Abstract
Objective
To assess seminal oxidation–reduction potential (ORP) and sperm DNA fragmentation (SDF) in male infertility and their relationships with sperm morphology in fertile and infertile men.
Patients and methods
Prospective case-control study comparing the findings of infertile men (n = 1168) to those of men with confirmed fertility (n = 100) regarding demographics and semen characteristics (conventional and advanced semen tests). Spearman rank correlation assessed the correlation between ORP, SDF, and different morphological indices. Means of ORP and SDF were assessed in variable levels of normal sperm morphology amongst all participants.
Results
Infertile patients had a significantly lower mean sperm count (32.7 vs 58.7 × 106 sperm/mL), total motility (50.1% vs 60.4%), and normal morphology (5.7% vs 9.9%). Conversely, infertile patients had significantly higher mean head defects (54% vs 48%), and higher ORP and SDF values than fertile controls. ORP and SDF showed significant positive correlations and significant negative correlations with sperm head defects and normal morphology in infertile patients, respectively. ORP and SDF were significantly inversely associated with the level of normal sperm morphology. Using receiver operating characteristic curve analysis, ORP and SDF threshold values of 1.73 mV/106 sperm/mL and 25.5%, respectively, were associated with 76% and 56% sensitivity and 72% and 72.2% specificity, respectively, in differentiating <4% from ≥4% normal morphology.
Conclusion
A direct inverse relationship exists between seminal ORP and SDF with various levels of normal sperm morphology. Using ORP and SDF measures in conjunction with standard semen morphology analysis could validate the result of the fertility status of patients.
Abbreviations: ART, assisted reproductive techniques; AUC, area under the curve; ICSI, intracytoplasmic sperm injection; IUI, intrauterine insemination; IVF, in vitro fertilisation; NPV, negative predictive value; ORP, oxidation–reduction potential; OS, oxidative stress; PPV, positive predictive value; ROC, receiver operating characteristic; ROS, reactive oxygen species; SCD, sperm chromatin dispersion
Keywords: Male infertility, Oxidation-reduction potential, Sperm, Sperm DNA fragmentation, Sperm morphology
Introduction
Infertility is the inability to conceive after at least 1 year of regular unprotected intercourse. About 15% of couples report this medical problem [1]. Globally, 30–50% of infertility cases are due to a male factor, reaching up to 60% where men are directly or partially responsible for the infertility [2]. Clinicians rely on conventional semen parameters for the initial evaluation of male fertility potential, and sperm morphology assessment is one of its cornerstones. Several approaches and classifications for sperm morphology assessment have been used including the strict criteria of the WHO classification, and the modified David classification [3], [4], [5]. The most recent fifth edition of the WHO manual recommends using strict criteria for examining sperm morphology, and provides a precise definition of a normal spermatozoon [3]. Multiple abnormal sperm morphology indices have been described in literature to ease interpretation of this semen parameter, including sperm deformity index (SDI), teratozoospermia index (TZI), and multiple abnormalities index (MAI). However, data on their clinical relevance remain scarce [6], [7].
Sperm morphology has been subject to considerable debate about its ability to accurately predict in vivo and in vitro conception. With the exception of some specific sperm morphology defects, which are often linked to genetic disorders (e.g. globozoospermia, macrocephaly, decapitated sperm syndrome, and fibrous sheath dysplasia), sperm morphology assessment has been suggested to have very poor sensitivity and specificity in the diagnosis of infertility [7]. Earlier studies have shown sperm morphology to be one of the most important semen parameters capable of predicting natural conception, intrauterine insemination (IUI), in vitro fertilisation (IVF), and intracytoplasmic sperm injection (ICSI) outcomes [8], [9]. Nonetheless, more recent studies failed to confirm such propositions [10], [11], where for instance, men with <1% normal forms were able to conceive without IVF, and even men with 0% normal forms had similar successful pregnancy outcome [12]. Furthermore, Hotaling et al. [11] investigated the impact of teratozoospermia (<5% normal forms) on assisted reproductive outcomes to report that isolated teratozoospermia was not associated with decreased clinical pregnancy rates in cases of IVF with or without ICSI. Hence, alternative methods such as natural conception or even IUI seem possible prior to seeking immediate IVF treatment in men with severe teratozoospermia. Such controversy highlights the uncertainty of conventional semen parameters in predicting true male fertility potential, and triggers the search for advanced tests of sperm function that could help improve diagnostic accuracy.
Seminal oxidative stress (OS) and sperm DNA fragmentation (SDF) are two advanced sperm function tests that are increasingly used in the evaluation of infertile men. OS has recently been identified as a major mediator in the various causes of male infertility [13]. High levels of reactive oxygen species (ROS) are found in the semen samples of 25–40% of infertile men [14]. Although small physiological levels of ROS are essential for normal sperm functions such as sperm capacitation, acrosome reaction, and fertilisation [15], elevated seminal ROS induce a state of OS that can cause sperm dysfunction through aggravating membrane lipid peroxidation, sperm DNA damage, and abortive apoptosis [16]. These consequences can affect sperm structural and functional integrity thereby altering motility, morphology, count, and viability [16]. Most importantly is the OS effect on the integrity of sperm DNA, where OS provokes nucleotide modifications, DNA strand breaks and chromatin cross-linking that result in SDF [17].
An accurate measure of OS is the oxidation–reduction potential (ORP), which provides an overview of the redox system through assessment of the net balance between oxidants and reductants in any given medium. Recently, ORP in the semen has been easily and comprehensively measured using the Male Infertility Oxidative System (MiOXSYS®; Aytu BioScience Inc., Englewood, CO USA) that enables wider application of OS analysis in clinical and research settings [20]. ORP results provided by the MiOXSYS are standardised, reliable, and reproducible compared to previously used ROS assays [20], [21].
Higher levels of ORP and SDF are associated with worse sperm quality and provide reliable information synergising the predictive value of semen analysis during male fertility evaluation [19], [21]. Whilst ORP and SDF have been previously correlated with different semen parameters, their relationship with various levels and types of sperm morphological anomalies has never been investigated. The present study assessed whether there is a significant correlation between sperm morphology on the one hand, and measures of OS and SDF in seminal plasma on the other. Given the aforementioned controversy between sperm morphology and fertility potential, studying the relationship between sperm morphology and these two advanced sperm function tests is of major importance, as this would help in better understanding the true clinical implication of sperm morphology on fecundity. Therefore, the specific objectives of the present study were to: (i) compare conventional semen parameters (semen volume, sperm concentration, motility, and normal morphology) and advanced sperm function tests (ORP and SDF) between infertile patients and fertile controls; (ii) explore the correlation between ORP, SDF, and different morphological abnormalities; (iii) examine the ORP and SDF levels associated with various degrees of normal sperm morphology; and (iv) assess the ORP and SDF threshold values differentiating ‘normal’ from ‘abnormal’ sperm morphology.
Patients and methods
Study design, sample and procedures
This prospective study was conducted at the specialised Male Fertility Unit of Hamad Medical Corporation in Qatar, an academic tertiary medical centre. Over a period of 12 months (June 2016–June 2017), 3142 infertile patients were evaluated. The study was approved by the Medical Research Centre (Institutional Review Board) at Hamad Medical Corporation in Qatar, and all participants provided signed informed consents before being enrolled.
Cases comprised all men with primary or secondary infertility attending the male infertility clinic. The exclusion criteria were as follows: azoospermia, use of antioxidant therapy (may bias the results of the study, particularly the ORP result), present/past history of pyospermia (leucocytospermia), presence of clinically palpable varicocele, history of chemical/radiation exposure based on patients’ occupation, and presence of a female factor of infertility. All female partners were evaluated by a fertility specialist whose assessment aided in selection of cases. Moreover, patients receiving medications such as ketotifen or NSAIDs or who had history of chronic disease (chronic lung diseases, chronic renal failure or liver failure) were also excluded. Of the initial 3142 patients, 1168 patients met the inclusion criteria and constituted the infertile patient group (cases).
The control group consisted of 100 men with confirmed fertility (established a pregnancy in the previous 24 months) and was recruited through an advertisement placed at the same tertiary medical centre. Controls were selected only after we received a proof of pregnancy in the previous 24 months (child’s birth certificate and medical report from the spouse’s gynaecologist stating that pregnancy was spontaneous). The same exclusion criteria previously mentioned were applied to the controls.
Data collection
Infertile patients and fertile controls were evaluated. Information on participants’ demographics, history of present illness, relevant past medical, surgical and family histories, results of the general and local genital clinical examinations, and the laboratory findings of conventional semen analysis, ORP and SDF were collected.
Semen analysis parameters
Each participant provided a semen sample after 2–7 days of sexual abstinence. Standard semen analysis was performed consistent with the fifth edition of the WHO manual [3], manually using a haemocytometer. Sperm motility was assessed and categorised as progressive or non-progressive. For sperm morphology assessment, air-dried semen smears were fixed and stained using the Diff-Quik kit (Baxter Healthcare Corporation, Englewood, Chicago, IL, USA) by a single experienced technician. The morphological abnormalities were examined according to strict criteria, with the very low normal sperm morphology threshold value of ≥4% [3]. A total of 5000 sperm were scored, and results were expressed as percentage normal morphology. The percentage of abnormality in the sperm head, neck or tail was recorded in each semen sample.
ORP
Consistent with others, ORP was measured in unprocessed post-liquefied semen to assess test reproducibility using the MiOXSYS [20]. The MiOXSYS comprises an electrochemical analyser (Aytu BioScience, cat. #100,229) and single-use disposable semen sensors (Aytu BioScience; cat. # 100,283). As the analyser applies a low-voltage steady current measured in millivolts (mV), OS reflects the relationship between sperm (producers of free radicals) and seminal plasma (an antioxidant reservoir), thus raw ORP values (mV) were normalised to sperm concentration, a value that reflects both semen volume and sperm number. Data for ORP are presented as mV/106 sperm/mL throughout [20].
SDF
SDF was evaluated in a subset of the study participants (n = 365) using the sperm chromatin dispersion (SCD) method or ‘Halosperm® kit’ (Halotech DNA, SL, Madrid, Spain) as per manufacturer’s instructions. The method is based on the SCD assay test [21]. Unfixed spermatozoa were immersed in an inert agarose microgel on a pretreated slide. An initial acid treatment denatures DNA in the spermatozoa with fragmented DNA. The lysing solution removes most of the nuclear proteins, and in the absence of large DNA breaks produces nucleoids with large halos of spreading DNA loops, emerging from a central core. Spermatozoa with fragmented DNA do not show dispersion halos or exhibit very small halos. Both, positive and negative controls were included. For positive control (sperm with halo), the acid denaturation (0.08 M HCl) step was omitted. For negative control (sperm without halo), after removing the coverslip, 10 µL undiluted denaturation solution was applied and a cover slip was gently placed without pressure and left for 5 min. A minimum of 500 spermatozoa were scored and reported as percentage of sperm with spermatozoa with fragmented DNA.
Statistical analyses
Analyses were performed using Statistical Package for the Social Sciences software (SPSS® version 24; SPSS Inc., IBM Corp., Armonk, NY, USA), with significance set at P < 0.05. Summaries of quantitative variables are reported as mean ± standard error (SE) for the infertile patients and fertile controls. Descriptive analysis showed that the data were not normally distributed; therefore the Mann–Whitney test was used to compare means of age, count, motility, normal morphology, abnormal morphological indices, SDF, and ORP between infertile patients and fertile controls. Spearman rank correlation examined the correlation (rs) between ORP, SDF, sperm morphology, and the different morphological indices for the fertile controls and the infertile patients. Using the whole sample, we assessed the mean ORP and SDF values associated with five increasing levels of normal sperm morphology 0, 1, 2, 3 and ≥4%. A receiver operating characteristic (ROC) curve was used to establish the threshold, sensitivity, specificity, area under the curve (AUC), accuracy, and the ability for ORP and SDF to differentiate men with normal sperm morphology (≥4%) and abnormal sperm morphology (<4%) [3].
Results
Characteristics of the sample
Table 1 describes the demographic, conventional semen analysis, and advanced sperm function characteristics of the infertile patients (n = 1168) and fertile controls (n = 100). Infertile patients were significantly, ∼3 years, older than fertile controls (P < 0.001). They had significantly lower mean conventional semen parameter values (lower sperm count, lower total motility, and lower normal morphology). However, the mean measures of head defects, ORP and SDF values were significantly higher amongst the infertile patients than the fertile controls (P < 0.001 for all; Table 1). The mean ORP (mV/106 sperm/mL) in the semen of the infertile patients was five-times higher than the fertile controls (P < 0.001), and head defects were significantly higher amongst infertile patients (54%) than fertile controls (48%) (P < 0.001).
Table 1.
Demographic, conventional semen analysis, and advanced sperm function characteristics of the sample.
| Variable, mean (SE) | Infertile patients (n = 1169) | Fertile controls (n = 100) | P |
|---|---|---|---|
| Demography | |||
| Age, years | 35.92 (0.22) | 32.32 (0.72) | <0.001 |
| Abstinence, days | 3.83 (0.05) | 3.53 (0.13) | 0.087 |
| Conventional semen analysis | |||
| Volume, mL | 3.17 (0.06) | 2.73 (0.12) | 0.067 |
| Count, ×106/mL | 32.65 (0.78) | 58.72 (2.5) | <0.001 |
| Total motility,% | 50.07 (0.54) | 60.41 (1.21) | <0.001 |
| Total morphology,% | 5.72 (0.22) | 9.86 (0.51) | <0.001 |
| Head defect,% | 53.76 (0.43) | 47.62 (0.67) | <0.001 |
| Neck defect,% | 23.79 (0.25) | 23.88 (0.59) | 0.916 |
| Tail defect,% | 17.10 (0.24) | 18.94 (0.71) | 0.328 |
| Advanced sperm function tests | |||
| SDF, % | 27.60 (1.02) | 15.68 (0.92) | <0.001 |
| ORP, mV/106 sperm/mL | 5.44 (0.34) | 1.18 (0.94) | <0.001 |
Mann–Whitney test; bolded cells indicate statistical significance.
Correlation between ORP, SDF and sperm morphology parameters
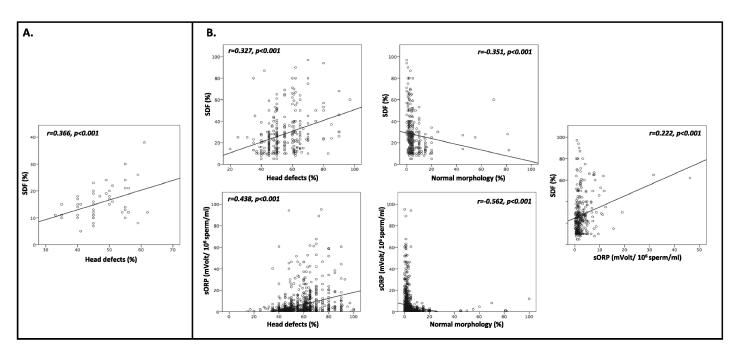
Table 2 shows that amongst the fertile controls, there was no correlation between ORP and SDF. The only significant correlation was between SDF and sperm head defects (rs = 0.36; P = 0.011; Fig. 1B). Both the correlations between normal morphology and ORP and SDF did not reach statistical significance (P = 0.052 and 0.081, respectively).
Table 2.
Correlations between ORP, SDF and sperm morphology amongst fertile controls and infertile patients.
| ORP | SDF, % | Morphology, % |
||||
|---|---|---|---|---|---|---|
| Abnormal |
Normal | |||||
| Neck defect | Head defect | Tail defect | ||||
| Infertile patients (n = 1169) | ||||||
| ORP | 1 | 0.222** | −0.051 | 0.438** | −0.228 | −0.562** |
| SD, % | 0.222** | 1 | −0.092 | 0.327** | −0.117 | −0.351** |
| Fertile controls (n = 100) | ||||||
| ORP | 1 | 0.004 | 0.121 | 0.127 | −0.033 | −0.195 |
| SDF, % | 0.004 | 1 | 0.092 | 0.366* | −0.190 | −0.257 |
ORP measured in mV/106 sperm/mL; *significant at 0.01 level; **significant at 0.001 level.
Fig. 1.
Significant correlations between ORP, SDF, and sperm morphology: (A) amongst infertile patients; (B) amongst fertile controls.
Table 2 and Fig. 1A also show a significant correlation between ORP and each of sperm head defects (0.438) and normal morphology (rs = −0.56) amongst the infertile patients. There were also significant correlations between SDF and ORP (rs = 0.22; P < 0.001), head defects (rs = 0.33; P < 0.001), and normal morphology (rs = −0.35; P < 0.001). The correlation was negative but not statistically significant for both ORP and SDF with sperm neck (rs = −0.05, P = 0.06 and rs = −0.23, P = 0.21) and sperm tail (rs = −0.09; P = 0.11 and rs = −0.12; P = 0.39) defects, respectively.
Assessment of ORP and SDF in different levels of normal sperm morphology
Table 3 depicts the mean ORP and SDF associated with five increasing levels of normal morphology across the whole sample. ORP was significantly inversely associated with the level of normal sperm morphology. Likewise, SDF was significantly inversely associated with the level of normal sperm morphology. The ORP was highest at 13.1 mV/106 sperm/mL in the 0% normal sperm morphology group; and lowest for men with ≥4% normal sperm morphology at 1.99 mV/106 sperm/mL. Similarly, the mean SDF rate was highest at 56% in the 0% normal sperm morphology group.
Table 3.
ORP and SDF associated with five increasing levels of normal morphology across the whole sample (N = 1269).
| Normal sperm forms morphology, mean (SE) |
P | |||||
|---|---|---|---|---|---|---|
| 0% | 1% | 2% | 3% | ≥4% | ||
| ORP | 13.12 (1.84) | 12.04 (1.27) | 7.30 (0.73) | 4.92 (0.55) | 1.99 (0.26) | <0.001 |
| SDF,% | 56.00 (8.18) | 38.55 (3.75) | 31.58 (2.71) | 26.53 (2.16) | 22.66 (1.04) | <0.001 |
Mann–Whitney test; ORP measured in mV/106 sperm/mL.
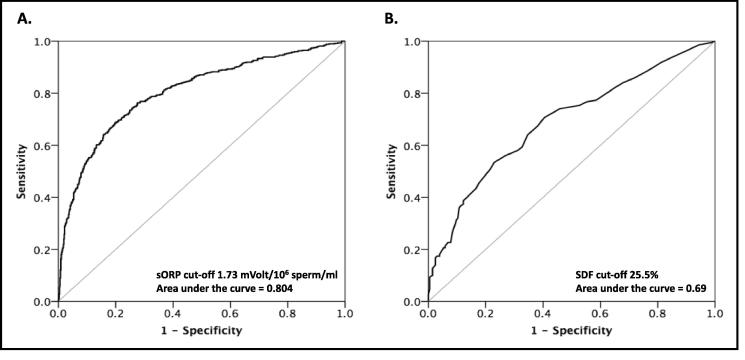
ROC curve analysis
ROC curves were generated both for ORP and SDF for normal (≥4%) and abnormal (<4%) sperm morphology (Fig. 2 A and B). ROC analysis suggested an ORP threshold of 1.73 mV/106 sperm/mL in semen. At this threshold, sensitivity was 76%, specificity 72%, positive predictive value (PPV) 69.2%, negative predictive value (NPV) 78.6%, and accuracy 73.9% (AUC of 0.8; P < 0.001; Fig. 2A). Likewise, a SDF threshold of 25.5% had 56% sensitivity, 72.2% specificity, 60.4% PPV, 69.4% NPV, and 65.9% accuracy (AUC of 0.69; P < 0.001; Fig. 2B).
Fig. 2.
ROC curves for: (A) ORP threshold; (B) SDF threshold against <4% or ≥4% normal sperm morphology.
Discussion
The present study is the very first in the literature to examine ORP and SDF levels at various intensities and types of sperm morphological abnormalities. Both ORP and SDF showed a significant inverse relationship with levels of normal sperm morphology. Furthermore, ORP and SDF had significant positive correlations with sperm head defects, but not with neck or tail defects.
Our present infertile patients had significantly lower conventional semen analysis parameters (sperm count, total motility, and normal morphology) than fertile controls, hence further validating the utility of semen analysis as a cornerstone for male fertility evaluation. However, despite the ability of conventional semen analysis to assess sperm quality, it does not precisely and accurately predict a man’s fertility [23] due to the many factors, in addition to sperm and semen quality that contribute to the ability of spermatozoa to fertilise an oocyte. Of the different semen analysis parameters, sperm morphology has been the most commonly investigated predictor of fertility, with remarkably contradictory results, probably due to the subjective nature of conventional sperm morphology testing, where many issues arise concerning how sperm morphology should be assessed, the test’s clinical relevance, and how to interpret the thresholds of normal forms [23].
In male infertility, the choice between various assisted reproductive techniques (ART), e.g. IUI, IVF or ICSI, seems not very dependent on sperm morphology, as there are no clear-cut recommendations, probably due to the lack of rigorous randomised controlled trials, the numerous biases inherent in most retrospective studies, and the wide variations of thresholds and variety of classifications used by different researchers. For instance, Van Waart et al. [8] compared IUI pregnancy rates of men with ≤4 vs >4% normal sperm morphology, where the meta-analysis revealed a risk difference in pregnancy success between these two levels of normal sperm morphology levels, and a significant improvement in pregnancy rate for the >4% threshold using the strict criteria. Others showed that the percentage of normal sperm morphology was positively associated with successful IVF (fertilisation rate and clinical pregnancy rate) when the 5% (strict criteria) or the 14% (WHO 1999) thresholds were used [9]. However, these studies did not examine isolated teratozoospermia, and important male and female characteristics were also lacking and hence not accounted for in the analyses. In contrast, more recent studies failed to find a statistically significant influence on ART outcomes between groups with and without isolated teratozoospermia [5]. For example, Hotaling et al. [11] examined, through four retrospective studies, the relationship between severely isolated teratozoospermia (<5% using the strict criteria) and clinical pregnancy rate after conventional IVF or ICSI, to report that teratozoospermia was not associated with lower clinical pregnancy rates in IVF or ICSI. Therefore, the predictive power of sperm morphology for pregnancy outcomes in patients undergoing ART is questioned [10], [11].
For different sperm morphological anomalies, assessment of the percentage of thin or amorphous head, or bent or asymmetrical neck may be of limited clinical significance, as their pathophysiologies remain not well explained, as they are mostly physiological traits. However, some sperm morphology defects may be associated with functional abnormalities e.g. chromatin condensation changes, acrosome reaction defects, tail motility problems, or even increased apoptosis or necrosis [26], [27]. Our present results showed that sperm head defects were significantly higher amongst infertile patients compared with fertile controls, highlighting the significance of the sperm head at the molecular and genetic level. The sperm head encompasses nuclear DNA, which is an integral element responsible for packaging all paternal genetic information needed for the fertilised egg’s early embryo development [28].
Such controversies in the predictive power of sperm morphology exemplify some of the shortcomings that still remain with conventional semen analysis testing, paving the way for investigating the value of advanced sperm function tests (ORP and SDF) for the evaluation of infertile men. We demonstrated that ORP and SDF were significantly higher amongst infertile patients compared with fertile controls, and in order to assess the association between sperm morphology and reproductive outcomes, we investigated, in-depth, the relationship between ORP and SDF and this semen parameter. OS is fundamental to male infertility pathophysiology, as spermatozoa produce small amounts of ROS during mitochondrial energy production, which are generally counterbalanced by antioxidants in the mitochondria and in seminal fluid [19]. The ROS antioxidants imbalance triggers OS, which may damage sperm DNA and impair fertility [19].
ORP showed significant negative correlations with normal sperm morphology and significant positive correlations with sperm head defects amongst our present infertile patients, where ORP significantly increased as the normal sperm morphology levels decreased, reaching 13.12 mV/106 sperm/mL at 0% normal sperm morphology. Our present ROC curve analysis for ORP threshold value that best differentiates <4% from ≥4% normal morphology depicted that an ORP threshold of 1.73 mV/106 sperm/mL was associated with 76% sensitivity, 72% specificity, 69.2% PPV, 78.6% NPV and 73.9% accuracy. We used the MiOXSYS for ORP measurement, which has been recently used for the evaluation of male infertility and its validity confirmed as an accurate measure of OS in semen [29]. We are in agreement with others, in that ORP was significantly higher amongst infertile men compared with fertile controls [29]. Whilst we found no published ROC curve analyses performed for ORP against sperm morphology in order to compare our present ROC findings with, nevertheless, ORP threshold values between 1.36 and 2.59 mV/106 sperm/mL have been reported to differentiate fertile from infertile men, or normal from abnormal semen [20], [22]. Our present sensitivity and specificity levels are promising and suggest that ORP can be used as a valuable tool in estimating sperm morphology.
Using the SCD method, we found that SDF also showed significant negative correlation with normal sperm morphology, significant positive correlation with sperm head defects, and SDF was significantly inversely associated with normal sperm morphology levels, reaching its highest level (56%) with the very low level (0%) of normal sperm morphology. ROC curve analysis examining the SDF threshold value that best differentiates <4% from ≥4% normal morphology, depicted a 25.5% SDF threshold level to be associated with 56% sensitivity, 72.2% specificity, 60.4% PPV, 69.4% NPV, and 65.9% accuracy. There is increasing evidence on the role of sperm DNA damage in infertility, and the possible consequences of damaged sperm chromatin to the offspring’s health [30]. Studies investigating SDF thresholds using the SCD method have reported almost identical values. For instance, recent research reported 80.8% sensitivity and 86.1% specificity when the 26.1% SDF threshold was used, with a 2.84 male infertility prevalence ratio [31]. Along the same lines, the SCD test had 86.2% sensitivity and 72.7% NPV (P = 0.02) in predicting successful ART treatment at 25.5% threshold value [31], [32]. Again, we are unable to directly compare our SDF ROC curve findings because previous analyses [32] were performed to predict the likelihood of fertility and were not intended for establishing a threshold value that distinguishes normal from abnormal sperm morphology.
Our present results highlight the importance of routinely incorporating advanced sperm function tests into the evaluation of infertile men. The combination of conventional sperm morphology indices with the advanced sperm function tests would help recognise sperm with greater reproductive potential despite having abnormal morphology; and, provide robust knowledge for developing novel sperm selection techniques that can be used during ART. Our current efforts to understand the clinical utility of sperm DNA damage and ORP in relation to sperm morphology in order to optimise reproductive outcomes seem promising. Future research will be needed to test our preliminary findings.
The present study has limitations. Only a subset (28%) of our participants had undertaken SDF testing, therefore, a smaller sample size (n = 365) for this particular test was used in the ROC curve analysis, which may have contributed to the lower diagnostic accuracy parameters in comparison with the ORP ROC curve analysis, which displayed superior diagnostic accuracy. However, SDF testing is still not routinely performed for the initial evaluation of all of our infertile men, and the more complex nature of the SDF test compared to the simpler ORP test, hence both such features prevented its utility for a larger group of our participants. In addition, the present study provides a snapshot in the lifetime of the infertile group, and follow-up of these patients could more accurately assess the real implications of sperm morphology, SDF and ORP on reproductive potential. Nevertheless, the present study is the first to assess, in depth, the relationship between advanced sperm function tests and sperm morphology and provides solid grounds for future studies in this particular aspect of male infertility.
Conclusion
Infertile patients had significantly lower conventional semen analysis parameters and significantly higher ORP and SDF levels compared with fertile controls. The infertile group showed significant negative correlation and significant positive correlation between ORP and SDF with normal sperm morphology and head defects, respectively. ORP and SDF were significantly associated with various intensities of normal sperm morphology. An ORP and SDF levels of 1.73 mV/106 sperm/mL and 25.5%, respectively, had the highest diagnostic accuracy in differentiating normal from abnormal sperm morphology. Using ORP and SDF measures in conjunction with standard semen morphology analysis could be used to validate the result of the fertility status of patients.
Conflict of interest
None.
Diagnosis
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Agarwal A., Mulgund A., Hamada A., Chyatte M.R. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurunath S., Pandian Z., Anderson R.A., Bhattacharya S. Defining infertility–a systematic review of prevalence studies. Hum Reprod Update. 2011;17:575–588. doi: 10.1093/humupd/dmr015. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva, Switzerland: 2010. WHO Laboratory manual for the examination and processing of human semen. [Google Scholar]
- 4.Check J.H., Adelson H.G., Schubert B.R., Bollendorf A. Evaluation of sperm morphology using Kruger's strict criteria. Arch Androl. 1992;28:15–17. doi: 10.3109/01485019208987674. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard M., Haguenoer K., Apert A., Poret H., Barthelemy C., Royere D. Sperm morphology assessment using David's classification: time to switch to strict criteria? Prospective comparative analysis in a selected IVF population. Int J Androl. 2011;34:145–152. doi: 10.1111/j.1365-2605.2010.01066.x. [DOI] [PubMed] [Google Scholar]
- 6.Aziz N., Buchan I., Taylor C., Kingsland C.R., Lewis-Jones I. The sperm deformity index: a reliable predictor of the outcome of oocyte fertilization in vitro. Fertil Steril. 1996;66:1000–1008. doi: 10.1016/s0015-0282(16)58697-x. [DOI] [PubMed] [Google Scholar]
- 7.Gatimel N., Moreau J., Parinaud J., Léandri R.D. Sperm morphology: assessment, pathophysiology, clinical relevance, and state of the art in 2017. Andrology. 2017;5:845–862. doi: 10.1111/andr.12389. [DOI] [PubMed] [Google Scholar]
- 8.Van Waart J., Kruger T.F., Lombard C.J., Ombelet W. Predictive value of normal sperm morphology in intrauterine insemination (IUI): a structured literature review. Hum Reprod Update. 2001;7:495–500. doi: 10.1093/humupd/7.5.495. [DOI] [PubMed] [Google Scholar]
- 9.Coetzee K., Kruge T.F., Lombard C.J. Predictive value of normal sperm morphology: a structured literature review. Hum Reprod Update. 1998;4:73–82. doi: 10.1093/humupd/4.1.73. [DOI] [PubMed] [Google Scholar]
- 10.Lockwood G.M., Deveneau N.E., Shridharani A.N., Strawn E.Y., Sandlow J.I. Isolated abnormal strict morphology is not a contraindication for intrauterine insemination. Andrology. 2015;3:1088–1093. doi: 10.1111/andr.12098. Epub 12015 Sep 12018. [DOI] [PubMed] [Google Scholar]
- 11.Hotaling J.M., Smith J.F., Rosen M., Muller C.H., Walsh T.J. The relationship between isolated teratozoospermia and clinical pregnancy after in vitro fertilization with or without intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2011;95:1141–1145. doi: 10.1016/j.fertnstert.2010.09.029. 1110.1016/j.fertnstert.2010.1109.1029.Epub 2010 Oct 1128. [DOI] [PubMed] [Google Scholar]
- 12.Kovac J., Smith R., Cajipe M., Ramasamy R., Dupree J., Langille G., Lamb D. Success rates of natural conception and intrauterine insemination in men with severely abnormal strict morphology (1% normal forms) suggests alternatives to immediate IVF. J Urol. 2014;191(Suppl.):e802. [Google Scholar]
- 13.Agarwal A., Virk G., Ong C., du Plessis S.S. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lamirande E., Gagnon C. Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod. 1995;10:15–21. doi: 10.1093/humrep/10.suppl_1.15. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal A., Majzoub A. Free radicals in andrology. In: Balercia G., Gandini L., Lenzi A., Lombardo F., editors. Antioxidants in andrology. Springer International Publishing; Switzerland: 2017. pp. 1–21. [Google Scholar]
- 16.Sharma R.K., Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–850. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 17.Sakkas D., Mariethoz E., Manicardi G., Bizzaro D., Bianchi P.G., Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–37. doi: 10.1530/ror.0.0040031. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal A., Majzoub A., Esteves S.C., Ko E., Ramasamy R., Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016;5:935–950. doi: 10.21037/tau.2016.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal A., Sharma R., Roychoudhury S., Du Plessis S., Sabanegh E. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril. 2016;106:566–573. doi: 10.1016/j.fertnstert.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez J.L., Muriel L., Rivero M.T., Goyanes V., Vazquez R., Alvarez J.G. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 22.Agarwal A., Roychoudhury S., Sharma R., Gupta S., Majzoub A., Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online. 2017;34:48–57. doi: 10.1016/j.rbmo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Wang C., Swerdloff R.S. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502–1507. doi: 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franken D.R. How accurate is sperm morphology as an indicator of sperm function? Andrologia. 2015;47:720–723. doi: 10.1111/and.12324. 710.1111/and.12324. [DOI] [PubMed] [Google Scholar]
- 27.Menkveld R., Stander F.S., Kotze T.J., Kruger T.F., van Zyl J.A. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586–592. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- 28.Marchesi D.E., Feng H.L. Sperm DNA integrity from sperm to egg. J Androl. 2007;28:481–489. doi: 10.2164/jandrol.106.002105. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal A., Roychoudhury S., Bjugstad K.B., Cho C.L. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol. 2016;8:302–318. doi: 10.1177/1756287216652779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majzoub A., Agarwal A., Esteves S.C. Sperm DNA fragmentation for the evaluation of male infertility: clinical algorithms. Transl Androl Urol. 2017;6(Suppl. 4):S405–S408. doi: 10.21037/tau.2017.03.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiweko B., Utami P. Predictive value of sperm deoxyribonucleic acid (DNA) fragmentation index in male infertility. Basic Clin Androl. 2017;27:1. doi: 10.1186/s12610-016-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez G., Lafuente R., Checa M.A., Carreras R., Brassesco M. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assisted reproduction treatment. Asian J Androl. 2013;15:790–794. doi: 10.1038/aja.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- 18.Ozmen B., Koutlaki N., Youssry M., Diedrich K., Al-Hasani S. DNA damage of human spermatozoa in assisted reproduction: origins, diagnosis, impacts and safety. Reprod Biomed Online. 2007;14:384–395. doi: 10.1016/s1472-6483(10)60883-8. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y., Li B., Fan L.Q., Zhu W.B., Chen X.J., Feng J.H. Does sperm morphology affect the outcome of intrauterine insemination in patients with normal sperm concentration and motility? Andrologia. 2012;44:299–304. doi: 10.1111/j.1439-0272.2012.01280.x. 210.1111/j.1439-0272.2012.01280.x. [DOI] [PubMed] [Google Scholar]
- 25.Keegan B.R., Barton S., Sanchez X., Berkeley A.S., Krey L.C., Grifo J. Isolated teratozoospermia does not affect in vitro fertilization outcome and is not an indication for intracytoplasmic sperm injection. Fertil Steril. 2007;88:1583–1588. doi: 10.1016/j.fertnstert.2007.01.057. [DOI] [PubMed] [Google Scholar]