence used in the original cDNA library construction. Mutagenesis of the carboxy terminal KDEL sequence to HDEL, KEQL, and KDELGL was achieved with the (complementary strand) primers 5′-ACTTGTGACCTAGAGTTCGTCATGTGCTGCTTC-3′, 5′-ACTTGTGACCTAGAGTTGCTCTTTTGCT-3′, and 5′-ACTTGTGACCTAGAGTCCGAGTTCGTCTTTTG-3′, respectively (mu-tagenicbases in italics), used in conjunction with the universal M13 forward primer. The primers 5′-ACTCTAGGTCACAAGTGT-3′ and 5′-TAGTAGTCCGGTACCAGCAGG-3′ (designated 3′Kpn) were used to amplify an overlapping fragment extending from the end of the coding region to an introduced KpnI site (underlined) within the 3′-untranslated region. Modified carboxy terminal fragments were combined with the 3′ fragment in PCRs containing overlapping PCR products as template and the flanking primers (M13 forward and 3′Kpn). The resultant products were digested with EcoRI and KpnI and ligated into pAux3 to replace the wild-type KDEL-encoding segment and 3′-untranslated region. The presence of the desired mutations and absence of undesired mutations were confirmed in each case by DNA sequencing.
Transfer vectors for the production of mutated ABP1s in the baculovirus system were constructed by inserting BamHI-KpnI fragments between the BglII and KpnI sites of pEVmXIV as described for the expression of wild-type ABP1 (Macdonald et al., 1994). Plasmids for plant transformation were constructed by inserting the same wild-type or mutated ABP1 fragments into the polylinker of a cauliflower mosaic virus-nos expression cassette in pBin19 (Bevan, 1984).
Plant Transformation
Plasmids were introduced into Agrobacterium tumefaciens strain LBA4404 by electroporation. Leaf pieces of tobacco (Nicotiana tabacum cv Samsun) were transformed by cocultivation essentially as described by Herrera-Estrella and Simpson (1988) except that the medium used was Murashige and Skoog basal medium with Gamborg's vitamins (Sigma, Poole, UK) containing 30 g L−1 Suc, 1 mg L−1 benzylaminopurine, and 0.1 mg L−1 naphthylacetic acid. Transgenic shoots were regenerated on Murashige and Skoog medium A (Sigma) containing 300 μg mL−1 kanamycin, and rooted on Murashige syngonium stage III medium (Sigma) containing 100 μg mL−1 kanamycin. Cefotaxime (250 μg mL−1) and nystatin (50 units mL−1) were included throughout the regeneration phase. All solid media contained 0.8% (w/v) agar.
Northern-Blot Analysis of Transgenic Tobacco
Total RNA was extracted from 1-g samples of young leaves by the method of Napoli et al. (1990). Ten-microgram samples were fractionated by electrophoresis in 3-[N-morpholino] propane-sulfonic acid-formaldehyde agarose gels and transferred to nylon membranes. Hybridization to DNA probes and washes of the RNA gel blots were as described for DNA gel blots by Lazarus and Macdonald (1996).
Analysis of Proteins in Microsomal Membranes
The extraction and electrophoretic fractionation of proteins was as described by Macdonald et al. (1994), starting from 4 g of leaf tissue. Western immunoblotting was also as described, except that alkaline phosphatase activity was detected by enhanced chemiluminescence (Amersham, Buckinghamshire, UK).
Baculovirus-Mediated Expression of ABP1 in Insect Cells
Constructs of all four forms of maize ABP1 expressed in tobacco plants were generated and expressed in Spodoptera frugiperda insect cells via baculovirus infection as previously described (Macdonald et al., 1994; Henderson et al., 1996). Secretion of recombinant ABP1 from insect cell cultures infected in 35-mm tissue culture plates (multiplicity of infection = 10) was followed over 120 h postinfection. Cells and medium were harvested every 24 h and analyzed separately by SDS-PAGE as described previously (Henderson et al., 1997).
Auxin-Binding Assays
Recombinant ABP1 was partially purified from infected insect cells by ion-exchange chromatography and assayed for auxin-binding activity by ammonium sulfate precipitation as described previously (Macdonald et al., 1994). Maize ABP1 partially purified from 5-d-old etiolated maize coleoptiles by ion-exchange chromatography was used for comparison (Napier et al., 1988).
Microscopy
For immunogold electron microscopy, root tips from stem cuttings grown hydroponically in aerated water supplemented with macronutrients [10 mm KCl, 1.5 mm CaCl2, 0.6 mm KH2PO4, 0.75 mm MgSO4, and 5 mm (NH4)2(SO4)], micronutrients (10 μm MnSO4, 1 μm CuSO4, 1 μm ZnSO4, 50 μm H3BO3, 100 μm NaCl, and 0.5 μm Na2MoO4), and 50 μm Fe-EDTA were subjected to fixation in 2% (w/v) formaldehyde in 50 mm phosphate-buffered saline (PBS) pH 7.0, for 20 h at 4°C. Root tips were dehydrated with progressive lowering of temperature, 15 min in 30% (w/v) ethanol at 0°C followed by 45 min in each of 50% (w/v) at −20°C, 70% (w/v), and 100% (w/v) ethanol at −35°C, respectively. The root tips were then embedded in Lowicryl HM20 resin and polymerized with UV for 24 h at −35°C (Villinger, 1991). Eighty-nanometer sections (Ultracut; Leica, Vienna, Austria), were collected on formvar-coated nickel grids and incubated in PBS with 1% (w/v) bovine seerum albumin (BSA) for 45 min before incubation with anti-ABP1 polyclonal IgG Fab fragments (1:30 in PBS-Tween 20 and 1% [w/v] BSA) for 2 h. After 3 × 5 min washes in PBS-Tween 20 with 0.1% (w/v) BSA, sections were incubated with 10-nm gold-linked anti-rabbit secondary antibody (BioCell, Cardiff, UK) diluted 1:30 in PBS-Tween 20 with 0.1% (w/v) BSA for 30 min. After three additional 5-min washes in PBS-Tween 20 with 0.1% (w/v) BSA and two brief washes in double-distilled water, sections were post-stained by incubation in aqueous OsO4 (4%, w/v) for 1 h, aqueous uranyl acetate (3%, w/v) for 5 min, and lead citrate (0.3%, w/v) for 5 min. Sections were viewed at 80 kV using an electron microscope (CM10; Philips, Eindhoven, The Netherlands). For testing the specificity of the signal, the antibody preparation was pre-incubated with 1 nm purified maize ABP1. SEIG EPOM was carried out on leaf mesophyll protoplasts according to the methods of Diekmann et al. (1995).
Electrophysiology
Epidermal strips from tobacco plants were prepared from newly expanded leaves of plants 4 to 6 weeks-old (Thiel et al., 1993; Blatt and Thiel, 1994). All operations were carried out on an Axiovert microscope (Zeiss, Oberkochen, Germany) fitted with Nomarski D.I.C. optics with strips bathed in rapidly flowing solutions (10 mL min−1 approximately 20 chamber volumes per minute) at 20°C to 22°C. The standard medium was prepared with MES [5 mm 2-(N-morpholino) propanesulfonic acid] titrated to its pKa (6.1) with Ca(OH)2 (final [Ca2+] approximately 1 mm). KCl and IAA were included as required. Buffers and salts were from Sigma Chemicals. Electrical recordings were achieved with double-barreled microelectrodes coated with paraffin to reduce electrode capacitance and filled with 200 mm KOAc to minimize salt leakage and salt-loading artifacts associated with the Cl− anion (Blatt and Armstrong, 1993). Connection to the amplifier headstage was via a 1-m KCl ‖Ag-AgCl halfcell, and a matching half-cell and 1 m KCl-agar bridge served as the reference (bath) electrode. Membrane currents were measured by voltage clamp under microprocessor control (LAB/LAN; WyeScience, Wye, UK) using three-pulse protocols (sampling frequency, 2 kHz) and bipolar staircase duty cycles.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Philip White and Dr. Steven Jackson for critical reading of the manuscript and helpful comments.
Footnotes
This work was jointly funded by the Biotechnology and Biological Science Research Council, the Gatsby Charitable Foundation, the Science and Engineering Research Council, and the EC.
LITERATURE CITED
- Abel S, Theologis A. Early genes and auxin action. Plant Physiol. 1996;111:9–17. doi: 10.1104/pp.111.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres DA, Rhodes JD, Meisel RL, Dixon JE. Characterization of the carboxyl-terminal sequences responsible for protein retention in the endoplasmic reticulum. J Biol Chem. 1991;266:14277–14282. [PubMed] [Google Scholar]
- Barbier-Brygoo H, Ephritikhine G, Klämbt D, Ghislain M, Guern J. Functional evidence for an auxin receptor at the plasmalemma of tobacco mesophyll protoplasts. Proc Natl Acad Sci USA. 1989;86:891–895. doi: 10.1073/pnas.86.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Brygoo H, Ephritikine G, Klämbt D, Maurel C, Palme K, Schell J, Guern J. Perception of the auxin signal at the plasma membrane of tobacco mesophyll protoplasts. Plant J. 1991;1:83–93. doi: 10.1042/bst0200059. [DOI] [PubMed] [Google Scholar]
- Barbier-Brygoo H, Zimmermann S, Thomine S, White IR, Millner P, Guern J. Elementary response chains at the plasma membrane involve external ABP1 and multiple electrogenic ion transport proteins. Pant Growth Regul. 1996;18:23–28. [Google Scholar]
- Bassham DC, Raikhel NV. Transport proteins in the plasma membrane and the secretory pathway. Trends Plant Sci. 1996;1:15–20. [Google Scholar]
- Batt S, Venis MA. Separation and localization of two classes of auxin-binding sites in corn coleoptile membranes. Planta. 1976;130:15–21. doi: 10.1007/BF00390839. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR, Armstrong F. K+ channels of stomatal guard cells: abscisic acid-evoked control of the outward rectifier mediated by cytoplasmic pH. Planta. 1993;191:330–341. [Google Scholar]
- Blatt MR, Thiel G. K+ channels of stomatal guard cells: bimodal control of the K+ inward-rectifier evoked by auxin. Plant J. 1994;5:55–68. doi: 10.1046/j.1365-313x.1994.5010055.x. [DOI] [PubMed] [Google Scholar]
- Bronsema FBF, van Oostveen WJF, van Lammeren AAM. Immunocytochemical localization of auxin-binding proteins in coleoptiles and embryos of Zea mays L. Protoplasma. 1998;202:65–75. [Google Scholar]
- Claussen M, Luthen H, Blatt M, Böttger M. Auxin-induced growth and its linkage to potassium channels. Planta. 1998;201:227–234. [Google Scholar]
- Cross JW, Briggs WR. Properties of solubilised microsomal auxin-binding protein from coleoptiles and primary leaves of Zea mays. Plant Physiol. 1978;62:152–157. doi: 10.1104/pp.62.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ. The plant hormones: their nature, occurrence and functions. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 5. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1–12. [Google Scholar]
- Denecke J, De Rycke R, Botterman J. Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 1992;11:2345–2355. doi: 10.1002/j.1460-2075.1992.tb05294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann W, Venis MA, Robinson DG. Auxins induce clustering of auxin-binding protein at the surface of maize coleoptile protoplasts. Proc Natl Acad Sci USA. 1995;92:3425–3429. doi: 10.1073/pnas.92.8.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, McConchie RM, Venis MA, Parish RW. Auxin-binding-protein antibodies and peptides influence stomatal opening and alter cytoplasmic pH. Planta. 1998;205:581–586. doi: 10.1007/s004250050359. [DOI] [PubMed] [Google Scholar]
- Guilfoyle T, Hagen G, Ulmasov T, Murfett J. How does auxin turn on genes? Plant Physiol. 1998;118:341–347. doi: 10.1104/pp.118.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J, Bauly JM, Ashford DA, Oliver SC, Hawes CR, Lazarus CM, Venis MA, Napier RM. Retention of maize auxin-binding protein in the endoplasmic reticulum: quantifying escape and the role of auxin. Planta. 1997;202:313–323. doi: 10.1007/s004250050133. [DOI] [PubMed] [Google Scholar]
- Henderson J, Macdonald H, Lazarus CM, Napier RM, Hawes CR. Protein retention in the endoplasmic reticulum is not compromised by baculovirus infection. Cell Biol Int. 1996;6:413–422. doi: 10.1006/cbir.1996.0052. [DOI] [PubMed] [Google Scholar]
- Herrera-Estrella L, Simpson J. Foreign gene expression in plants. In: Shaw CH, editor. Plant Molecular Biology: A Practical Approach. Oxford: IRL Press; 1988. pp. 131–160. [Google Scholar]
- Hertel R. Auxin-binding protein is a red herring. J Exp Bot. 1995;46:461–462. [Google Scholar]
- Hesse T, Feldwisch J, Balshüsemann D, Puype M, Vandekerckhove J, Löbler M, Klämbt D, Schell J, Palme K. Molecular cloning and structural analysis of a gene from Zea mays (L.) coding for a putative receptor for the plant hormone auxin. EMBO J. 1989;8:2453–2461. doi: 10.1002/j.1460-2075.1989.tb08380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM. Auxin-binding proteins. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:393–420. [Google Scholar]
- Jones AM, Herman EM. KDEL-containing auxin-binding protein is secreted to the plasma membrane and cell wall. Plant Physiol. 1993;101:595–606. doi: 10.1104/pp.101.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Im K-H, Savka MA, Wu M-J, DeWitt G, Shillito R, Binns AN. Auxin-dependent cell expansion mediated by over-expressed auxin-binding protein 1. Science. 1998;282:1114–1117. doi: 10.1126/science.282.5391.1114. [DOI] [PubMed] [Google Scholar]
- Kim J, Harter A, Theologis A. Protein-protein interactions among the Aux/IAA proteins. Proc Natl Acad Sci USA. 1997;94:11786–11791. doi: 10.1073/pnas.94.22.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus CM, Macdonald H. Characterization of a strawberry gene for auxin-binding protein, and its expression in insect cells. Plant Mol Biol. 1996;31:267–277. doi: 10.1007/BF00021789. [DOI] [PubMed] [Google Scholar]
- Lazarus CM, Napier RM, Yu L-X, Lynas C, Venis MA. Auxin-binding proteins: antibodies and genes. In: Jenkins GI, Schuch W, editors. Molecular Biology of Plant Development. Cambridge, UK: Company of Biologists; 1991. pp. 129–148. [PubMed] [Google Scholar]
- Leblanc N, Perrot-Rechenmann C, Barbier-Brygoo H. The auxin-binding protein Nt-ERabp1 alone activates an auxin-like transduction pathway. FEBS Letters. 1999;449:57–60. doi: 10.1016/s0014-5793(99)00398-1. [DOI] [PubMed] [Google Scholar]
- Löbler M, Klämbt D. Auxin-binding protein from coleoptile membranes of corn (Zea mays L.): I. Purification by immunological methods and characterization. J Biol Chem. 1985;260:9848–9853. [PubMed] [Google Scholar]
- Macdonald H. Auxin perception and signal transduction. Physiol Plant. 1997;100:423–430. [Google Scholar]
- Macdonald H, Henderson J, Napier RM, Venis MA, Hawes C, Lazarus CM. Authentic processing and targeting of active maize auxin-binding protein in the baculovirus expression system. Plant Physiol. 1994;105:1049–1057. doi: 10.1104/pp.105.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memelink J, Hoge JHC, Schilperoot RA. Cytokinin stress changes the developmental regulation of several defense-related genes in tobacco. EMBO J. 1987;6:3579–3583. doi: 10.1002/j.1460-2075.1987.tb02688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJP. A reassessment of the binding of naphthaleneacetic acid by membrane preparations from maize. Planta. 1980;149:417–426. doi: 10.1007/BF00385742. [DOI] [PubMed] [Google Scholar]
- Napier RM, Fowke LC, Hawes C, Lewis M, Pelham HRB. Immunological evidence that plants use both HDEL and KDEL for targeting proteins to the endoplasmic reticulum. J Cell Sci. 1992;102:261–271. doi: 10.1242/jcs.102.2.261. [DOI] [PubMed] [Google Scholar]
- Napier RM, Venis MA, Bolton MA, Richardson LI, Butcher GW. Preparation and characterization of monoclonal and polyclonal antibodies to maize auxin-binding protein. Planta. 1988;176:519–526. doi: 10.1007/BF00397659. [DOI] [PubMed] [Google Scholar]
- Napoli C, Lemieux C, Jorgenson R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible cosuppression of homologous genes in trans. Plant Cell. 1990;2:279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HRB. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Pelham HRB. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990;15:483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Luthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Bottger M, Becker D, Hedrich R. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PM, Dohrmann U, Hertel R. Characterization of naphthaleneacetic acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977a;59:357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PM, Dohrmann U, Hertel R. Specificity of auxin-binding sites on maize coleoptile membranes as possible receptor sites for auxin action. Plant Physiol. 1977b;60:585–591. doi: 10.1104/pp.60.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle DL, Cleland RE. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Hoh B, Hinz G, Jeong B-K. One vacuole or two vacuoles: do protein storage vacuoles arise de novo during pea cotyledon development? J Plant Physiol. 1995;145:654–664. doi: 10.1242/jcs.108.1.299. [DOI] [PubMed] [Google Scholar]
- Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser HMO. Changes in auxin response from mutations in an AUX/IAA gene. Science. 1998;279:1372–1373. doi: 10.1126/science.279.5355.1371. [DOI] [PubMed] [Google Scholar]
- Rück A, Palme K, Venis MA, Napier RM, Felle H. Patch-clamp analysis establishes a role for an auxin binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant J. 1993;4:41–46. [Google Scholar]
- Shimomura S, Sotobayashi T, Futai M, Fukui T. Purification and properties of an auxin-binding protein from maize shoot membranes. J Biochem. 1986;99:1513–124. doi: 10.1093/oxfordjournals.jbchem.a135621. [DOI] [PubMed] [Google Scholar]
- Shorrosh Expression and localization of plant protein disulfide isomerase. Plant Physiol. 1993;103:719–726. doi: 10.1104/pp.103.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon F, Perrot-Rechenmann Expression of auxin-regulated genes. Physiol Plant. 1997;100:443–455. [Google Scholar]
- Sönnichsen B, Füllekrug J, Nguyen Van P, Diekmann W, Robinson DG, Mieskes G. Retention and retrieval: both mechanisms cooperate to maintain calreticulin in the endoplasmic reticulum. J Cell Sci. 1994;107:2705–2717. doi: 10.1242/jcs.107.10.2705. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Nomoto H, Cutler DF, Hopkins CR. Anterograde and retrograde traffic between the rough endoplasmic reticulum and the Golgi complex. J Cell Biol. 1995;131:1387–1401. doi: 10.1083/jcb.131.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G, Blatt MR, Fricker MD, White IR, Millner P. Modulation of K+ channels in Vicia stomatal guard cells by peptide homologues to the auxin-binding protein C terminus. Proc Natl Acad Sci USA. 1993;90:11493–11497. doi: 10.1073/pnas.90.24.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venis MA. Auxin binding protein 1 is a red herring? Oh no it isn't! J Exp Bot. 1995;46:463–465. [Google Scholar]
- Venis MA, Napier RM. Auxin receptors and auxin binding proteins. Crit Rev Plant Sci. 1995;14:27–47. [Google Scholar]
- Venis MA, Napier RM, Barbier-Brygoo H, Maurel C, Perrot-Rechenmann C, Guern J. Antibodies to a peptide from the maize auxin-binding protein have auxin agonist activity. Proc Natl Acad Sci USA. 1992;89:7208–7212. doi: 10.1073/pnas.89.15.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villinger W. Lowicryl resins. In: Hayat MA, editor. Colloidal Gold. Vol. 3. London: Academic Press; 1991. pp. 59–71. [Google Scholar]
- Vuori K, Pihlajaniemi T, Myllyla R, Kivirikko KI. Site-directed mutagenesis of human protein disulphide isomerase: effect on the assembly, activity and endoplasmic-reticulum retention of human prolyl 4-hydroxylase in Spodoptera frugiperda insect cells. EMBO J. 1992;11:4213–4217. doi: 10.1002/j.1460-2075.1992.tb05515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L, Estelle M. Molecular mechanisms of auxin action. Curr Opin Plant Biol. 1998;1:434–439. doi: 10.1016/s1369-5266(98)80269-0. [DOI] [PubMed] [Google Scholar]