Abstract
Objective
In this review, we investigate the advantage of varicocele repair prior to assisted reproductive technologies (ART) for infertile couples and provide cost analysis information.
Materials and methods
We searched the following electronic databases: PubMed, Medline, Excerpta Medica Database (Embase), Cumulative Index to Nursing and Allied Health Literature (CINAHL). The following search strategy was modified for the various databases and search engines: ‘varicocele’, ‘varicocelectomy’, ‘varicocele repair’, ‘ART’, ‘in vitro fertilisation (IVF)’, ‘intracytoplasmic sperm injection (ICSI)’.
Results
A total of 49 articles, including six meta-analyses, 32 systematic reviews, and 11 original articles, were included in the analysis. Bypassing potentially reversible male subfertility factors using ART is currently common practice. However, varicocele may be present in 35% of men with primary infertility and 80% of men with secondary infertility. Varicocele repair has been shown to be an effective treatment for infertile men with clinical varicocele, thus should play an important role in the treatment of such patients due to the foetal/genetic risks and high costs that are associated with increased ART use.
Conclusion
Varicocele repair is a cost-effective treatment method that can improve semen parameters, pregnancy rates, and live-birth rates in most infertile men with clinical varicocele. By improving semen parameters and sperm structure, varicocele repair can decrease or even eliminate ART requirement.
Abbreviations: ART, assisted reproductive technologies; CINAHL, Cumulative Index to Nursing and Allied Health Literature; Embase, Excerpta Medica Database; ICSI, intracytoplasmic sperm injection; IUI, intrauterine insemination; IVF, in vitro fertilisation; NOA, non-obstructive azoospermia; ROS, reactive oxygen species; SDF, sperm DNA fragmentation; TESE, testicular sperm extraction; TMSC, total motile sperm count
Keywords: Assisted reproductive technology, In vitro fertilisation, Intracytoplasmic sperm injection, Varicocele, Varicocelectomy
Introduction
Testicular varicocele is the abnormal expansion of the pampiniform plexus, which provides testicle venous drainage. It is the most common treatable cause of male infertility worldwide. It is detected in 40% of men with infertility and nearly 15% of adult men generally [1]. Varicocele may cause testicular atrophy, discomfort, infertility, and hypogonadism. Varicocele aetiology is not entirely clear, with venous reflux thought to be the main cause of varicocele-related testicular dysfunction [1], [2]. There are three hypotheses for venous blood drainage impairment: (i) lack of or functional disorder in the venous valves, (ii) differences in the attachment of the testicular veins to the left renal vein and vena cava, and (iii) renal vein compression between the upper mesenteric artery and aorta (the ‘nutcracker’ effect) [2], [3], [4]. Intratesticular temperature increase, testicle hypoxia, oxidant accumulation in the semen, renal and adrenal metabolite reflux, and anti-sperm antibodies may result in varicocele-related testicular dysfunction [5], and these are all a reflection of venous reflux effects. Varicocele may cause changes at the cellular level, which may induce testicular cell apoptosis and increase reactive oxygen species (ROS), decrease testicular DNA polymerase activity, change Sertoli cellular function, and decrease testosterone production by Leydig cells [6]. These, secondary to varicocele, can result in infertility.
Recent studies have shown that in infertile men with abnormal semen parameters, varicocele repair is an efficient treatment method [7], [8], [9]. Since the advent of in vitro fertilisation (IVF) at the end of the 1970s, fertility treatment has generally been provided through assisted reproductive technologies (ART), rather than specific treatments for male infertility. However, varicocele is present in 35% of men with primary infertility and 80% of men with secondary infertility [10]. Even though ART allow infertile couples to become biological parents, there are associated disadvantages, such as increases in multi-pregnancy-related birth defects, ovary hyperstimulation, and high costs. Amongst the IVF methods currently used, intracytoplasmic sperm injection (ICSI) is applied most commonly, at a rate of 76% [11]. ICSI pregnancies more commonly involve chromosomal anomaly, autism, mental disability, and birth defects than pregnancies resulting from conventional IVF, as the natural selection process is disabled [6].
Varicocele repair should have an important role in the treatment of infertile patients with clinical varicocele, due to the foetal/genetic risks and high costs that are associated with increased ART use. Varicocele repair should provide nearly two-times more advantage in improving sperm quality and quantity for ART, thus decreasing the need for using ART and increasing spontaneous pregnancy rates [6].
In this review, we detail the advantages of varicocele repair before ART for infertile couples and provide cost analysis information.
Materials and methods
Search strategy
We searched the following electronic databases from 1993 to 2017: PubMed, Medline, Excerpta Medica Database (Embase), and Cumulative Index to Nursing and Allied Health Literature (CINAHL). The following search term strategy was modified for the various databases and search engines: ‘varicocele’, ‘varicocelectomy’, ‘varicocele repair’, ‘IVF’, ‘ICSI’, and ‘ART’. We also searched amongst the references of the identified articles. If it was not clear from the abstract whether the paper contained relevant data, the full paper was assessed. Along with Medical Subject Headings (MeSH) terms and relevant keywords, we used the Cochrane Highly Sensitive Search Strategy to identify articles in PubMed. We restricted the search to articles published in the English language that reported on varicocele, varicocelectomy, varicocele repair, IVF, ICSI, and ART. Studies for which the full text was inaccessible and articles written before 1993 were excluded. A total of 48 original articles, systematic reviews, and meta-analyses were included in this review.
Data extraction and management
Based on the pre-determined selection criteria, two authors (M.G.S. and A.H.H.) independently selected all trials retrieved from the databases and bibliographies. Disagreements between evaluators were resolved via discussion. Studies were reviewed to determine their relevance to varicocele treatment, interventions (varicocele and/or infertility), and their outcome measures. We retrieved full-text copies of the articles identified as potentially relevant by either one or both review authors.
Results
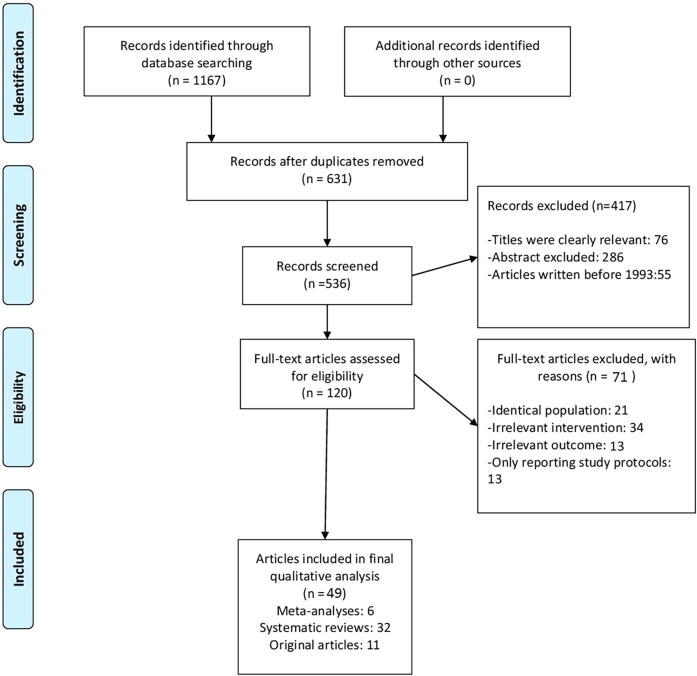
In all, 1167 studies were identified from the search of the PubMed, Medline, Embase, and CINAHL databases. In all, 1047 of these articles were not directly related to the subject based on the titles and abstracts, were written before 1993, or had full texts that could not be accessed. These articles were thus excluded from the analysis. Of the remaining 120 articles, 71 were excluded because they had identical populations, irrelevant interventions, or irrelevant outcomes or only reported the study protocols. As a result, a total of 49 articles, including six meta-analyses, 32 systematic reviews, and 11 original articles, were included in the analysis. The flow of the study selection is described in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram (Fig. 1). The data are reported in a narrative manner. Two tables were created to illustrate the results in short: Table 1 is titled ‘Effects of varicocele repair on sperm parameters’ [7], [8], [9], [15], [16], [17], [18], [19], [20], [21], [22] and Table 2 is titled ‘Effect of varicocelectomy on ART’ [26], [27], [28], [29], [30], [31].
Fig. 1.
Search strategy and selection process.
Table 1.
Studies evaluating the effects of varicocele repair on sperm parameters.
| Reference | Number of studies evaluated | Number of patients | Groups | Parameters | Outcomes |
|---|---|---|---|---|---|
| Abdel-Meguid et al. [7] | 1 | 145 | Varicocelectomy (n = 73) vs control (n = 72) | Pregnancy rate, Semen quality | Spontaneous pregnancy rate 32.9% vs 13.9% (P = 0.01). Significant recovery in semen parameters (P < 0.001) |
| Kroese et al. [8] | 10 | 894 | Varicocele occlusion (n = 449) vs control (n = 445) | Pregnancy rate, Semen quality | Significant recovery in treatment group (P = 0.03) |
| Baazeem et al. [9] | 22 | – | Pre-varicocelectomy vs post-varicocelectomy | Semen quality, sperm DNA damage, seminal oxidative stress | Significant recovery in sperm concentration and sperm progressive motility, decrease in sperm DNA damage and seminal oxidative stress (P < 0.001, P < 0.001, P = 0.003, respectively) |
| Marmar et al. [15] | 5 | 570 | Varicocelectomy (n = 396) vs control (n = 174) | Spontaneous pregnancy rate | Spontaneous pregnancy rate: 33% vs 15% (P = 0.007) |
| Barekat et al. [16] | 1 | 35 | Post-varicocelectomy + N-acetyl-L-cysteine vs post-varicocelectomy without N-acetyl-L-cysteine | Semen quality, sperm DNA integrity, oxidative stress | No significant difference in sperm parameters, significant decrease in DNA fragmentation and oxidative stress (P < 0.05) |
| Agarwal et al. [17] | 10 | 1231 | Pre-varicocelectomy vs post-varicocelectomy | Semen quality | The sperm concentration increased by 12.03 106/mL, motility increased by 11.72% (P = 0.002), morphology increased by 3.16% (P = 0.01) |
| Chen et al. [18] | 1 | 30 | Pre-varicocelectomy vs post-varicocelectomy | Semen quality, sperm mitochondrial DNA deletion | 73% recovery in semen quality, significant decrease in sperm mitochondrial DNA deletion: 40% vs 13.3% (P < 0.001) |
| Zini et al. [19] | 1 | 25 | Pre-varicocelectomy vs post-varicocelectomy | Semen quality, sperm chromatin and DNA integrity | Sperm concentration, progressive motility, sperm chromatin and DNA integrity significant recovery (P < 0.05, P < 0.05, P < 0.001, P = 0.004, respectively) |
| Smit et al. [20] | 1 | 49 | Pre-varicocelectomy vs post-varicocelectomy | Semen quality, DNA fragmentation index, pregnancy rate | Sperm concentration and sperm progressive motility significant recovery (both P < 0.001), DNA fragmentation index decrease (35.2% vs 30.2% (P = 0.019), spontaneous pregnancy rate: 37% |
| Li et al. [21] | 1 | 19 | Pre-varicocelectomy vs post-varicocelectomy | Semen quality, DNA fragmentation index | Sperm concentration and sperm progressive motility significant recovery, DNA fragmentation index decrease (28.4% vs 22.4%) (P = 0.009, P = 0.029, P = 0.018, respectively) |
| Wang et al. [22] | 7 | 476 | Varicocele patients (n = 240) vs controls (n = 176) | Sperm DNA damage | In varicocele patients group, high sperm DNA damage: 9.84% more (P < 0.001) |
Table 2.
Studies evaluating effect of varicocelectomy on ART.
| Study | Number of patients | Groups | Parameters | Outcomes |
|---|---|---|---|---|
| Daitch et al. [26] | 58 | IUI with prior varicocelectomy (n = 34) vs IUI without prior varicocelectomy (n = 24) | Live-birth and pregnancy rates | Pregnancy rate/cycle: 11.8% vs 6.3% (P = 0.04) Live-birth rate/cycle: 11.8% vs 2.1% (P = 0.007) Live-birth rate/couple: 32.4% vs 4.2% (P = 0.001) |
| Cayan et al. [27] | 540 | Pre-varicocelectomy vs post-varicocelectomy | Spontaneous pregnancy, changes in ART candidacy after varicocelectomy | Spontaneous pregnancy rate: 36.6% 31% of preoperative IVF and ICSI candidates became IUI or spontaneous pregnancy candidates after varicocelectomy 42% of IUI candidates gained the potential for spontaneous pregnancy |
| Esteves et al. [28] | 242 | ICSI with prior varicocelectomy (n = 80) vs ICSI without prior varicocelectomy (n = 162) | Live-birth, clinical pregnancy, miscarriage and fertilisation rates | Live-birth rate: 46.3% vs 1.5% (P = 0.03) Clinical pregnancy rate: 60.0% vs 45.1% (P = 0.04) Miscarriage rate: 22.9% vs 30.1% (P = 0.46) Fertilisation rate:78% vs 66% (P = 0.04) |
| Pasqualotto et al. [29] | 248 | ICSI with prior varicocelectomy (n = 169) vs ICSI without prior varicocelectomy (n = 79) | Clinical pregnancy, implantation, miscarriage and fertilisation rates | Clinical pregnancy rate: 30.9% vs 31.1% (P = 0.98) Implantation rate: 17.3% vs 22.1% (P = 0.58) Miscarriage rate: 23.9% vs 21.7% (P = 0.84) Fertilisation rate:64.9% vs 73.2% (P = 0.03) |
| Gokce et al. [30] | 306 | ICSI with prior varicocelectomy (n = 168) vs ICSI without prior varicocelectomy (n = 138) | Live-birth, clinical pregnancy and miscarriage rates | Live-birth rate: 47.6% vs 29.0% (P < 0.001) Clinical pregnancy rate: 62.5% vs 47.1% (P = 0.001) Miscarriage rate: 14.9% vs 18.1% (P = 0.057) |
| Zini et al. [31] | 393 | Varicocelectomy (n = 251) vs control (n = 142) | Pregnancy rates, ART utilisation | Spontaneous pregnancy rate: 39% vs 32% (P > 0.05) ART utilisation rate: 38% vs 54% (P = 0.002) |
Discussion
When should varicocele repair be performed?
Although varicocele repair is generally known to be advantageous for infertile men, the determination of the specific patients and couples who most benefit from surgical intervention remains controversial [12].
The American Society for Reproductive Medicine updated its suggestions regarding varicocele management for infertile couples in 2014, and these suggestions were included in the American Urological Association (AUA) Clinical Guideline [13]. The application committee suggested that varicocele repair to overcome infertility should be performed under four conditions: (a) infertility in both partners, (b) palpable (clinical) varicocele in the male partner, (c) a female partner with normal fertility or treatable infertility, (d) at least one anomaly in terms of the semen parameters of the male partner (except isolated teratozoospermia).
Effects of varicocele repair on sperm
Surgical repair can be successful in clearing the enlarged veins of the spermatic cord, but the main result that infertile partners demand is increased fertility. Improvements in post-varicocelectomy sperm parameters can easily be evaluated, but it may not be possible to discuss complete success if these improvements are not effective in terms of improved live-birth rates or the level of ART given to couples. Oxidative stress and sperm DNA fragmentation (SDF) are major contributing factors in the pathophysiology of varicocele. Although sperm with fragmented DNA can fertiliseoocytes at a similar rate to that of sperm without DNA fragmentation, it has been found that increased SDF negatively affects embryo development and may endanger pregnancies in patients receiving ART [14]. There are many studies showing that the surgical repair of clinical varicocele improves sperm parameters, decreases seminal oxidative stress and SDF, and increases seminal antioxidants [8], [15], [16], [17]. Molecular and ultrastructural evaluation may represent more sensitive alternative methods of evaluating the effects of repair. Surgical repair typically results in a decrease in ROS levels and SDF [18], [19], [20]. Various studies have shown that men with varicocele have more sperm DNA damage than control patients; this difference was 9.84% on average [20], [21], [22]. In a meta-analysis, it was shown that varicocelectomy decreased SDF, with average fragmentation decreasing by 3.37% as compared with controls [22].
Zini et al. [19] evaluated the effect of varicocelectomy on sperm chromatin and DNA integrity, and detected significant improvements in sperm chromatin structure assay parameters, sperm DNA integrity, sperm concentration, and progressive motility at6months after varicocelectomy. This study showed that varicocele-caused SDF can be reversed by varicocelectomy.
One of the key events in the pathology of varicocele is the excessive production of ROS. In terms of pathological conditions, two roles have been envisaged for the overproduction of ROS: ROS-induced damage to the sperm membrane reduces sperm motility and the ability of the sperm to fuse with the oocyte, and ROS directly damages sperm DNA and subsequently affects the genomic integrity of the embryo. Thus, pregnancy results may be negatively affected in patients with varicocele and patients using ART. Varicocelectomy and antioxidant therapy may overcome the deleterious effects of ROS in individuals with varicocele [16], [17], [18]. Barekat et al. [16] suggested the use N-acetyl-L-cysteine, as an antioxidant, after varicocelectomy, and reported that post-operation antioxidant treatment reduced ROS levels and improved chromatin integrity and pregnancy rates.
Ultrastructural studies have shown the positive effects of varicocele repair on sperm structure. Reichart et al. [23] examined sperms’ subcellular organelles in males with treated and untreated varicocele. Whilst no change was found in the subcellular organelles in the tail section after treatment, a significant recovery was seen in the normal acrosome structure, chromatin concentration, and sperm head section. Reichart et al. [23] commented that especially given that semen parameters did not change amongst groups, ultramorphology may be a more sensitive tool with which to evaluate sperm pathology in men with varicocele. In a meta-analysis of prospective studies, it was reported that varicocele repair caused the reversal of sperm head organelle ultrastructural defects in infertile men [9]. Richardson et al. [24] evaluated 2291 couples in 24 studies and reported an average natural birth rate of 39.5%, in addition to the recovery of sperm parameters, after varicocele repair. Abdel-Meguid et al. [7] considered 145 male patients, with a minimum of 1 year of infertility, who were followed-up without varicocele repair (n = 72) or with varicocele repair (n = 73). Whilst the natural pregnancy rate during follow-up was 13.9% in the group without varicocele repair, it was 32.9% in the varicocele repair group [7]. Despite all these studies, the question of whether the application of varicocelectomy before ART improves treatment results in infertile men with clinical varicocele remains controversial.
Effects of varicocele repair on ART
Although the advantages of varicocele repair in couples using ART remain to be clarified, studies in the literature assert that varicocele repair before ART may have a positive effect on general pregnancy and live-birth rates. Studies have claimed that the high spontaneous pregnancy rates seen after varicocele repair, as high as 37%, can decrease or even eliminate ART use [25], [26], [27].
In a study retrospectively examining 58 couples who underwent intrauterine insemination (IUI), microsurgical varicocelectomy was performed in 34 couples with varicocele. In this study, it was found that the varicocelectomy group had higher pregnancy (11.8% vs 6.3%) and live-birth rates (11.8% vs 1.6%) [26].
Cayan et al. [27] reported improvements in the total number of motile sperm of >50% in 50% of patients (271/540) in a prospective evaluation performed after varicocelectomy in 540 males with clinical varicocele. This study reported a general pregnancy rate of 36.6% after the seventh month.
Esteves et al. [28] found that 80 men who underwent varicocelectomy before ICSI had higher pregnancy and live-birth rates and lower miscarriage rates as compared with 162 men without varicocelectomy. Additionally, the total number of motile sperm was found to have increased in the varicocelectomy group.
Pasqualotto et al. [29] compared 169 couples, in which the man underwent varicocele repair before ICSI with 79 couples who did not undergo varicocelectomy, in a study of male partners with clinical varicocele. No significant differences were found for spontaneous implantation, pregnancy, and miscarriage rates. However, a significant difference (73.2% vs 64.9%) was found for the recovery of fertilisation rates between the two groups, and they suggested that varicocele repair should always be performed before ICSI.
Gokce et al. [30] compared 168 couples who underwent varicocelectomy before ICSI with 138 couples who did not undergo varicocelectomy, and reported that the varicocelectomy group had increased pregnancy and live-birth rates and a lower miscarriage rate.
Only one meta-analysis examining the effect of varicocelectomy on ART has been published. A total of 870 ICSI cycles were evaluated in this study. In all, 438 patients who underwent varicocelectomy before ICSI and 432 patients who underwent ICSI without varicocelectomy were compared. In the ICSI and untreated varicocele group, the live-birth rate was 24.5–31.5%, the clinical pregnancy rate was 28.3–47.1%, and the miscarriage rate was 18.1–30.1%. In the ICSI after varicocelectomy group, the live-birth rate was 46.3–52.3%, the clinical pregnancy rate was 30.9–62.5%, and the miscarriage rate was 14.9–23.9%. This meta-analysis found that patients who underwent varicocelectomy before ICSI had higher pregnancy and live-birth rates and lower miscarriage rates as compared with those who did not [14].
In terms of contrasting results, Zini et al. [31] evaluated 610 infertile males; 363 underwent varicocelectomy, and 247 did not undergo surgery and were observed. The prevalence of primary infertility was reported to be higher in patients who underwent surgical treatment (80% vs 71%) and in those who had bilaterally smaller testicles, lower sperm concentrations, and lower sperm motilities. However, couples who responded more positively to surgical varicocelectomy were excluded from the final analysis because this resulted in early spontaneous pregnancy without ART. This affected the surgery-related results in a negative way.
More well-designed studies are needed given that the above-mentioned studies did not completely address the effect of varicocelectomy on semen, had a non-randomised and non-retrospective nature, and had conflicting results, as well as the fact that couples’ decision-making processes are influenced by economic issues and other factors.
In addition, because the initial semen parameters of IUI/IVF/ICSI candidates are very different, the studies involving patients using ART are heterogeneous. Therefore, the results obtained from most studies are not comparable. Comparable studies with patients separated into homogenous groups are needed.
ART and varicocele repair in men with non-obstructive azoospermia (NOA)
Although the use of ART is inevitable in men with spermatogenic deficiency, varicocele repair can recover healthy sperm in the ejaculate of infertile men with NOA and clinical varicocele, and thus lower the need for ART and the associated costs [1]. The chance of sperm being present in the ejaculate is related to testicle histology. Whilst the chance of recovery increases amongst men with hypospermatogenesis and late maturation arrest, there is no recovery of the semen parameters in men with early maturation arrest or Sertoli-cell-only histology [32], [33]. Schlegel and Kaufmann [34] reported that <10% of men with NOA with varicocele had an adequate number of motile sperms for ICSI after varicocele repair, but there was no significant difference in sperm acquirement ratios during testicular sperm extraction (TESE) when these patients were compared to a group without varicocelectomy. Current studies show that sperm acquirement ratios are higher in patients with NOA after varicocele repair [35], [36]. Studies have determined that there is intermittent sperm in the ejaculates of 5–35% of patients with NOA who go without any treatment [34], [37], [38]. However, the presence of sperm rate in the ejaculate of NOA patients after varicocelectomy was found to be between 19% and 22% [34], [39]. The gradual regression of spermatogenesis and the reversal to azoospermia were reported at a rate of 55.5% in patients with NOA at 1 year after varicocelectomy. This shows that varicocele repair does not have a long-term positive effect in patients with NOA [37].
Spermatogenesis is recovered in a minority of men after varicocelectomy, and a significant number of these lose their spermatogenic abilities again in the long term. Thus, it is suggested to freeze and retain the sperm after the initial recovery after varicocelectomy [40].
Varicocele repair before ART can allow for successful pregnancy in such individuals and decrease the costs associated with pregnancy by lowering the need for ICSI. However, ART is necessary in most patients with NOA, as the chance of sperm in the ejaculate after varicocele repair is low. Therefore, varicocele repair may not be a cost-effective solution for these patients.
When should varicocele repair be performed along with ART?
The question of whether ART should be applied first in infertile oligospermic and azoospermic men with varicocele or varicocele repair should be performed before ART remains controversial.
Samplaski et al. [41] divided 373 clinical varicocele patients into three groups according to total motile sperm counts (TMSC). For the analyses, men with a TMSC of <5 million were considered candidates for IVF, those with 5–9 million for IUI, and those with >9 million for natural pregnancies. After varicocelectomy, 38 of 66 men (57.6%) who would have initially been candidates for IUI were upgraded to natural pregnancy candidacy, and 74 of 139 men (53.2%) who would initially have been candidates for IVF were upgraded to IUI or natural pregnancy candidacy. By contrast, 40 of 168 men (23.8%) showed enough decline in their TMSC after treatment and they were downgraded either from natural pregnancy to IUI or from IUI to IVF.
Kirby et al. [42] estimated the pregnancy rates for oligospermic men undergoing IVF/ICSI after varicocelectomy to be 49.1%. This same meta-analysis estimates the pregnancy rate for men with uncorrected varicoceles to be 42.1%.
Dubin et al. [43] evaluated 17 men with a TMSC of <2 million who underwent varicocelectomy. After varicocelectomy, 14/17 men had an improvement in TMSC, with 10 having a TMSC of >2 million. Of the 10 men, one achieved spontaneous pregnancy and seven underwent a cycle of IUI; two of the seven men achieved successful pregnancy with IUI.
These studies show that varicocele repair before ART potentially reduces the need for IVF and IUI, and will change the ART method to be performed, in addition to increasing the pregnancy rate. For this reason, we believe that varicocele repair should be done before ART.
In a current review, the results of varicocele repair + ART and ART-only treatments were compared in infertile oligospermic and NOA patients with clinical varicocele. The pregnancy rates for the partners of oligospermic men were varicocele repair + IUI: 7.7–50%, only IUI: 10–16.7%, varicocele repair + ICSI/IVF: 30.9–62.5%, only ICSI/IVF: 31.1–47.1%. Amongst the partners of men with NOA, the pregnancy rates were varicocele repair + ICSI/IVF: 31.4–74.2%, only ICSI/IVF: 22.2–52.3%. Because pregnancy rates are better for the varicocele repair + ART treatment group, we believe that performing varicocele repair in infertile oligospermic and azoospermic men prior to ART would be the more advantageous option [44].
Cost analysis in varicocele repair and the use of ART
Whilst the cost per varicocelectomy was found to be $26 668 in a study performed in 1994 in the USA, the cost of ICSI was much higher at $89 091. Spontaneous pregnancy rates were reported to be 30% for varicocelectomy and 28% for a cycle of ICSI [45]. In an analysis performed in 2013 in the Korean health system, the cost of varicocelectomy was reported to be $10 534, and the cost of ICSI was reported to be $14 893 [46]. These two analyses have shown that varicocelectomy is more cost-effective than ICSI. Meng et al. [47] created a decision analysis model for infertile couples with varicocele, and direct institutional costs of $4500 for varicocele repair, $10 000 for an ICSI cycle, and $500 for an IUI cycle. The results stated that varicocele repair was more affordable than ICSI when the total motile number of sperm before surgery was <10 million. Because the postoperative pregnancy rate after varicocele repair was >45% when the total motile sperm number was >10 million, it was calculated to be more cost-effective than IUI. Lee et al. [48] state that microsurgical TESE is more cost-effective than varicocelectomy in patients with NOA. The cost of TESE was $65 515 and that of varicocelectomy was $76 578 in 1999. In 2005, the cost of TESE was $69 731, and that of varicocelectomy was $79 576.
Penson et al. [49] compared the cost-effectiveness of four potential treatment strategies for varicocele-related infertility. These treatment strategies included an observation group, varicocele repair and then the application of a maximum of three IVF cycles after that (or double this if pregnancy did not result in the first year after varicocelectomy), the application of three cycles of ovarian stimulation and IUI, and then the application of three IVF cycles if IUI failed, and the immediate application of a maximum of three cycles of IVF. Live births were observed at a rate of 14% in couples in the observation group. Even though indirect costs were not included, it was reported that the direct transition to IVF was the most cost-effective management strategy for infertility. However, the live-birth rate in patients who underwent direct transition to IVF was determined to be 61%, and it was shown that this strategy was less successful as compared to varicocele repair (72%) or IUI (73%). Whilst the average cost of treatment for the varicocele group per live birth was $32 171, this value was calculated to be $36 232 in the IUI group.
Finally, Dubin et al. [43] have reported that varicocelectomy in combination with IUI in men with clinical varicocele is more cost-effective than direct IVF/ICSI ($35 924 vs $45 795).
The number of children desired by the couples, the cost of birth defects, ART-related complications, and the differences in costs across countries and institutions affect the objectivity of the results. However, it is accepted as more cost-effective for both institutions and patients when varicocele repair is used by itself or in combination with IVF in an attempt to begin pregnancy, and varicocele repair accompanied by IVF has been shown to be more cost-effective than varicocele repair alone.
Conclusion
Varicocele repair is a cost-effective treatment method that can improve semen parameters, pregnancy rates, and live-birth rates in most infertile men with clinical varicocele. By improving semen parameters and sperm structure, varicocele repair may increase the possibility of fertilisation during IVF and ICSI, and may decrease the need for use of ART.
Conflicts of interest
None.
Source of funding
None.
Management
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Pastuszak A.W., Wang R. Varicocele and testicular function. Asian J Androl. 2015;17:659–667. doi: 10.4103/1008-682X.153539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheehan M.M., Ramasamy R., Lamb D.J. Molecular mechanisms involved in varicocele-associated infertility. J Assist Reprod Genet. 2014;31:521–526. doi: 10.1007/s10815-014-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenberg M.L., Lipshultz L.I. Varicocele-induced infertility: newer insights into its pathophysiology. Indian J Urol. 2011;27 doi: 10.4103/0970-1591.78428. 58–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naughton C.K., Nangia A.K., Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001;7:473–481. doi: 10.1093/humupd/7.5.473. [DOI] [PubMed] [Google Scholar]
- 5.Chiles K.A., Schlegel P.N. Cost-effectiveness of varicocele surgery in the era of assisted reproductive technology. Asian J Androl. 2016;18:259–261. doi: 10.4103/1008-682X.172644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pathak P., Chandrashekar A., Hakky T.S., Pastuszak A.W. Varicocele management in the era of in vitro fertilization/intracytoplasmic sperm injection. Asian J Androl. 2016;18:343–348. doi: 10.4103/1008-682X.178482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Meguid T.A., Al-Sayyad A., Tayib A., Farsi H.M. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol. 2011;59:455–461. doi: 10.1016/j.eururo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Kroese A.C., de Lange N.M., Collins J., Evers J.L. Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev. 2012;10:CD000479. doi: 10.1002/14651858.CD000479.pub5. [DOI] [PubMed] [Google Scholar]
- 9.Baazeem A., Belzile E., Ciampi A., Dohle G., Jarvi K., Salonia A. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Gorelick J.I., Goldstein M. Loss of fertility in men with varicocele. Fertil Steril. 1993;59:613–616. [PubMed] [Google Scholar]
- 11.Boulet S.L., Mehta A., Kissin D.M., Warner L., Kawwass J.F., Jamieson D.J. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. 2015;313:255–263. doi: 10.1001/jama.2014.17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlegel P.N. Contemporary issues in varicocele management. Curr Opin Urol. 2012;22:487–488. doi: 10.1097/MOU.0b013e328359735a. [DOI] [PubMed] [Google Scholar]
- 13.Practice Committee of the American Society for Reproductive Medicine. Society for Male Reproduction and Urology. Report on varicocele and infertility: a committee opinion. Fertil Steril 2014;102:1556–60. [DOI] [PubMed]
- 14.Esteves S.C., Roque M., Agarwal A. Outcome of assisted reproductive technology in men with treated and untreated varicocele: systematic review and meta-analysis. Asian J Androl. 2016;18:254–258. doi: 10.4103/1008-682X.163269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marmar J.L., Agarwal A., Prabaskan S., Agarwal R., Short R.A., Benoff S. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril. 2007;88:639–648. doi: 10.1016/j.fertnstert.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Barekat F., Tavalaee M., Deemeh M.R., Bahreinian M., Azadi L., Abbasi H. A preliminary study: N-acetyl-L-cysteine improves semen quality following varicocelectomy. Int J Fertil Steril. 2016;10:120–126. doi: 10.22074/ijfs.2016.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal A., Deepinder F., Cocuzza M., Agarwal R., Short R.A. Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology. 2007;70:532–538. doi: 10.1016/j.urology.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Chen S.S., Huang W.J., Chang L.S., Wei Y.H. Attenuation of oxidative stress after varicocelectomy in subfertile patients with varicocele. J Urol. 2008;179:639–642. doi: 10.1016/j.juro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 19.Zini A., Azhar R., Baazeem A., Gabriel M.S. Effect of microsurgical varicocelectomy on human sperm chromatin and DNA integrity: a prospective trial. Int J Androl. 2011;34:14–19. doi: 10.1111/j.1365-2605.2009.01048.x. [DOI] [PubMed] [Google Scholar]
- 20.Smit M., Romijn J.C., Wildhagen M.F., Veldhoven J.L., Weber R.F., Dohle G.R. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2013;189:S146–50. doi: 10.1016/j.juro.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Li F., Yamaguchi K., Okada K., Matsushita K., Ando M., Chiba K. Significant improvement of sperm DNA quality after microsurgical repair of varicocele. Syst Biol Reprod Med. 2012;58:274–277. doi: 10.3109/19396368.2012.692431. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y.J., Zhang R.Q., Lin Y.J., Zhang R.G., Zhang W.L. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online. 2012;25:307–314. doi: 10.1016/j.rbmo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Reichart M., Eltes F., Soffer Y., Zigenreich E., Yogev L., Bartoov B. Sperm ultramorphology as a pathophysiological indicator of spermatogenesis in males suffering from varicocele. Andrologia. 2000;32:139–145. doi: 10.1046/j.1439-0272.2000.00355.x. [DOI] [PubMed] [Google Scholar]
- 24.Richardson I., Grotas A.B., Nagler H.M. Outcomes of varicocelectomy treatment: an updated critical analysis. Urol Clin North Am. 2008;35:191–209. doi: 10.1016/j.ucl.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 25.McIntyre M., Hsieh T.C., Lipshultz L. Varicocele repair in the era of modern assisted reproductive techniques. Curr Opin Urol. 2012;22:517–520. doi: 10.1097/MOU.0b013e328358e191. [DOI] [PubMed] [Google Scholar]
- 26.Daitch J.A., Bedaiwy M.A., Pasqualotto E.B., Hendin B.N., Hallak J., Falcone T. Varicocelectomy improves intrauterine insemination success rates in men with varicocele. J Urol. 2001;165:1510–1513. [PubMed] [Google Scholar]
- 27.Cayan S., Erdemir F., Ozbey I., Turek P.J., Kadioğlu A., Tellaloğlu S. Can varicocelectomy significantly change the way couples use assisted reproductive technologies? J Urol. 2002;167:1749–1752. doi: 10.1016/s0022-5347(05)65192-0. [DOI] [PubMed] [Google Scholar]
- 28.Esteves S.C., Oliveira F., Bertolla R.P. Clinical outcome of intracytoplasmic sperm injection in infertile men with treated and untreated clinical varicocele. J Urol. 2010;184:1442–1446. doi: 10.1016/j.juro.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Pasqualotto F.F., Braga D.P., Figueira R.C., Setti A.S., Iaconelli A., Jr, Borges E., Jr. Varicocelectomy does not impact pregnancy outcomes following intracytoplasmic sperm injection procedures. J Androl. 2012;33:239–243. doi: 10.2164/jandrol.110.011932. [DOI] [PubMed] [Google Scholar]
- 30.Gokce M.I., Gulpinar O., Suer E., Mermerkaya M., Aydos K., Yaman O. Effect of performing varicocelectomy before intracytoplasmic sperm injection on clinical outcomes in non-azoospermic males. Int Urol Nephrol. 2013;45:367–372. doi: 10.1007/s11255-013-0394-2. [DOI] [PubMed] [Google Scholar]
- 31.Zini A., Boman J., Baazeem A., Jarvi K., Libman J. Natural history of varicocele management in the era of intracytoplasmic sperm injection. Fertil Steril. 2008;90:2251–2256. doi: 10.1016/j.fertnstert.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 32.Kim E.D., Leibman B.B., Grinblat D.M., Lipshultz L.I. Varicocele repair improves semen parameters in azoospermic men with spermatogenic failure. J Urol. 1999;162:737–740. doi: 10.1097/00005392-199909010-00031. [DOI] [PubMed] [Google Scholar]
- 33.Weedin J.W., Khera M., Lipshultz L.I. Varicocele repair in patients with nonobstructive azoospermia: a meta-analysis. J Urol. 2010;183:2309–2315. doi: 10.1016/j.juro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Schlegel P.N., Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril. 2004;81:1585–1588. doi: 10.1016/j.fertnstert.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 35.Inci K., Hascicek M., Kara O., Dikmen A.V., Gürgan T., Ergen A. Sperm retrieval and intracytoplasmic sperm injection in men with nonobstructive azoospermia, and treated and untreated varicocele. J Urol. 2009;182:1500–1505. doi: 10.1016/j.juro.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Haydardedeoglu B., Turunc T., Kilicdag E.B., Gul U., Bagis T. The effect of prior varicocelectomy in patients with nonobstructive azoospermia on intracytoplasmic sperm injection outcomes: a retrospective pilot study. Urology. 2010;75:83–86. doi: 10.1016/j.urology.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 37.Pasqualotto F.F., Sobreiro B.P., Hallak J., Pasqualotto E.B., Lucon A.M. Induction of spermatogenesis in azoospermic men after varicocelectomy repair: an update. Fertil Steril. 2006;85:635–639. doi: 10.1016/j.fertnstert.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 38.Gat Y., Bachar G.N., Everaert K., Levinger U., Gornish M. Induction of spermatogenesis in azoospermic men after internal spermatic vein embolization for the treatment of varicocele. Hum Reprod. 2005;20:1013–1017. doi: 10.1093/humrep/deh706. [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Meguid T.A. Predictors of sperm recovery and azoospermia relapse in men with nonobstructive azoospermia after varicocele repair. J Urol. 2012;187:222–226. doi: 10.1016/j.juro.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 40.Cocuzza M., Cocuzza M.A., Bragais F.M., Agarwal A. The role of varicocele repair in the new era of assisted reproductive technology. Clinics (Sao Paulo) 2008;63:395–404. doi: 10.1590/S1807-59322008000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samplaski M.K., Lo K.C., Grober E.D., Zini A., Jarvi K.A. Varicocelectomy to “upgrade” semen quality to allow couples to use less invasive forms of assisted reproductive technology. Fertil Steril. 2017;108:609–612. doi: 10.1016/j.fertnstert.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Kirby E.W., Wiener L.E., Rajanahally S., Crowell K., Coward R.M. Undergoing varicocele repair before assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligospermic men with a varicocele: a systematic review and meta-analysis. Fertil Steril. 2016;106:1338–1343. doi: 10.1016/j.fertnstert.2016.07.1093. [DOI] [PubMed] [Google Scholar]
- 43.Dubin J.M., Greer A.B., Kohn T.P., Masterson T.A., Ji L., Ramasamy R. Men with severe oligospermia appear to benefit from varicocele repair: a cost-effectiveness analysis of assisted reproductive technology. Urology. 2018;111:99–103. doi: 10.1016/j.urology.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Kohn T.P., Kohn J.R., Pastuszak A.W. Varicocelectomy before assisted reproductive technology: are outcomes improved? Fertil Steril. 2017;108:385–391. doi: 10.1016/j.fertnstert.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 45.Schlegel P.N. Is assisted reproduction the optimal treatment for varicocele-associated male infertility? A cost-effectiveness analysis. Urology. 1997;49:83–90. doi: 10.1016/S0090-4295(96)00379-2. [DOI] [PubMed] [Google Scholar]
- 46.Kim J. Surgical managements versus artificial reproductive technology in male infertility: cost effectiveness in Korea. Clin Exp Reprod Med. 2013;40:30–35. [Google Scholar]
- 47.Meng M.V., Greene K.L., Turek P.J. Surgery or assisted reproduction? A decision analysis of treatment costs in male infertility. J Urol. 2005;174:1926–1931. doi: 10.1097/01.ju.0000176736.74328.1a. [DOI] [PubMed] [Google Scholar]
- 48.Lee R., Li P.S., Goldstein M., Schattman G., Schlegel P.N. A decision analysis of treatments for nonobstructive azoospermia associated with varicocele. Fertil Steril. 2009;92:188–196. doi: 10.1016/j.fertnstert.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 49.Penson D.F., Paltiel D.A., Krumholz H.M., Palter S. The cost-effectiveness of treatment for varicocele related infertility. J Urol. 2002;168:2490–2494. doi: 10.1016/S0022-5347(05)64175-4. [DOI] [PubMed] [Google Scholar]