Abstract
Objectives
To highlight alternative treatment options other than exogenous testosterone administration for hypogonadal men with concomitant infertility or who wish to preserve their fertility potential, as testosterone replacement therapy (TRT) inhibits spermatogenesis, representing a problem for hypogonadal men of reproductive age.
Materials and methods
We performed a comprehensive literature review for the years 1978–2017 via PubMed. Also abstracts from major urological/surgical conferences were reviewed. Review was consistent with the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) criteria. We used Medical Subject Heading terms for the search including ‘testosterone replacement therapy’ or ‘TRT’ and ‘male infertility’.
Results
In all, 91 manuscripts were screened and the final number used for the review was 56. All studies included were performed in adults, were written in English and had an abstract available.
Conclusions
Exogenous testosterone inhibits spermatogenesis. Hypogonadal men wanting to preserve their fertility and at the same time benefiting from TRT effects can be prescribed selective oestrogen receptor modulators or testosterone plus low-dose human chorionic gonadotrophin (hCG). Patients treated for infertility with hypogonadotrophic hypogonadism can be prescribed hCG alone at first followed by or in combination from the start with follicle-stimulating hormone preparations.
Abbreviations: CC, clomiphene citrate; E2, oestradiol; hCG, human chorionic gonadotrophin; hMG, human menopausal gonadotrophin; HPG, hypothalamic–pituitary–gonadal; PRISMA, Preferred Reporting Items for Systemic Reviews and Meta-Analyses; SERM, selective oestrogen receptor modulator; SHBG, sex hormone-binding globulin; TRT, testosterone replacement therapy
Keywords: Gonadotrophins, Hypogonadism, Infertility, Systematic review, Testosterone therapy
Introduction
Male hypogonadism is characterised by low serum testosterone and associated with symptoms such as fatigue, decreased libido, erectile dysfunction, concentration difficulty, sleep disturbance, and loss of lean body mass or weight gain [1].
The prevalence of male hypogonadism is reported to be 37% in the USA and a higher prevalence is seen with increasing age [2], [3], [4]. Another large population-based study in the USA showed the prevalence of symptomatic hypogonadism to be 5.6% in men aged 30–79 years [5].
The impact of testosterone deficiency on the overall health of men was recently examined in a meta-analysis. Hypogonadism was found to be linked to cardiovascular mortality, metabolic syndrome, osteoporosis, frailty, non-insulin dependent diabetes, and depression [6]. Treatment for hypogonadism typically includes testosterone replacement therapy (TRT), which results in satisfactory amelioration of symptoms and normalisation of serum testosterone. However, treatment with exogenous testosterone decreases serum gonadotrophins, impairs normal spermatogenesis and suppresses intra-testicular testosterone. Azoospermia develops in up to 40% of patients on TRT and, as a result, treatment of hypogonadal men desiring to reproduce whilst on TRT remains a challenge [7].
Testosterone deficiency may result in some clinical manifestations as shown in Table 1, which may affect sexual health, reproductive health, and overall quality of life. These clinical symptoms and signs improve dramatically with TRT [8].
Table 1.
Symptoms, signs, and conditions indicative of testosterone deficiency.
| Vasomotor and nervous symptoms: |
| • Hot flushes (similar to those of menopause in women) |
| • Episodes of sweating |
| • Insomnia and disturbed sleep rhythm |
| • Nervousness |
| Mood disorders and cognitive functions: |
| • Irritability and lethargy |
| • Decreased sense of well-being |
| • Lack of motivation |
| • Difficulties with short-term memory |
| • Depressive symptoms |
| Masculinity/virility: |
| • Decreased vigour and physical energy |
| • Diminished muscle mass (sarcopenia) and strength (sarcoasthenia) |
| • Loss of sexual body hair |
| • Abdominal obesity |
| • Gynaecomastia |
| Sexuality: |
| • Decreased interest or desire for sex |
| • Reduction of sexual activity |
| • Poor erectile function |
| • Limited quality of orgasm (unpleasurable orgasm) |
| • Weakness or reduction of ejaculation |
Infertility is defined as the inability of sexually active non-contracepting couples to achieve clinical pregnancy within 1 year. It affects 10–15% of all couples who are seeking conception within the first year and seek medical treatment for infertility [9].
Amongst all infertile couples, male factor represents 40–50% of all causes of infertility. Several causes have been attributed to reduced male fertility including; congenital and acquired urogenital abnormalities, genital tract infections, genetic and chromosomal anomalies, varicocele immunological factors, exposure to gonadotoxins, and endocrine disturbances. No causal factor is found in 30–40% of cases and thus known as idiopathic male infertility [10].
Physiology of hypothalamic–pituitary–gonadal (HPG) axis
The hypothalamus, the pituitary, and the testes form the HPG axis, which acts in harmony and synchronisation to achieve adequate secretion of androgens and normal spermatogenesis. Under the influence of neuropeptides (i.e. kisspeptin, noradrenaline and leptin with stimulatory effects, and prolactin, dopamine, serotonin, γ-aminobutyric acid (GABA) and interleukin 1 being inhibitory), the arcuate nucleus and preoptic area in the hypothalamus secrete GnRH in a pulsatile manner that in turn stimulates the gonadotrophs in the anterior pituitary gland to release the gonadotrophins; FSH and LH [11].
FSH acts directly on the germinal epithelium stimulating spermatogenesis; also it stimulates Sertoli cells to support spermatogenesis and secretes inhibin B, which negatively regulates FSH secretion. On the other hand, LH stimulates the secretion of testosterone by Leydig cells from its precursor (cholesterol) that in turn stimulates sperm production and virilisation, in addition to providing feedback to the hypothalamus and pituitary to regulate GnRH secretion [12].
It is well-known that the GnRH pulse generator is the main regulator of puberty; however, its production of GnRH starts early in foetal life. As a result, gonadotrophin levels undergo drastic changes during foetal development, childhood, puberty, and adulthood. Male infants exhibit a unique phenomenon known as a ‘mini-puberty’ during the first 6 months of life, during which gonadal function can be clinically detected in response to gonadotrophin stimulation. After that period, serum gonadotrophin levels drop and can only be detected again with the onset of puberty [13].
Endocrine causes of male infertility are uncommon, representing only 2% of patients with an abnormal semen analysis due to primary endocrinal defects along the HPG axis leading to hypogonadism [14].
Hypogonadism can be simply classified into two main categories: (i) hypogonadotrophic hypogonadism due to dysfunction in the hypothalamus and/or pituitary gland and (ii) hypergonadotrophic hypogonadism due to testicular failure.
Hypogonadotrophic hypogonadism
This type of hypogonadism is also known as secondary hypogonadism and is caused by deficient secretion of GnRH from the hypothalamus and/or deficient secretion of gonadotrophins (FSH and LH) from the pituitary gland. As a result of these deficient hormones, impairment of testicular functions occurs (spermatogenesis and/or steroidogenesis). Hormonal evaluation of hypogonadotrophic hypogonadism is characterised by low-normal or low levels of FSH, LH, and testosterone [15].
Several causes have been attributed to hypogonadotrophic hypogonadism including various congenital and acquired conditions.
Hypergonadotrophic hypogonadism
The term primary hypergonadotrophic hypogonadism refers to testicular disorders and is characterised by low serum testosterone despite high levels of FSH and LH. Low testosterone production results in impaired spermatogenesis (primary testicular failure) as seen in congenital anorchia, undescended testis, Sertoli cell only syndrome (germ cell aplasia), or after testicular injury from trauma, infection, surgery, exposure to chemo/radiotherapy or drug induced (e.g. ketoconazole, flutamide, spironolactone, etc.). In addition, genetic causes for primary testicular failure are described such as numerical chromosome aberrations including: Klinefelter syndrome, XX-male syndrome, XYY syndrome and Y chromosome microdeletions [16].
Hormonal evaluation
Hormonal evaluation is the key diagnostic tool for the assessment of hypogonadism and the differentiation between hypogonadotrophic and hypergonadotrophic hypogonadism. Initial laboratory assessment of the HPG axis includes the measurement of serum levels of LH, FSH, and total testosterone. More specialised hormonal evaluation including free testosterone, oestradiol (E2), prolactin, and sex hormone-binding globulin (SHBG), may be required upon suspicion after proper clinical history taking and physical examination [17].
Several clinical conditions, hormones, and certain drugs that may affect the circulating level of SHBG and thus affect the bioavailable fractions of testosterone in men are summarised in Table 2 [18], [19]. In addition, some genetic variations may also be responsible for inter-individual variations [20].
Table 2.
Factors increasing and decreasing SHBG and affecting free testosterone level.
| Factors that increase SHBG | Factors that decrease SHBG |
|---|---|
| Hyperthyroidism | Hypothyroidism |
| Ageing males (PADAM) | Obesity |
| Oestrogens | Androgens |
| Hepatocellular dysfunction | Hyperinsulinaemia and insulin resistance |
| Anti-epileptic drugs | Hyperprolactinaemia |
| Tamoxifen | Acromegaly |
| Hypercorticism |
PADAM, partial androgen deficiency of the ageing male.
Amongst different methods for proper estimation of serum total testosterone levels, radioimmunoassays and chemiluminescence immunoassays are the most widely used methods. However, liquid chromatography–tandem mass spectrometry is considered the gold-standard method to measure testosterone, but is only available in some reference and research laboratories [21].
Free or bioavailable testosterone can be measured directly using radioimmunoassay or by means of calculation using a formula incorporating SHBG. Measurement using equilibrium dialysis, ultrafiltration, and steady-state gel filtration is not recommended, because these methods are lengthy, require a high degree of skill, are unsuited for routine use, and lack evidence of superiority over radioimmunoassay [22].
All testosterone formulations share as a common shortcoming some suppression of the HPG axis if this axis was previously, at least partly, functional. This suppression results from a negative feedback mechanism causing decreased secretion of testosterone and spermatozoa. This negative impact on fertility is transient and disappears some months after discontinuation of testosterone therapy. Agents that stimulate the endogenous production of testosterone include human chorionic gonadotrophin (hCG) or in the mild cases clomiphene [23]. Other anti-oestrogens would theoretically avoid this effect in men with secondary testosterone deficiency (hypogonadotrophic testosterone deficiency). In young men with secondary hypogonadism/testosterone deficiency (hypogonadotrophic testosterone deficiency), long-term testosterone does not compromise future responsiveness to LH and FSH when they will require fertility, and may even, in some cases, be followed by recovery of pulsatile secretion of gonadotrophins with testosterone secretion and spermatogenesis [24]. However, a recent report indicates that prior androgen therapy is independently associated with a decrease in the likelihood of achieving conception [25]. Several therapeutic approaches have been suggested to manage hypogonadal infertile men by positively manipulating the HPG axis, thus improving endogenous testosterone. However, all of them are used off-label and have no USA Food and Drug Administration (FDA) approval as a specific treatment of male infertility.
The main objective of the present review article is to highlight alternative treatment options other than exogenous testosterone administration for hypogonadal men with concomitant infertility or who wish to preserve their fertility potential.
Material and methods
We performed a comprehensive literature review for the years 1978–2017 via PubMed and Medline. Also abstracts from major urological/surgical conferences were reviewed. Review was consistent with the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) criteria. We used Medical Subject Headings terms for the search including ‘testosterone replacement therapy’, or ‘TRT’, and ‘male infertility’. Exclusion criteria were: animal studies, studies involving children, female studies, and articles written in a language other than English.
Results
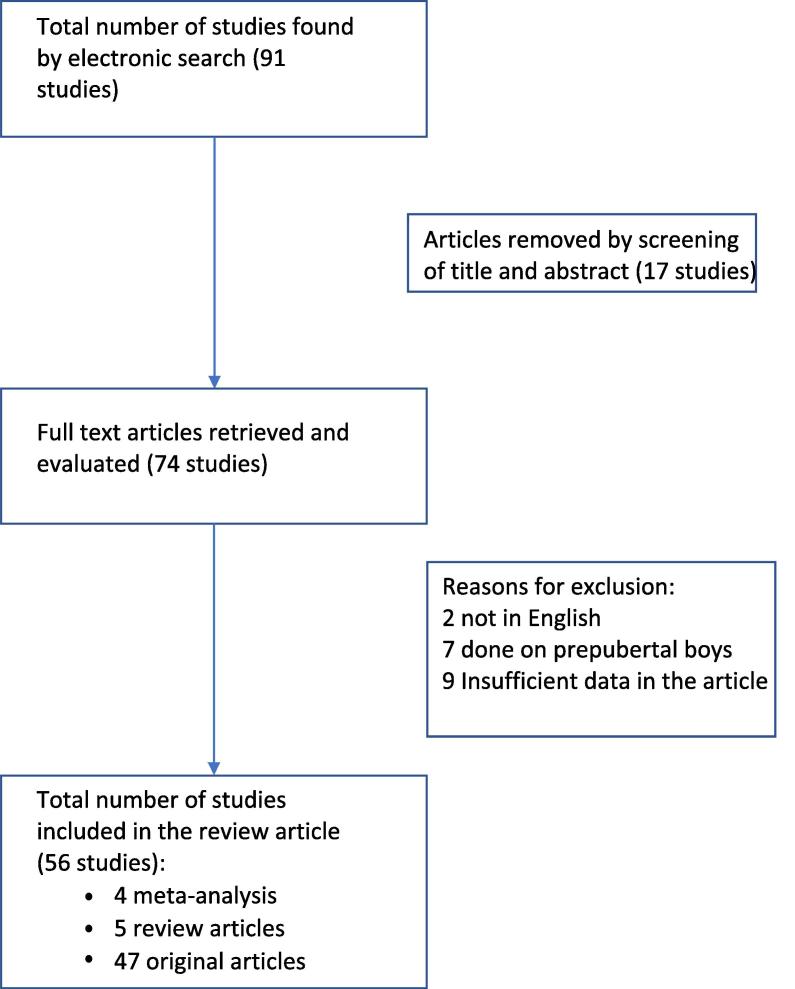
The total number of manuscripts screened was 91 and the final number of manuscripts used for the review was 56. All studies included were performed in adults, were written in English and had an abstract available. Review of the abstract denoted that 17 articles were irrelevant. The remaining 74 articles were further screened by the authors. Further exclusion was due to two articles being in language other than English, seven articles relating to prepubertal children, and nine articles had insufficient data (Fig. 1).
Fig. 1.
Flowchart illustrating selection criteria.
The following therapeutic approaches were introduced:
Selective oestrogen receptor modulators (SERMs)
SERMs are group of pharmaceutical agents that act by the competitive inhibition of oestrogen receptors in the hypothalamus and pituitary with subsequent increased release of GnRH and gonadotrophins thus increase intra-testicular testosterone production and enhance spermatogenesis. In addition, it remains uncertain whether there is also an additional direct effect of anti-oestrogens on spermatogenesis or steroidogenesis at the testicular level [26].
Clomiphene citrate (CC) as a chemical compound is composed mainly of a racemic mixture of two isoforms; enclomiphene and zuclomiphene. Several studies have used CC as an anti-oestrogen in the treatment of azoospermia, oligozoospermia and unexplained infertility, as well as for the treatment of hypogonadism independent from infertility. They demonstrated that CC causes moderate elevations in LH, FSH, total testosterone and sperm concentrations in such patients [27].
Concerning the impact of CC therapy on the pregnancy rate, two studies reported increased rates. Ghanem et al. [28] evaluated the combination of CC and vitamin E vs placebo and found that pregnancy rates were significantly higher in the treatment groups (P = 0.037). In addition, Chua et al. [29] conducted a meta-analysis of oestrogen antagonists that showed a statistically significant increase in sperm concentration as well as an increased pregnancy rate.
In hypogonadism and oligozoospermia, CC is used as a monotherapy. The starting dose is 25 mg once daily; as CC is normally available in 50 mg tablets, a starting dose might be 50 mg every other day. When testosterone remains low, CC can be titrated up to 100 mg daily [30].
In cases with late onset hypogonadism, CC was introduced as a TRT instead of testosterone compounds. It was stated that CC was effective in significantly increasing testosterone levels in at either a 25 or 50 mg dose. However, the rise of testosterone was not maintained after cessation of CC therapy, indicating that long-term therapy was needed [31].
In addition, CC has also been used on hypogonadal patients with azoospermia. Increases in intratesticular testosterone levels might favour production of sufficient sperm in the ejaculate. Accordingly, tamoxifen is used for the same indications, at a dose of 10 mg/day [31].
Recently, enclomiphene citrate, one of the single isomers of CC, has been tried in the treatment of male infertility. This isomer has more selective oestrogen antagonism compared to CC, which possesses both agonistic and antagonistic properties. To date, only a few studies have been conducted on the efficacy of enclomiphene citrate for treating male infertility and they have shown promising results with increased morning testosterone levels, LH, FSH, dihydrotestosterone, oestrogen, whilst preserving spermatogenesis [32], [33], [34].
In general, CC is a well-tolerated pharmacotherapeutic agent. The more common side-effects include: gastrointestinal distress, dizziness, hair loss, gynaecomastia, and minimal weight gain. In addition, a few case reports have mentioned that some patients have encountered visual disturbances such as blurred vision, photophobia, and diplopia. Fortunately, they are reversible with cessation of the medication [35]. It will be interesting to see whether future studies using a non-racemic mixture of pure enclomiphene will avoid these occasional negative side-effects.
On the other hand, tamoxifen citrate and other similar compounds toremifene and raloxifene are non-steroidal SERMs with a similar mechanism of action as CC at the level of the hypothalamus and pituitary. Tamoxifen citrate improves spermatogenesis by increasing FSH, Leydig cells sensitivity to LH, and testosterone levels, which lacks an intrinsic oestrogenic effect, so it may be more appropriate to use in male infertility [36], [37].
Previous studies showed that tamoxifen significantly increased sperm concentration in infertile men with oligozoospermia, but did not affect other semen values, such as volume, pH, motility, morphology, or viability, because of tamoxifen’s effectiveness on the seminiferous tubules during the early stages of spermatogenesis [38], [39].
Many randomised controlled trials in men with oligozoospermia or azoospermia evaluating the efficacy of tamoxifen (20 mg daily) or toremifene and raloxifene (60 mg daily) have reported improvements in semen parameters and pregnancy rates after 3 months of treatment [31], [40]. By contrast, some studies have shown improvements in the hormone profile with no effect on semen parameters or pregnancy rates [41].
Aromatase inhibitors
Aromatase inhibitors are group of drugs used in the treatment of male infertility that act through inhibiting aromatase P450 enzymes, thus normalising the testosterone-to-E2 ratio with subsequent stimulation of spermatogenesis [42]. They are classified chemically into steroidal and non-steroidal groups. Currently available aromatase inhibitors include: testolactone, anastrozole and letrozole [43].
Testolactone, as an aromatase inhibitor, has been shown to be effective in alleviating infertility as a result of hypogonadotrophic hypogonadism of obese men at a dose of 1 g daily for 6 weeks [44]. On the other hand, Raman and Schlegel [45] have conducted a study on a total of 140 subfertile men with abnormal testosterone-to-E2 ratios of <10:1 to evaluate the effect of administration of anastrozole at a dose of 100–200 mg daily on hormone profiles as well as semen parameters in non-obstructive azoospermic patients who presented with normal or decreased levels of testosterone and elevated levels of E2. Anastrozole treatment was found to be effective in normalising the testosterone-to-E2 ratio and total testosterone levels, thus improving semen parameters.
Additionally, letrozole has been suggested to normalise serum testosterone levels in severely obese men with hypogonadotrophic hypogonadism, with short-term letrozole treatment normalising serum testosterone levels in obese men [46], [47]. Variable dosing regimens have been tried ranging from 2.5 mg daily to 2.5 mg 3 times/week for 3–6 months [48]; however, the clinical significance of this intervention remains to be established in controlled, long-term studies [49].
Anastrozole and letrozole are the most widely used non-steroidal aromatase inhibitors in the clinical management of male infertility. They act by inhibiting aromatase enzyme thus limiting the conversion of testosterone to E2, thus increasing the production of gonadotrophins [43], [50].
Some men with severely defective sperm production having excessive aromatase activity, low serum testosterone, elevated E2 levels or disturbed testosterone-to-E2 ratio (i.e. <10) might benefit from aromatase inhibitors as a treatment of infertility. Anastrozole (1 mg once daily) and letrozole (2.5 mg once daily) are used for treatment of impaired spermatogenesis, although this represents an off-label use [51], [52]. They can increase endogenous testosterone production and serum testosterone levels. Treatment of infertile males with aromatase inhibitors has been associated with increased sperm production and return of sperm to the ejaculate in men with non-obstructive azoospermia [53].
Generally, no grave side-effects have been reported with the use of aromatase inhibitors in elevating serum testosterone levels. No significant changes in liver functions, serum haematocrit or LUTS; however, a slight insignificant increase in serum PSA level has been reported in one study amongst elderly men [54].
GnRH and gonadotrophin therapy
Gonadotrophin therapy is mainly indicated in management of infertile patients with low testosterone levels aiming at restoring normal spermatogenesis and improving fertility potential. In such cases, the stimulation of sperm production requires treatment with hCG alone or combined with recombinant FSH, urinary FSH, or human menopausal gonadotrophins (hMGs).
Therapy is usually initiated with hCG for 6–12 months to stimulate testosterone secretion before adding hMG or FSH. Occasionally, hCG alone may result in the spontaneous appearance of sperm in the ejaculate [55]. Pulsatile GnRH therapy is also used for hypothalamic disorders, but its advantage over gonadotrophins is debated. Some reports showed improved results with GnRH, whilst others showed no difference. The variation in response to hormonal treatment amongst patients with hypogonadotrophic hypogonadism may be related to residual function of the pituitary gland that varies individually. Those who do not respond to one regimen may benefit from another [56].
In most cases, prolonged continuous hormone replacement therapy is necessary to achieve satisfactory outcomes. The time to first appearance of sperm in the ejaculate varies considerably from a few months (3–6 months) up to >36 months [57]. Typically, many patients do not reach normal values. Despite low semen quality, pregnancy can still occur in a high proportion of cases [57]. On the other hand prolonged treatment, full spermatogenesis and pregnancy may not be achieved in all cases.
Gonadotrophin therapy usually starts with the administration of 1000–2500 IU of isolated hCG twice a week for 8–12 weeks. This initial phase is the induction phase, which is crucial for allowing testosterone levels to increase. In certain cases, hCG alone can induce spermatogenesis. In individuals who do not have sufficient endogenous FSH, treatment can be continued with the co-administration of 75–150 IU hMG three times/week for up to 18 months, as the presence of FSH is crucial for stimulating spermatogenesis. Recombinant FSH can be used in place of hMG, with patients receiving 150 IU three times/week for the same duration. This combined treatment provides considerable testicular growth in most patients, in addition to spermatogenesis in up to 90% of patients [58], [59].
The success rate and induction of spermatogenesis using the hCG in combination with hMG regimen as shown by positive sperm count vary between 40% and 75% [60]. However, the retrospective nature of many of the studies makes assessment of the rate of noncompliance and the true success rate rather difficult. Those patients discontinuing treatment at early stages are unlikely to be included in retrospective studies, which primarily look at long-term outcomes such as initiation of spermatogenesis and pregnancy [61].
Concomitant i.m. hCG and TRT
A retrospective study reviewed the records of 26 hypogonadal men treated with TRT and concomitant low dose hCG. TRT consisted of daily topical gel or weekly i.m. injection of hCG (500 IU) every other day. They concluded that low-dose hCG appears to maintain semen parameters in hypogonadal men on TRT. Concurrent TRT and hCG use may preserve fertility in hypogonadal males who desire fertility preservation whilst on TRT [6].
Coviello et al. [62] studied the level of intra-testicular testosterone in testicular biopsies from healthy men after the administration of hCG with different doses (125, 250, or 500 IU hCG every other day for 3 weeks) vs placebo in patients receiving 200 mg testosterone enanthate weekly, their results show that relatively low-dose hCG maintains intra-testicular testosterone within the normal range in healthy men with gonadotrophin suppression.
To date, there is insufficient information about the therapeutic and adverse effects of long-term hCG treatment. This type of treatment can therefore not be recommended for male hypogonadism, except in patients in whom fertility treatment is an issue [63].
Conclusion
Exogenous testosterone inhibits spermatogenesis. Hypogonadal men wanting to preserve their fertility and at the same time benefiting from TRT effects can be prescribed SERMs or testosterone plus low-dose hCG. Patients treated for infertility with hypogonadotrophic hypogonadism can be prescribed hCG alone at first followed by or in combination from the start with FSH preparations.
Conflict of interest
None.
Management
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Mulligan T., Frick M.F., Zuraw Q.C., Stemhagen A., McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–769. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corona G., Monami M., Rastrelli G., Aversa A., Tishova Y., Saad F. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8:272–283. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 3.Corona G., Rastrelli G., Monami M., Guay A., Buvat J., Sforza A. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 4.Zarrouf F.A., Artz S., Griffith J., Sirbu C., Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15:289–305. doi: 10.1097/01.pra.0000358315.88931.fc. [DOI] [PubMed] [Google Scholar]
- 5.Araujo A.B., Esche G.R., Kupelian V., O'Donnell A.B., Travison T.G., Williams R.E. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh T.C., Pastuszak A.W., Hwang K., Lipshultz L.I. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013;189:647–650. doi: 10.1016/j.juro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 7.de Souza G.L., Hallak J. Anabolic steroids and male infertility: a comprehensive review. BJU Int. 2011;108:1860–1865. doi: 10.1111/j.1464-410X.2011.10131.x. [DOI] [PubMed] [Google Scholar]
- 8.Buvat J., Maggi M., Guay A., Torres L.O. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J Sex Med. 2013;10:245–284. doi: 10.1111/j.1743-6109.2012.02783.x. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . Cambridge University Press; Cambridge: 2000. WHO Manual for the Standardised Investigation and Diagnosis of the Infertile Couple. [Google Scholar]
- 10.Tournaye H. Male factor infertility and ART. Asian J Androl. 2012;14:103–108. doi: 10.1038/aja.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggi R. Physiology of gonadotropin-releasing hormone (GnRH): beyond the control of reproductive functions. MOJ Anat Physiol. 2016;2:00063. [Google Scholar]
- 12.Guyton A.C. Textbook of medical physiology. 8th ed. WB Saunders Company; Philadelphia: 1991. Reproductive and hormonal functions of the male. [Google Scholar]
- 13.Copeland K.C., Chernausek S. Mini-puberty and growth. Pediatrics. 2016;138:e20161301. doi: 10.1542/peds.2016-1301. [DOI] [PubMed] [Google Scholar]
- 14.Jarow J.P. Endocrine causes of male infertility. Urol Clin North Am. 2003;30:83–90. doi: 10.1016/s0094-0143(02)00117-9. [DOI] [PubMed] [Google Scholar]
- 15.Fraietta R., Zylberstejn D.S., Esteves S.C. Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo) 2013;68(Suppl. 1):81–88. doi: 10.6061/clinics/2013(Sup01)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halder A., Jain M., Kumar P. Primary testicular failure: an overview. J Clin Diagn Res. 2015;3:e105. [Google Scholar]
- 17.McCabe M.J., Bancalari R.E., Dattani M.T. Diagnosis and evaluation of hypogonadism. Pediatr Endocrinol Rev. 2014;11(Suppl. 2):214–229. [PubMed] [Google Scholar]
- 18.Bhasin S., Cunningham G.R., Hayes F.J., Matsumoto A.M., Snyder P.J., Swerdloff R.S. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 19.Buvat J., Maggi M., Gooren L., Guay A.T., Kaufman J., Morgentaler A. Endocrine aspects of male sexual dysfunctions. J Sex Med. 2010;7:1627–1656. doi: 10.1111/j.1743-6109.2010.01780.x. [DOI] [PubMed] [Google Scholar]
- 20.Vanbillemont G., Bogaert V., De Bacquer D., Lapauw B., Goemaere S., Toye K. Polymorphisms of the SHBG gene contribute to the interindividual variation of sex steroid hormone blood levels in young, middle-aged and elderly men. Clin Endocrinol (Oxf) 2009;70:303–310. doi: 10.1111/j.1365-2265.2008.03365.x. [DOI] [PubMed] [Google Scholar]
- 21.Fanelli F., Gambineri A., Mezzullo M., Vicennati V., Pelusi C., Pasquali R. Revisiting hyper- and hypo-androgenism by tandem mass spectrometry. Rev Endocr Metab Disord. 2013;14:185–205. doi: 10.1007/s11154-013-9243-y. [DOI] [PubMed] [Google Scholar]
- 22.Dean J.D., McMahon C.G., Guay A.T., Morgentaler A., Althof S.E., Becher E.F. The International Society for Sexual Medicine’s process of care for the assessment and management of testosterone deficiency in adult men. J Sex Med. 2015;12:1660–1686. doi: 10.1111/jsm.12952. [DOI] [PubMed] [Google Scholar]
- 23.Katz D.J., Nabulsi O., Tal R., Mulhall J.P. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int. 2012;110:573–578. doi: 10.1111/j.1464-410X.2011.10702.x. [DOI] [PubMed] [Google Scholar]
- 24.Raivio T., Falardeau J., Dwyer A., Quinton R., Hayes F.J., Hughes V.A. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357:863–873. doi: 10.1056/NEJMoa066494. [DOI] [PubMed] [Google Scholar]
- 25.Liu P.Y., Baker H.W., Jayadev V., Zacharin M., Conway A.J., Handelsman D.J. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009;94:801–808. doi: 10.1210/jc.2008-1648. [DOI] [PubMed] [Google Scholar]
- 26.Damber J.E., Abramsson L., Duchek M. Tamoxifen treatment of idiopathic oligozoospermia: effect on hCG-induced testicular steroidogenesis and semen variables. Scand J Urol Nephrol. 1989;23:241–246. doi: 10.3109/00365598909180331. [DOI] [PubMed] [Google Scholar]
- 27.Chehab M., Madala A., Trussell J.C. On-label and off-label drugs used in the treatment of male infertility. Fertil Steril. 2015;103:595–604. doi: 10.1016/j.fertnstert.2014.12.122. [DOI] [PubMed] [Google Scholar]
- 28.Ghanem H., Shaeer O., El-Segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertil Steril. 2010;93:2232–2235. doi: 10.1016/j.fertnstert.2009.01.117. [DOI] [PubMed] [Google Scholar]
- 29.Chua M.E., Escusa K.G., Luna S., Tapia L.C., Dofitas B., Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology. 2013;1:749–757. doi: 10.1111/j.2047-2927.2013.00107.x. [DOI] [PubMed] [Google Scholar]
- 30.Roth L.W., Ryan A.R., Meacham R.B. Clomiphene citrate in the management of male infertility. Semin Reprod Med. 2013;31:245–250. doi: 10.1055/s-0033-1345271. [DOI] [PubMed] [Google Scholar]
- 31.Moein M.R., Tabibnejad N., Ghasemzadeh J. Beneficial effect of tamoxifen on sperm recovery in infertile men with nonobstructive azoospermia. Andrologia. 2012;44(Suppl. 1):194–198. doi: 10.1111/j.1439-0272.2011.01163.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaminetsky J., Werner M., Fontenot G., Wiehle R.D. Oral enclomiphene citrate stimulates the endogenous production of testosterone and sperm counts in men with low testosterone: comparison with testosterone gel. J Sex Med. 2013;10:1628–1635. doi: 10.1111/jsm.12116. [DOI] [PubMed] [Google Scholar]
- 33.Niederberger C. Re: Oral enclomiphene citrate raises testosterone and preserves sperm counts in obese hypogonadal men, unlike topical testosterone: restoration instead of replacement. J Urol. 2017;197:233. doi: 10.1016/j.juro.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Wiehle R.D., Fontenot G.K., Wike J., Hsu K., Nydell J., Lipshultz L. Enclomiphene citrate stimulates testosterone production while preventing oligospermia: a randomized phase II clinical trial comparing topical testosterone. Fertil Steril. 2014;102:720–727. doi: 10.1016/j.fertnstert.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Patel D.P., Brant W.O., Myers J.B., Presson A.P., Johnstone E.B., Dorais J.A. The safety and efficacy of clomiphene citrate in hypoandrogenic and subfertile men. Int J Impot Res. 2015;27:221–224. doi: 10.1038/ijir.2015.21. [DOI] [PubMed] [Google Scholar]
- 36.Buvat J., Ardaens K., Lemaire A., Gauthier A., Gasnault J.P., Buvat-Herbaut M. Increased sperm count in 25 cases of idiopathic normogonadotropic oligospermia following treatment with tamoxifen. Fertil Steril. 1983;39:700–703. doi: 10.1016/s0015-0282(16)47069-x. [DOI] [PubMed] [Google Scholar]
- 37.Kadioglu T.C., Köksal I.T., Tunç M., Nane I., Tellaloglu S. Treatment of idiopathic and postvaricocelectomy oligozoospermia with oral tamoxifen citrate. BJU Int. 1999;83:646–648. doi: 10.1046/j.1464-410x.1999.00976.x. [DOI] [PubMed] [Google Scholar]
- 38.Vermeulen A., Comhaire F. Hormonal effects of an antiestrogen, tamoxifen, in normal and oligospermic men. Fertil Steril. 1978;29:320–327. doi: 10.1016/s0015-0282(16)43160-2. [DOI] [PubMed] [Google Scholar]
- 39.Kotoulas I.G., Cardamakis E., Michopoulos J., Mitropoulos D., Dounis A. Tamoxifen treatment in male infertility. I. Effect on spermatozoa. Fertil Steril. 1994;61:911–914. doi: 10.1016/s0015-0282(16)56705-3. [DOI] [PubMed] [Google Scholar]
- 40.AinMelk Y., Belisle S., Carmel M., Jean-Pierre T. Tamoxifen citrate therapy in male infertility. Fertil Steril. 1987;48:113–117. doi: 10.1016/s0015-0282(16)59299-1. [DOI] [PubMed] [Google Scholar]
- 41.Tsourdi E., Kourtis A., Farmakiotis D., Katsikis I., Salmas M., Panidis D. The effect of selective estrogen receptor modulator administration on the hypothalamic-pituitary-testicular axis in men with idiopathic oligozoospermia. Fertil Steril. 2009;91(Suppl.):1427–1430. doi: 10.1016/j.fertnstert.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro M.A., Gameiro L.F., Scarano W.R., Briton-Jones C., Kapoor A., Rosa M.B. Aromatase inhibitors in the treatment of oligozoospermic or azoospermic men: a systematic review of randomized controlled trials. JBRA Assist Reprod. 2016;20:82–88. doi: 10.5935/1518-0557.20160019. [DOI] [PubMed] [Google Scholar]
- 43.Schlegel P.N. Aromatase inhibitors for male infertility. Fertil Steril. 2012;98:1359–1362. doi: 10.1016/j.fertnstert.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Zumoff B., Miller L.K., Strain G.W. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism. 2003;52:1126–1128. doi: 10.1016/s0026-0495(03)00186-0. [DOI] [PubMed] [Google Scholar]
- 45.Raman J.D., Schlegel P.N. Aromatase inhibitors for male infertility. J Urol. 2002;167:624–629. doi: 10.1016/S0022-5347(01)69099-2. [DOI] [PubMed] [Google Scholar]
- 46.Loves S., Ruinemans-Koerts J., de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol. 2008;158:741–747. doi: 10.1530/EJE-07-0663. [DOI] [PubMed] [Google Scholar]
- 47.Saylam B., Efesoy O., Cayan S. The effect of aromatase inhibitor letrozole on body mass index, serum hormones, and sperm parameters in infertile men. Fertil Steril. 2011;95:809–811. doi: 10.1016/j.fertnstert.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 48.Stephens S.M., Polotsky A.J. Big enough for an aromatase inhibitor? How adiposity affects male fertility. Semin Reprod Med. 2013;31:251–257. doi: 10.1055/s-0033-1345272. [DOI] [PubMed] [Google Scholar]
- 49.Du Plessis S.S., Cabler S., McAlister D.A., Sabanegh E., Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol. 2010;7:153–161. doi: 10.1038/nrurol.2010.6. [DOI] [PubMed] [Google Scholar]
- 50.Elkhiat Y., Fahmy I. Aromatase inhibitors in the treatment of male infertility. Hum Androl. 2011;1:35–38. [Google Scholar]
- 51.Patry G., Jarvi K., Grober E.D., Lo K.C. Use of the aromatase inhibitor letrozole to treat male infertility. Fertil Steril. 2009;92(829):e821–822. doi: 10.1016/j.fertnstert.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Shoshany O., Abhyankar N., Mufarreh N., Daniel G., Niederberger C. Outcomes of anastrozole in oligozoospermic hypoandrogenic subfertile men. Fertil Steril. 2017;107:589–594. doi: 10.1016/j.fertnstert.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Dabaja A.A., Schlegel P.N. Medical treatment of male infertility. Transl Androl Urol. 2014;3:9–16. doi: 10.3978/j.issn.2223-4683.2014.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leder B.Z., Rohrer J.L., Rubin S.D., Gallo J., Longcope C. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. J Clin Endocrinol Metab. 2004;89:1174–1180. doi: 10.1210/jc.2003-031467. [DOI] [PubMed] [Google Scholar]
- 55.Chudnovsky A., Niederberger C.S. Gonadotropin therapy for infertile men with hypogonadotropic hypogonadism. J Androl. 2007;28:644–646. doi: 10.2164/jandrol.107.003400. [DOI] [PubMed] [Google Scholar]
- 56.Bakircioglu M.E., Erden H.F., Ciray H.N., Bayazit N., Bahceci M. Gonadotrophin therapy in combination with ICSI in men with hypogonadotrophic hypogonadism. Reprod Biomed Online. 2007;15:156–160. doi: 10.1016/s1472-6483(10)60703-1. [DOI] [PubMed] [Google Scholar]
- 57.Buchter D., Behre H.M., Kliesch S., Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998;139:298–303. doi: 10.1530/eje.0.1390298. [DOI] [PubMed] [Google Scholar]
- 58.Zitzmann M., Nieschlag E. Hormone substitution in male hypogonadism. Mol Cell Endocrinol. 2000;161:73–88. doi: 10.1016/s0303-7207(99)00227-0. [DOI] [PubMed] [Google Scholar]
- 59.Han T.S., Bouloux P.M. What is the optimal therapy for young males with hypogonadotropic hypogonadism? Clin Endocrinol (Oxf) 2010;72:731–737. doi: 10.1111/j.1365-2265.2009.03746.x. [DOI] [PubMed] [Google Scholar]
- 60.Liu L., Banks S.M., Barnes K.M., Sherins R.J. Two-year comparison of testicular responses to pulsatile gonadotropin-releasing hormone and exogenous gonadotropins from the inception of therapy in men with isolated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1988;67:1140–1145. doi: 10.1210/jcem-67-6-1140. [DOI] [PubMed] [Google Scholar]
- 61.Haidl G. Management strategies for male factor infertility. Drugs. 2002;62:1741–1753. doi: 10.2165/00003495-200262120-00004. [DOI] [PubMed] [Google Scholar]
- 62.Coviello A.D., Matsumoto A.M., Bremner W.J., Herbst K.L., Amory J.K., Anawalt B.D. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005;90:2595–2602. doi: 10.1210/jc.2004-0802. [DOI] [PubMed] [Google Scholar]
- 63.Dohle GR, Arver S, Bettocchi C, Jones TH, Kliesch S. EAU Guidelines on Male Hypogonadism. Available at: http://uroweb.org/guideline/male-hypogonadism/. Accessed November 2017.