Abstract
Background. Little is known about the health risks of air pollution and cardiorespiratory diseases, globally, across regions and populations, which may differ because of external factors.
Objectives. We systematically reviewed the evidence on the association between air pollution and cardiorespiratory diseases (hospital admissions and mortality), including variability by energy, transportation, socioeconomic status, and air quality.
Search Methods. We conducted a literature search (PubMed and Web of Science) for studies published between 2006 and May 11, 2016.
Selection Criteria. We included studies if they met all of the following criteria: (1) considered at least 1 of these air pollutants: carbon monoxide, sulfur dioxide, nitrogen dioxide, ozone, or particulate matter (PM2.5 or PM10); (2) reported risk for hospital admissions, mortality, or both; (3) presented individual results for respiratory diseases, cardiovascular diseases, or both; (4) considered the age groups younger than 5 years, older than 65 years, or all ages; and (5) did not segregate the analysis by gender.
Data Collection and Analysis. We extracted data from each study, including location, health outcome, and risk estimates. We performed a meta-analysis to estimate the overall effect and to account for both within- and between-study heterogeneity. Then, we applied a model selection (least absolute shrinkage and selection operator) to assess the modifier variables, and, lastly, we performed meta-regression analyses to evaluate the modifier variables contributing to heterogeneity among studies.
Main Results. We assessed 2183 studies, of which we selected 529 for in-depth review, and 70 articles fulfilled our study inclusion criteria. The 70 studies selected for meta-analysis encompass more than 30 million events across 28 countries. We found positive associations between cardiorespiratory diseases and different air pollutants. For example, when we considered only the association between PM2.5 and respiratory diseases (Figure 1, we observed a risk equal to 2.7% (95% confidence interval = 0.9%, 7.7%). Our results showed statistical significance in the test of moderators for all pollutants, suggesting that the modifier variables influence the average cardiorespiratory disease risk and may explain the varying effects of air pollution.
Conclusions. Variables related to aspects of energy, transportation, and socioeconomic status may explain the varying effect size of the association between air pollution and cardiorespiratory diseases.
Public Health Implications. Our study provides a transferable model to estimate the health effects of air pollutants to support the creation of environmental health public policies for national and international intervention.
PLAIN-LANGUAGE SUMMARY
We assessed the global association of air pollution and cardiorespiratory diseases by summarizing the available evidence from the literature, and investigated whether variables representing different aspects of energy, transportation, and socioeconomic factors could explain the varying effects of this association. Our results showed that clean electricity production, consumption of biofuels, and urban population account for 69% of the heterogeneity of the exposure to particulate matter of 2.5 micrometers or less (PM2.5; hospital admissions). For mortality attributable to PM2.5 exposure, clean electricity, consumption of motor gasoline, consumption of cooking fuel, population density, and education accounted for 64% of the heterogeneity.
Cardiorespiratory diseases are an escalating public health issue worldwide.1,2 The World Health Organization (WHO) estimates that in 2012 cardiovascular and respiratory diseases were responsible for 17.5 million and 4 million deaths globally, respectively.3 Extensive evidence indicates that environmental factors are associated with several adverse health effects, including cardiorespiratory disease risk.4–8
Air pollution is a major environmental health risk. In 2012, 7 million deaths all-cause and all-age worldwide were linked to ambient air pollution.9 Particulate matter of 2.5 micrometers or less (PM2.5) contributes to approximately 2 million premature deaths per year, ranking it as the 13th leading cause of worldwide mortality.10 The WHO estimates that 80% of outdoor air pollution–related premature deaths are associated with ischemic heart disease and strokes, 14% with chronic obstructive pulmonary disease and acute lower respiratory infections, and 6% with lung cancer.11 Even low levels of air pollutants have been associated with increases in cardiorespiratory diseases.12
Governments and health organizations have implemented regulations to reduce air pollution levels to protect human health.13–16 The WHO assessed air pollution data from more than 3000 cities worldwide and found that half of the cities in high-income countries and one third of those in low- and middle-income countries reduced air pollution levels by more than 5% between 2011 and 2016. All of these cities have addressed air pollution with the implementation of policies in sectors such as transport, energy, or urban planning.17 For example, efforts taken by cities include prioritizing rapid urban transit and fuels with reduced emissions,6,18,19 use of renewable energy sources,16 and land use management.20,21
Although several studies have examined the relationship between air pollution and cardiorespiratory diseases, less is known about the health risks across regions and populations from a global perspective. Varying health responses across individuals may be influenced by external factors, such as socioeconomic status and air pollution sources (e.g., transportation, energy generation). These factors are also called effect modifiers of the air pollution–health relationship.22 Understanding the global health risk can provide the knowledge for implementing regulations and improving urban air quality. Therefore, we systematically reviewed the evidence on the association between air pollution and cardiorespiratory diseases and investigated the effect modifiers of the air pollution–health relationship by considering 4 groups of variables: (1) energy, (2) transportation, (3) socioeconomic factors, and (4) air quality control variables. This study improves the regional transferability of health effect estimates by controlling for modifying variables, which should allow more effective air pollution policies and standards.
METHODS
We performed this study in 5 stages. First, we systematically reviewed the literature to identify research on the association between air pollution and cardiorespiratory diseases. In the second stage, we performed a meta-analysis to estimate the overall effect of air pollution on the composite outcome of any cardiovascular or respiratory disease (hospital admissions and mortality) and to account for both within- and between-study heterogeneity. In the third stage, we created a data set with potential modifier variables on the association between air pollution and cardiorespiratory diseases. Then we applied a model selection with regression shrinkage to identity the modifier variables that may be associated with cardiorespiratory diseases and, finally, we performed meta-regression analyses to evaluate the modifier variables that contribute to heterogeneity among studies.
Search Strategy, Selection Criteria, and Data Extraction
We searched PubMed and Web of Science by using the following keywords: “air pollution,” “cardiovascular diseases,” “respiratory diseases,” “mortality,” and “hospital admissions.” We limited our search to studies published between 2006 and May 11, 2016 (to access modifier variables), and to studies in English, Portuguese, or Spanish. We considered peer-reviewed original articles only. In Appendix A (available as a supplement to the online version of this article at http://www.ajph.org), we present the full search criteria and the number of studies identified, excluded, and included from our search.
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, 2 of the authors (W. R. and M. A.) first independently reviewed article titles and abstracts. They based the final inclusion of studies on full-text evaluation. In case of disagreement, a third researcher (A. A.) resolved any discrepancies.
We included studies if they met all of the following criteria:
considered at least 1 of these air pollutants: carbon monoxide (CO), sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3), particulate matter 2.5 micrometers or less (PM2.5), or particulate matter 10 micrometers or less (PM10);
reported risk for hospital admissions, mortality, or both;
presented individual results for respiratory diseases (International Classification of Diseases, Ninth Revision [ICD-9] 460–519 or ICD-10 J00–J98), cardiovascular diseases (ICD-9 390–459 or ICD-10 I00–I99), or both23,24;
considered the age groups younger than 5 years, older than 65 years, or all ages; and
did not segregate the analysis by gender.
Finally, we selected significant results from a single pollutant model and with lag 0.
We extracted the following data from each study included in our analysis: first author, publication year, period of analysis, pollutant (CO, SO2, NO2, O3, PM2.5, or PM10), health outcome (mortality or hospital admissions), disease (respiratory or cardiovascular), age (< 5 years, > 65 years, or all ages), location (city and country), study design (time–series, case–crossover, or study cohort), number of events (when not stated in the paper, we estimated from mean daily values and the study period), risk estimates, measure of uncertainty (95% confidence interval [CI]), and the confounders included in each study (e.g., temperature, humidity, season). We contacted authors for additional data or clarification when necessary.
With regard to the location, we included both single-city and multicity studies. However, we included city-specific results when a single study reported results from multiple cities. With regard to O3, we converted results from 1-hour and 8-hour maximum to the daily average. We converted this value by using a relationship of 20 (1-hour maximum):15 (8-hour maximum):8 (daily average). This approach has been used in previous studies.25
We expressed all risk estimates as the percentage change in the number of health outcomes associated with every 10 micrograms per cubic meter change in pollutant concentration. We converted results reported in parts per billion or parts per million to micrograms per cubic meter assuming the standard pressure and temperature conditions. We standardized results on the basis of different values from 10 micrograms per cubic meter (e.g., interquantile range).
Meta-Analysis
First, we calculated the effect size and variance, which is needed as input data for the meta-analysis. Because all risk estimates were based on a Poisson or logistic regression, we calculated the effect size converting the risks to log scale. We calculated the variance (Vi) on the basis of the CI’s upper (A), and lower (B) bounds:
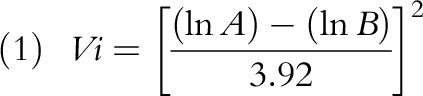 |
We used a random-effects model to perform the meta-analysis. This model assumes that average effect size in the population varies randomly from study to study.26–28 The random-effects model assumes that
where 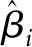 is the estimated effect size; i is the study included in the analysis, which is equal to 1,2,3, …, n, where n is the number of the enrolled studies;
is the estimated effect size; i is the study included in the analysis, which is equal to 1,2,3, …, n, where n is the number of the enrolled studies; 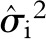 is the estimated variance; β is the overall effect; τi is the mean effect at the study level; τ2 is the variability of τi around Β (between-study variance); and εi and ui are random error normally distributed and mutually independent.
is the estimated variance; β is the overall effect; τi is the mean effect at the study level; τ2 is the variability of τi around Β (between-study variance); and εi and ui are random error normally distributed and mutually independent.
We assessed heterogeneity with the I2 test, in which I2 values of 25%, 50%, and 75% correspond to cut-off points for low, moderate, and high degrees of heterogeneity.29 We assessed the publication bias by using Egger’s regression test and normal quantile plot.29,30 We performed analyses in R version 3.3.0 (R Foundation, Vienna, Austria).
Modifier Variables
We created a data set with 14 potential modifier variables on the association between air pollution and cardiorespiratory diseases. We grouped the modifier variables into 4 categories. The first category was energy use, which included measurements of clean electricity production, dirty electricity production, and total electricity net consumption. The second category was transportation, which included the consumption of biofuels, consumption of distillate fuel oil, consumption of motor gasoline, and motor vehicles. The third category, socioeconomic status indicators, included population density, urban population, gross domestic product, and education. The last category included air quality control variables: CO2 emissions, prevalence of smoking, and solid fuels as main cooking fuel.
We calculated these variables at the national level and their period coincided with the study period applied in each applicable research paper. In Appendix B (available as a supplement to the online version of this article at http://www.ajph.org), we present the details of each variable, including the units and the data source.
Statistical Analysis
First we assessed the association between 14 modifier variables and cardiorespiratory diseases by using a model selection with regression shrinkage. Then we performed a meta-regression analysis to evaluate the significant modifier variables (indicated by the model selection) that contribute to heterogeneity among studies.
Selecting predictors from a large collection of possible covariates is a difficult process. Many methods (e.g., stepwise) are empirical and ignore stochastic errors during the statistical modeling.31 To overcome these potential issues, we applied the least absolute shrinkage and selection operator (LASSO) to select the modifier variables that may be associated with cardiorespiratory diseases.
LASSO is an approach to select a parsimonious set of variables and estimate coefficients simultaneously. The LASSO approach performs variable selection by constraining the sum of the magnitudes of the coefficients and applying a penalty to the component regression coefficients. The penalty is an estimator that minimizes the sum of squared errors subject to the sum of the absolute values of the regression coefficients to be less than a fixed value.31,32 Briefly, LASSO coefficient 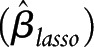 is expressed as:
is expressed as:
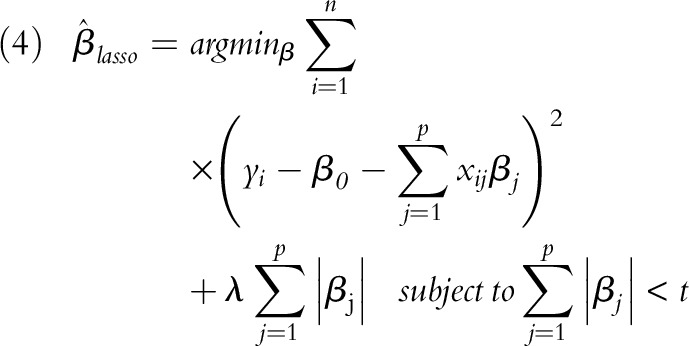 |
where n is the observations (number of studies), p is the covariate (total of 14), yi is the outcome (cardiorespiratory disease risk), x is the covariate vector for the ith case, t is the parameter that determines the amount of regularization, and λ is the penalty parameter.
We selected the modifier variables with non-0 coefficients and we chose the λ having the smallest Bayesian information criterion. Then we used a meta-regression (mixed effects model) to explore where variables representing different aspects of energy, transportation, socioeconomic status, and air quality control variables could explain the varying effects of air pollution on cardiorespiratory diseases. We performed LASSO and meta-regression analyses in R version 3.3.0.
RESULTS
We assessed the title and abstract of 2183 studies. From those studies we selected 529 for text review from which 70 articles fulfilled the inclusion criteria. In Figure A (available as a supplement to the online version of this article at http://www.ajph.org), we present a flowchart that shows the number of studies identified, included, and excluded from analysis. In Appendix C (available as a supplement to the online version of this article at http://www.ajph.org), we present the details of the 70 articles including the bibliographic information, the period of study, pollutant(s) observed, health outcome measure, disease, age categories, city, country, and study design for the articles that met the inclusion criteria.
Two studies reported results for CO, 23 for NO2, 7 for O3, 36 for PM10, 28 for PM2.5, and 14 for SO2. With regard to the health outcome, 34 studies evaluated hospital admissions and 39 mortality. For type of disease, 53 studies estimated cardiovascular diseases and 46 respiratory diseases. Regarding age groups, 5 studies showed results for people aged younger than 5 years, 26 for people aged older than 65 years, and 55 for all ages. For study design, 26 studies are case–crossover, 9 cohort, 34 time series, and 1 case–control (Table B, available as a supplement to the online version of this article at http://www.ajph.org). Most of the studies reported results for more than 1 category of pollutant, health outcome, disease, or age. The 70 studies selected for meta-analysis incorporated more than 30 million events across 28 countries.
Meta-Analysis
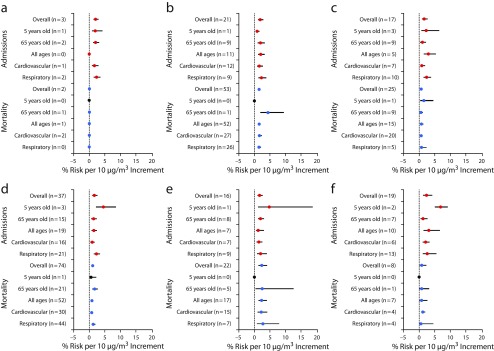
In Appendix D (available as a supplement to the online version of this article at http://www.ajph.org) we report forest plots for each pollutant by health outcome, and in Appendix E (available as a supplement to the online version of this article at http://www.ajph.org) we present the pooled risk by location. Figure 2 shows the pooled risk by pollutant, age group, and health outcome. The overall risk estimated for hospital admissions was larger than mortality for all pollutants, except PM2.5.
FIGURE 2—
Global Association Between Air Pollution and Health Stratified by Age, Health Outcome, and Diseases, for (a) Carbon Monoxide, (b) Nitrogen Dioxide, (c) Ozone, (d) PM10, (e) PM2.5, and (f) Sulphur Dioxide: Studies Published Between 2006 and May 11, 2016
Note. PM10 = fine particulate matter of ≤ 10 µm; PM2.5 = fine particulate matter of ≤ 2.5 µm. Because most of the studies reported results for more than 1 category of pollutant, health outcome, disease, or age, “n” here represents the number of reports, and not the number of studies. Red circle = hospital admissions; blue circle = mortality; black circle = there is no report; whiskers = 95% confidence interval.
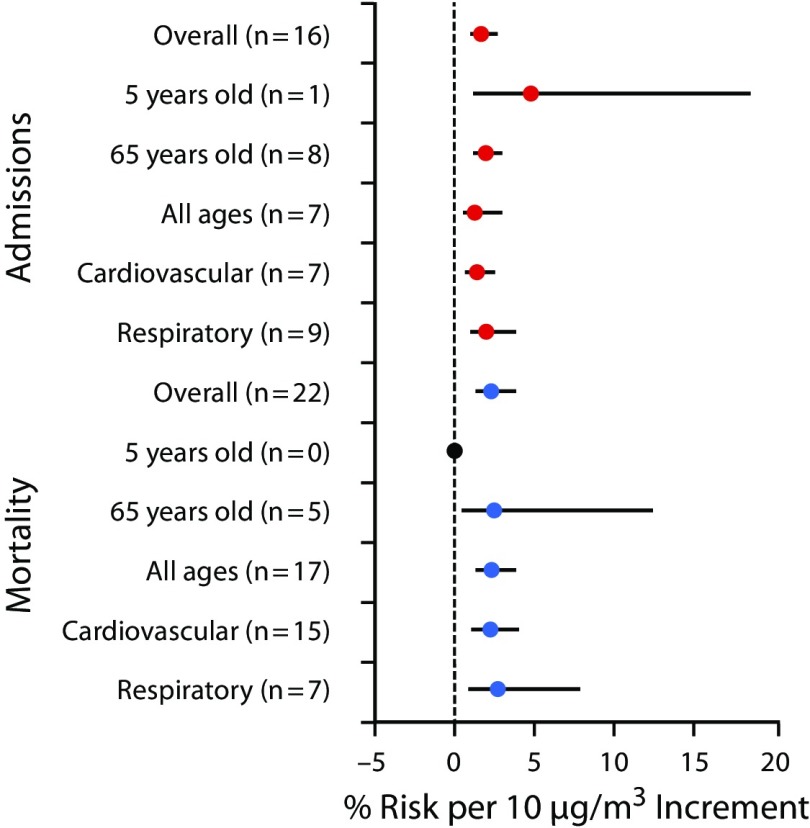
FIGURE 1—
Global Association Between Air Pollution (Fine Particulate Matter ≤ 2.5 µm) and Health
With regard to hospital admissions, the youngest age group (aged < 5 years) demonstrated the highest risk across all pollutants, except NO2 and CO. Respiratory diseases showed the strongest association, especially for O3 and PM10, for which we found a risk equal to 2.4% (95% CI = 1.6%, 3.7%) and 2.3% (95% CI = 1.6%, 3.2%), respectively (Figure 2).
For mortality, the oldest age group (aged > 65 years) showed the highest risk for PM10, whereas the youngest age group demonstrated the highest risk for O3. There are no studies reporting association between children (aged < 5 years) and the pollutants PM2.5 and SO2. Respiratory diseases showed the highest risk for PM10 (1.3%; 95% CI = 0.9%, 1.7%), PM2.5 (2.7%; 95% CI = 0.9%, 7.7%), and O3 (0.8%; 95% CI = 0.2%, 2.3%), whereas cardiovascular diseases demonstrated highest risk for SO2 (1.1%; 95% CI = 0.8%, 1.6%) and NO2 (1.6%; 95% CI = 1.2%, 2.2%). There is no report presenting association between respiratory diseases and CO (Figure 2).
In Appendix F (available as a supplement to the online version of this article at http://www.ajph.org) we report I2 test and Egger’s regression test results stratified by pollutant, age, and disease. When we considered the overall risk for hospital admissions, we observed high heterogeneity between studies for CO, PM10, PM2.5, and SO2 (I2 > 75%), and moderate for NO2 and O3 (I2 = 50%, 75%). Publication bias (Egger’s test for asymmetry) was significant for O3 (P = .005), PM10 (P = .033), and PM2.5 (P = .038), and nonsignificant for NO2 (P = .054), CO (P = .791), and SO2 (P = .339).
When we observed the overall risk for mortality (Table D, available as a supplement to the online version of this article at http://www.ajph.org), we found high heterogeneity for PM10, PM2.5, and SO2 (I2 > 75%), and moderate for NO2 and O3 (I2 = 50%, 71%). Publication bias was significant only for O3 (P = .048). For CO, the number of reports is very low (n = 2), to which it is not possible to apply I2 test and Egger’s regression test.
In Appendix J (available as a supplement to the online version of this article at http://www.ajph.org) we include a normal quantile plot for overall risk by pollutants, and health outcome (hospital admissions and mortality), which can help to identify whether all studies come from a single population (search for publication bias as well).
Effect Modifiers
We present in Appendix G (available as a supplement to the online version of this article at http://www.ajph.org) the LASSO coefficient paths for each pollutant, except for CO because the number of observations is low (n = 5). Coefficients are expressed as the change in mean cardiorespiratory diseases per variation in the modifier. Each plot in Figure D indicates the rate at which the modifier’s coefficient shrinks toward 0 as λ increases.
When λ is equal to 0, there is no shrinkage, and the model is just the ordinary mixed effect regression of the fixed modifiers. Our results showed that when λ is equal to 0, all modifiers have non-0 coefficients (Figure D, available as a supplement to the online version of this article at http://www.ajph.org).
We selected the modifier variables with non-0 coefficients at the λ with the smallest Bayesian information criterion, and then we performed the meta-regression analysis. The modifiers selected from each pollutant are presented in Appendix H (available as a supplement to the online version of this article at http://www.ajph.org). Clean electricity production was the most important modifier; it was selected for all pollutants except SO2 and PM10 (when we considered hospital admissions) and O3 (when we considered mortality). We did not apply meta-regression to the age group and disease group because of few observations.
In the meta-regression analysis, we observed a statistically significant effect (P < .05) in the test of moderators in all pollutants, suggesting that the modifier variables influence the average cardiorespiratory disease risk and may explain the varying effects of air pollution among studies. When we considered hospital admissions, the modifiers contribute to high heterogeneity between studies for NO2 (I2 = 94%) and SO2 (I2 = 92%); moderate heterogeneity for O3 (I2 = 59%) and PM2.5 (I2 = 69%); and low heterogeneity for PM10 (I2 = 44%). When we observed only mortality, we found high heterogeneity for NO2 (I2 = 96%), O3 (I2 = 78%), and SO2 (I2 = 96%), and moderate heterogeneity for PM10 (I2 = 54%) and PM2.5 (I2 = 64%; Figure E, available as a supplement to the online version of this article at http://www.ajph.org).
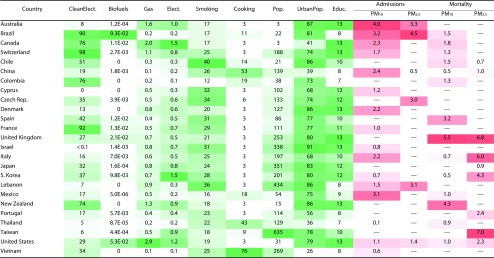
We plotted the modifier variables with significant coefficients (P < .05) in a global map (Appendix I, available as a supplement to the online version of this article at http://www.ajph.org). In addition, we show in this global map the cardiorespiratory diseases risk attributable to exposure to gaseous and particulate air pollutants. We present in Figure 3 these modifier variables and the cardiorespiratory disease risk associated with particulate emissions.
FIGURE 3—
Cardiorespiratory Disease Risk (%) Attributable to Exposure to Particulate Air Pollutants and Distribution of Significant Modifier Variables by Country, in Studies Published Between 2006 and May 11, 2016
Note. Biofuels = consumption of biofuels, 1000 barrels per day/100 000 people; Clean Elect. = clean electricity production, %; Cooking = percentage of population using solid fuels as main cooking fuel; Educ. = education, mean years of schooling; Elect. = total electricity net consumption, annual billion kWh per 100 000 people; Gas = consumption of motor gasoline, 1000 barrels per day/100 000 people; PM10 = fine particulate matter of ≤ 10 µm; PM2.5 = fine particulate matter of ≤ 2.5 µm; Pop. = population density; Smoking = prevalence of smoking, %; Urban Pop. = urban population, %. We selected the modifier variables shown here on the basis of the modifiers that presented significant coefficients (Appendix H, available as a supplement to the online version of this article at http://www.ajph.org). We calculated the mean value for each modifier variable by considering the study period stated in the literature. According to our selection criteria, there are no studies reporting risk for the specific particulate and country (—). Green color scheme represents the range values of each modifier variable, for which dark green is the highest value. Red color scheme represents the range values of each health variable (cardiorespiratory disease risk), for which dark red is the highest value.
DISCUSSION
We assessed the global association of air pollution and cardiorespiratory diseases by summarizing the available evidence from the literature, and investigated whether variables representing different aspects of energy, transportation, and socioeconomic factors could explain the varying effects of this association. We found positive associations between cardiorespiratory diseases and all air pollutants.
Other studies have reported global associations between air pollution and health. Song et al.33 showed that each increment of 10 micrograms per cubic meter of PM10 was associated with an increase in obstructive pulmonary disease mortality in China, United States, and the European Union. Shah et al.34 performed a systematic review and meta-analysis to assess the global association between air pollution and heart failure. The authors found that heart failure was associated with increases in CO, SO2, NO2, PM10, PM2.5, but not O3. Atkinson et al.35 showed that a 10 microgram per cubic meter increment in PM2.5 was associated with a 1.04% (95% CI = 0.52%, 1.56%) increase in the risk of death for cardiorespiratory diseases. In our study, we found a 1.64% (95% CI = 1.06%, 2.53%) increase in hospital admissions attributable to cardiorespiratory diseases per 10 microgram per cubic meter increment in PM2.5. With regard to mortality, we found a 2.29% (95% CI = 1.36%, 3.85%) increase in cardiorespiratory diseases per 10 microgram per cubic meter increment in PM2.5. Atkinson et al. also showed that associations for respiratory causes of death were larger than for cardiovascular causes. We found the same effect in our study.
Globally, we found the effect of air pollution on health outcomes to spatially vary across the 28 countries assessed. The highest risks occurred in Brazil (CO, hospital admissions; PM10, hospital admissions), China (CO, mortality), United States (NO2, mortality; O3, mortality), India (SO2 and O3, hospital admissions), United Kingdom (PM10, mortality), and Taiwan (PM2.5, mortality). Our results are in accordance with a global burden study that assessed the ambient air pollution exposure and reported that exposure to PM2.5 increased by 20.4% driven by trends in South Asia (e.g., Taiwan).36 In India, the exposure to O3 increased by 20.2% between 1990 and 2013. Lelieveld et al.37 identified that the United States population has an elevated exposure to NO2, for which the emission sources are primarily from traffic and power generation. We identified no studies that provide a global perspective on the health effects from CO, SO2, and PM10 exposure.
Our findings showed that variables related to aspects of energy, transportation, and socioeconomic status may explain the varying effect size of the association between air pollution and cardiorespiratory diseases. These modifier effects agree with previous localized studies. Our results showed that clean electricity production, consumption of biofuels, and urban population are significant modifier variables that account for 69% of the heterogeneity of the PM2.5 exposure (hospital admissions). When we considered mortality attributable to PM2.5 exposure, the significant modifier variables were clean electricity, consumption of motor gasoline, cooking fuel, population density, and education. These variables accounted for 64% of the heterogeneity.
In a rural Chinese population, energy generation and electric vehicle adoption were strongly associated with the variation in the exposure to PM2.5 resulting in a varied geographic distribution of health impacts.38 In Santiago, Chile, fuel consumption was a significant factor to understand PM2.5 trends and to inform air quality control efforts.39 Lelieveld et al.37 found that emissions from residential energy use such as heating and cooking contribute significantly to PM2.5, which is the largest driver for global premature mortality. Villeneuve et al.40 also found that cigarette smoking and sociodemographic characteristics are associated with ambient PM2.5 and mortality in Canada.
In our study, we observed that clean electricity production, consumption of motor gasoline, total electricity consumption, and smoking account for 59% of the heterogeneity of the O3 health outcomes (hospital admissions). When we considered mortality attributable to O3 exposure, the significant modifier variables were consumption of motor gasoline, total electricity consumption, population density, and education, which accounted for 78% of the heterogeneity. Razeghi et al.41 examined the association between power generation, transportation, and air quality in the United States, and they found that O3 and PM2.5 decreased with the introduction of wind energy to the grid mix and the use of plug-in electric vehicles. Tessum et al.42 evaluated air quality (PM2.5 and O3)–related human health impacts of fuel type including the use of electric vehicles, and the use of electricity from a range of conventional and renewable sources. The authors found that the health impacts varied by 80% depending on the use of fuel and electricity source. Electric vehicles powered by clean electricity (wind, water, or solar power) reduced environmental health impacts by at least 50%.
Our findings showed that significant modifier variables related to energy, transportation (e.g., fuel type, including biofuels, and consumption of distillate fuel oil), and socioeconomic factors contribute to 94% for heterogeneity of the NO2 exposure when one considers hospital admissions, and 96% when one considers mortality. Jacobson43 estimated that both gasoline and ethanol combustion are anticipated to cause at least 10 000 premature deaths in the United States in 2020. Jacobson projected that in Los Angeles, California, emissions from gasoline cars will contribute to 68 900 tons of NO2 in 2020. Tsao et al.44 identified a significant association between NOx emissions and biofuels (sugar-cane ethanol) in Brazil. Pinault et al.45 observed that socioeconomic aspects are important to assess differences in exposure to NO2 in 3 Canadian cities: Toronto, Montreal, and Vancouver.
Strengths and Limitations
The main strengths of our study are as follows: First, our findings add to the evidence that, from an international perspective, there is important heterogeneity in the effects of air pollution on cardiorespiratory diseases. Second, to our knowledge, this is the first study that estimated effects from energy, transportation, and socioeconomic characteristics on human exposure to air pollutants from a global perspective. Third, we applied the LASSO approach to investigate the global relationship between air pollution and cardiorespiratory diseases. This method has advantages over other conventional methods in linear regression because they are empirical and ignore stochastic errors, which may reduce power because of collinearity among components.
On the other hand, limitations to our study include that we did not quantify temporal effects of air pollution. We only assessed studies in English, Portuguese, and Spanish. This may increase the probability of publication bias. However, we must consider that most of the studies are in English. We have not considered the phenotype of pre-existing cardiorespiratory diseases, because these data are not available. The risk estimated by our study may be greater in patients with pre-existing cardiorespiratory diseases.46 We did not consider personal exposures and therefore we may underestimate the risk.47,48 We have considered only single pollutants, which does not take into consideration potential additive effects of multiple pollutants.49 Although mortality is often used as surrogate for incidence, we must be cautious about using mortality, especially for cardiorespiratory disease, as it may not be well-reported on death certificates. We observed significant heterogeneity in our analyses. This could indicate differences in ambient air pollution, population characteristics, and environmental exposure. However, pooled risk presented consistency and the effect direction was not altered in our additional analyses. The modifiers refer to the country level. We were unable to access modifier data at the local level from each study (most of the studies reported results at the city level). Finally, to interpret our results, we have to consider the residual confounding and the ecological fallacy.
Conclusions
The association of air pollution and cardiorespiratory diseases is a global concern. Hospitalizations and mortality attributable to this association have serious economic consequences. Factors related to energy, transportation, and socioeconomic conditions are important variables that explain the varying effects of air pollution on cardiorespiratory diseases. The findings from our study provide a mechanism to estimate the health effects of air pollutants in cities globally while controlling for modifier effects. The model presented in the article can be used to provide support for the creation of effective environmental health public policies for national and international intervention.
ACKNOWLEDGMENTS
This work was supported by the Social Sciences and Humanities Research Council of Canada (grant 886-2013-0001) and by the US Environmental Protection Agency (grants RD-834798 and RD-835872).
Note: The contents of this report are solely the responsibility of the grantee and do not necessarily represent the official views of the US Environmental Protection Agency. Furthermore, the agency does not endorse the purchase of any commercial products or services mentioned in the publication.
HUMAN PARTICIPANT PROTECTION
Human participant protection was not required because human participants were not involved.
REFERENCES
- 1.Laumbach RJ, Kipen HM. Respiratory health effects of air pollution: update on biomass smoke and traffic pollution. J Allergy Clin Immunol. 2012;129(1):3–11. doi: 10.1016/j.jaci.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newby DE, Mannucci PM, Tell GS et al. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36(2):83–93b. doi: 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Health Observatory. 2014. Available at: http://www.who.int/gho/ncd/mortality_morbidity/en. Accessed June 10, 2014.
- 4.Branis M, Linhartova M. Association between unemployment, income, education level, population size and air pollution in Czech cities: evidence for environmental inequality? A pilot national scale analysis. Health Place. 2012;8(5):1100–1104. doi: 10.1016/j.healthplace.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Makri A, Stilianakis N. Vulnerability to air pollution health effects. Int J Hyg Environ Health. 2008;211(3-4):326–336. doi: 10.1016/j.ijheh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Ji S, Cherry CR, Bechle MJ, Wu Y, Marshall JD. Electric vehicles in China: emissions and health impacts. Environ Sci Technol. 2012;46(4):2018–2024. doi: 10.1021/es202347q. [DOI] [PubMed] [Google Scholar]
- 7.Réquia Júnior WJ, Roig HL, Koutrakis P. A novel land use approach for assessment of human health: the relationship between urban structure types and cardiorespiratory disease risk. Environ Int. 2015;85:334–342. doi: 10.1016/j.envint.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 8.van den Berg AE, Maas J, Verheij RA, Groenewegen PP. Green space as a buffer between stressful life events and health. Soc Sci Med. 2010;70(8):1203–1210. doi: 10.1016/j.socscimed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. 7 million premature deaths annually linked to air pollution. 2014. Available at: http://www.who.int/mediacentre/news/releases/2014/air-pollution/en. Accessed April 20, 2016. [PubMed]
- 10.Lozano R, Naghavi M, Foreman K et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010 [erratum: Lancet. 2013 doi: 10.1016/S0140-6736(12)61728-0. 381(9867):628]. Lancet. 2012;380(9859):2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Ambient (outdoor) air quality and health. 2014. Available at: http://www.who.int/mediacentre/factsheets/fs313/en. Accessed June 2, 2016.
- 12.Schwartz J, Bind M-A, Koutrakis P. Estimating causal effects of local air pollution on daily deaths: effect of low levels. Environ Health Perspect. 2017;125(1):23–29. doi: 10.1289/EHP232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Williams G, Guo Y. Health benefits from improved outdoor air quality and intervention in China. Environ Pollut. 2016;214:17–25. doi: 10.1016/j.envpol.2016.03.066. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Wang S, Hao J et al. Air pollution and control action in Beijing. J Clean Prod. 2015;112:1519–1527. [Google Scholar]
- 15.Feng L, Liao W. Legislation, plans, and policies for prevention and control of air pollution in China: achievements, challenges, and improvements. J Clean Prod. 2014;112:1549–1558. [Google Scholar]
- 16.Zheng S, Yi H, Li H. The impacts of provincial energy and environmental policies on air pollution control in China. Renew Sustain Energy Rev. 2015;49:386–394. [Google Scholar]
- 17.World Health Organizaiton. Air pollution levels rising in many of the world’s poorest cities. 2016. Available at: http://www.who.int/mediacentre/news/releases/2016/air-pollution-rising/en. Accessed June 2, 2016.
- 18.Buekers J, Van Holderbeke M, Bierkens J, Int Panis L. Health and environmental benefits related to electric vehicle introduction in EU countries. Transp Res Part D Transp Environ. 2014;33(February):26–38. [Google Scholar]
- 19.Krause R, Lane B, Carley S, Graham JD. Assessing demand by urban consumers for plug-in electric vehicles under future cost and technological scenarios. Int J Sustain Transport. 2016;8318(March):742–751. [Google Scholar]
- 20.Fraser I, Stevens C. Nitrogen deposition and loss of biological diversity: agricultural land retirement as a policy response. Land Use Policy. 2008;25(4):455–463. [Google Scholar]
- 21.Barton H. Land use planning and health and well-being. Land Use Policy. 2009;26(suppl 1):115–123. [Google Scholar]
- 22.Bell ML, Zanobetti A, Dominici F. Who is more affected by ozone pollution? A systematic review and meta-analysis. Am J Epidemiol. 2014;180(1):15–28. doi: 10.1093/aje/kwu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Classification of Diseases, Ninth Revision. Geneva, Switzerland: World Health Organization; 1980. [Google Scholar]
- 24.International Classification of Diseases, Tenth Revision. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 25.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the National Morbidity, Mortality, and Air Pollution Study. Epidemiology. 2005;16(4):436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viechbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 27.Hedges LV, Vevea JL. Fixed and random effects models in meta-analysis. Psychol Methods. 1998;3(4):486–504. [Google Scholar]
- 28.Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care. 1990;6(1):5–30. doi: 10.1017/s0266462300008916. [DOI] [PubMed] [Google Scholar]
- 29.Field AP, Gillett R. How to do a meta-analysis. Br J Math Stat Psychol. 2010;63(pt 3):665–694. doi: 10.1348/000711010X502733. [DOI] [PubMed] [Google Scholar]
- 30.Wang MC, Bushman BJ. Using the normal quantile plot to explore meta-analytic data sets. Psychol Methods. 1998;3(1):46–54. [Google Scholar]
- 31.Fan Jianqing LR. Variable selection via nonconcave penalized and its orable properties. J Am Stat Assoc. 2001;96(456):1348–1360. [Google Scholar]
- 32.Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regressions, and Survival Analysis. 1st ed. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 33.Song Q, Christiani DC, XiaorongWang, Ren J. The global contribution of outdoor air pollution to the incidence, prevalence, mortality and hospital admission for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Environ Res Public Health. 2014;11(11):11822–11832. doi: 10.3390/ijerph111111822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah AS, Langrish JP, Nair H et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382(9897):1039–1048. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014;69(7):660–665. doi: 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brauer M, Freedman G, Frostad J et al. Ambient air pollution exposure estimation for the Global Burden of Disease 2013. Environ Sci Technol. 2016;50(1):79–88. doi: 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- 37.Lelieveld J, Evans J, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 38.Ji S, Cherry CR, Zhou W et al. Environmental justice aspects of exposure to PM2.5 emissions from electric vehicle use in China. Environ Sci Technol. 2015;49(24):13912–13920. doi: 10.1021/acs.est.5b04927. [DOI] [PubMed] [Google Scholar]
- 39.Jhun I, Oyola P, Moreno F, Castillo MA, Koutrakis P. PM. 2.5 mass and species trends in Santiago, Chile, 1998 to 2010: the impact of fuel-related interventions and fuel sales. J Air Waste Manag Assoc. 2013;63(2):161–169. doi: 10.1080/10962247.2012.742027. [DOI] [PubMed] [Google Scholar]
- 40.Villeneuve PJ, Goldberg MS, Burnett RT, van Donkelaar A, Chen H, Martin RV. Associations between cigarette smoking, obesity, sociodemographic characteristics and remote-sensing-derived estimates of ambient PM2.5: results from a Canadian population-based survey. Occup Environ Med. 2011;68(12):920–927. doi: 10.1136/oem.2010.062521. [DOI] [PubMed] [Google Scholar]
- 41.Razeghi G, Carreras-Sospedra M, Brown T, Brouwer J, Dabdub D, Samuelsen S. Episodic air quality impacts of plug-in electric vehicles. Atmos Environ. 2016;137:90–100. [Google Scholar]
- 42.Tessum CW, Hill JD, Marshall JD. Life cycle air quality impacts of conventional and alternative light-duty transportation in the United States. Proc Natl Acad Sci U S A. 2014;111(52):18490–18495. doi: 10.1073/pnas.1406853111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobson MZ. Effects of ethanol (E85) versus gasoline vehicles on cancer and mortality in the United States. Environ Sci Technol. 2007;41(11):4150–4157. doi: 10.1021/es062085v. [DOI] [PubMed] [Google Scholar]
- 44.Tsao C-C, Campbell JE, Mena-Carrasco M, Spak SN, Carmichael GR, Chen Y. Increased estimates of air-pollution emissions from Brazilian sugar-cane ethanol. Nat Clim Chang. 2012;2(4):294. [Google Scholar]
- 45.Pinault L, Crouse D, Jerrett M, Brauer M, Tjepkema M. Spatial associations between socioeconomic groups and NO2 air pollution exposure within three large Canadian cities. Environ Res. 2016;147(2):373–382. doi: 10.1016/j.envres.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 46.Mills NL, Donaldson K, Hadoke PW et al. Adverse cardiovascular effects of air pollution. Nat Clin Pr Cardiovasc Med. 2009;6(1):36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- 47.Borgini A, Tittarelli A, Ricci C, Bertoldi M, De Saeger E, Crosignani P. Personal exposure to PM2.5 among high-school students in Milan and background measurements: the EuroLifeNet study. Atmos Environ. 2011;45(25):4147–4151. [Google Scholar]
- 48.Gerharz LE, Klemm O, Broich AV, Pebesma E. Spatio-temporal modelling of individual exposure to air pollution and its uncertainty. Atmos Environ. 2013;64(2):56–65. [Google Scholar]
- 49.Winquist A, Klein M, Tolbert P, Sarnat SE. Power estimation using simulations for air pollution time-series studies. Environ Health. 2012;11(1):68. doi: 10.1186/1476-069X-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]