Abstract
Objectives
To review the literature and provide an updated summary on the role of reactive oxygen species (ROS) in male infertility.
Methods
A review of PubMed, Cochrane review, and Web of Science databases for full-text English-language articles published between 1943 and 2017 was performed, focusing on the aetiology of ROS, physiological role of ROS on spermatic function, pathological role of ROS in infertility, evaluation of ROS, and role of antioxidants in oxidative stress.
Results
ROS play a role in spermatic function and fertilisation. The literature describes both a physiological and a pathological role of ROS in fertility. A delicate balance between ROS necessary for physiological activity and antioxidants to protect from cellular oxidative injury is essential for fertility.
Conclusion
Although elevated levels of ROS are implicated as a cause of infertility, there is no consensus on selecting patients to test for ROS, which test to perform, or if treatment for ROS can have a positive impact on infertility rates and pregnancy.
Abbreviations: 4-HNE, 4 hydroxy-nonenal; ATP, adenosine triphosphate; CAT, catalase; ESR, electron spin resonance; G-6-PDH, glucose-6-phosphate dehydrogenase; GPX, glutathione peroxidase; MAGI, male accessory gland infections; MDA, malondialdehyde; NADH, nicotinamide adenine dinucleotide; NO, nitric oxide; SOD, superoxide dismutase; ROS, reactive oxygen species
Keywords: Reactive oxygen species, Male infertility, Free radicals, Antioxidants
Introduction
Infertility affects up to 15% of the population globally [1]. Male infertility is the cause in about 20% of cases but may contribute to 40% of infertile couples [2]. Reactive oxygen species (ROS), as a potential contributor to male infertility, have been reported in the literature since the 1940s [3]. Oxidative stress leading to defective sperm function was demonstrated in early studies illustrating the toxic effect of endogenously generated hydrogen peroxide (H2O2) on sperm metabolism and motility [4]. There has been a vast improvement in understanding the effects of ROS on infertility and spermatic function since these early studies. The current literature reports that ROS may be a contributing factor in 30–80% of infertile men [5]. The present article reviews the literature for updates on the aetiology of ROS, the role of ROS on sperm development and function, ROS as part of infertility evaluation, available ROS testing, and the physiological role of antioxidants.
Methods
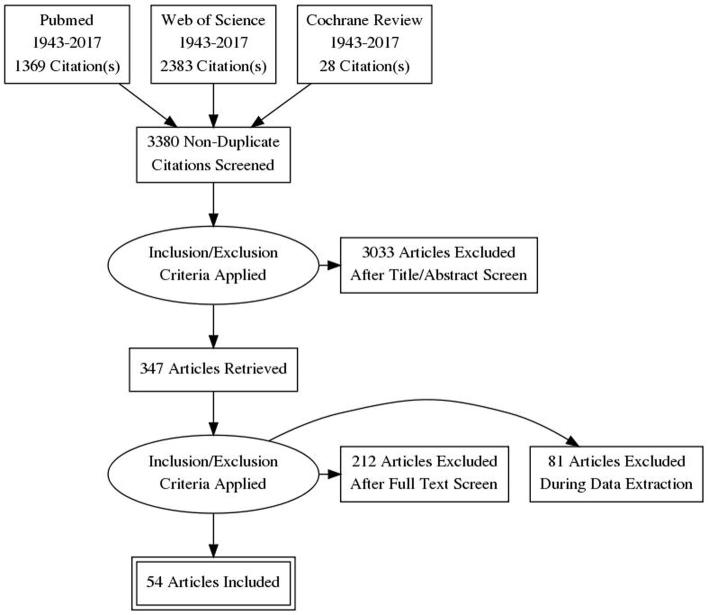
A search of PubMed, Cochrane review, and Web of Science databases for full-text English-language articles published between 1943 and 2017 was performed. Various combinations of the following search terms were used: ‘spermatozoa’, ‘male infertility’, ‘reactive oxygen species’, ‘oxidative stress’, ‘antioxidants’, ‘antioxidant paradox’, ‘seminal oxidative stress’, ‘peroxidative damage’, ‘apoptosis in sperm’, ‘sperm deoxyribonucleic acid (DNA) fragmentation’, ‘oxidative DNA damage’, and ‘infertility testing’. For the present review, we included original research studies and selected reviews. Emphasis was placed on studies addressing the following topics: aetiology of ROS, physiological role of ROS on spermatic function, pathological role of ROS in infertility, evaluation of ROS, and physiological role of antioxidants in oxidative stress. We excluded commentaries and case reports. In all, 3780 articles were identified. Eighty-three articles were excluded as they were written in a language other than English and 400 were found to be duplicates. A total of 3297 articles were reviewed and 2950 of these were excluded after review of the title or the abstract. Thus, 347 articles were screened, of these 293 were excluded as not applicable (Fig. 1). The reference lists of the 54 selected articles were also reviewed for relevant publications.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of article selection.
Biochemical review
A free radical is a molecule or element with an unpaired electron that is extremely reactive in an attempt to reach an electronically stable state. ROS are free radical derivatives of oxygen (O2) containing molecules. However, not all ROS are true free radicals. For example, H2O2 does not have an unpaired electron but is highly reactive. Some of the clinically important ROS identified in infertility include: peroxyl (•ROO−) and hydroxyl (•OH) radicals, superoxide (•O2−) anion, and H2O2 [6]. Although not technically a ROS, nitrogen compounds such as nitric oxide (NO) and peroxynitrite anion (ONOO−) also appear to play a role in oxidation and reduction reactions in infertility.
Oxidative stress describes when a system has an imbalance between oxidation and reduction reactions, leading to generation of excess oxidants or molecules that accept an electron from another reactant [6]. Common molecules that receive the unpaired electron are lipids in membranes and carbohydrates in nucleic acids [7]. This leads to potential cellular and DNA damage when ROS are greater than the antioxidant carrying capacity.
Intrinsic production of ROS
Aerobic metabolism in cells creates ROS as a by-product. The process of mitochondrial oxidative phosphorylation uses nicotinamide adenine dinucleotide (NADH) as an electron donor and O2 as an electron acceptor in the electron transport chain, coupling both reduction and oxidation reactions with the synthesis of adenosine triphosphate (ATP). These redox reactions occur in the mitochondria with 1–5% O2 consumed by aerobic metabolism transformed into a ROS as a by-product [8]. Another intrinsic source of spermatic ROS production is cytoplasmic glucose-6-phosphate dehydrogenase (G-6-PDH). This cytoplasmic source of ROS may explain why increased spermatic cytoplasm could be linked to infertility [9].
Spermatic leucocytes are another intrinsic source of ROS, producing up to 1000-fold more ROS than aerobic metabolism [10]. Infection has also been implicated as a source of ROS, as inflammation and infection can attract leucocytes to the affected region. The prevalence of subclinical infection in infertile males varies between 10% and 35% [6], [11]. ROS are higher in patients with male accessory gland infections (MAGI) including the urethra, prostate, seminal vesicles, deferent ducts, Cowper’s glands, epididymis, or testes [12]. The definition of MAGI is based on WHO criteria of oligo-, astheno-, or teratozoopsermia with a combination of the following criteria: (i) history of urinary infection, epididymitis, or sexually transmitted disease, or physical findings of abnormal rectal examination, thickened or tender epididymis or vas deferens, (ii) abnormal prostatic fluid or urine after prostate examination, (iii) abnormal ejaculate including peroxidase positive leucocytes, culture with pathogenic bacteria, or abnormal seminal plasma [12].
Patients with varicoceles have a higher oxidative stress factor and higher levels of ROS-induced spermatic DNA damage [13]. These patients have improved semen parameters after treatment of their varicocele [13]. There is not only improvement in semen parameters after varicocelectomy, but also decreased levels of seminal lipid peroxidation and sperm DNA damage [14]. Varicoceles also cause higher scrotal temperature [14]. Heat has been independently associated with increased ROS in semen analysis as well as other negative changes in semen factors [7], [15].
Another important medical cause of ROS is hyperglycaemia. Patients with pre-diabetes or diabetes mellitus also have increased oxidative stress with a high correlation of oxidative damage and decreased sperm quality [16].
Extrinsic factors of ROS
Industrial exposures, although uncommon, are a cause of increased ROS production and sperm DNA damage. Exposure to heavy metals (e.g., cadmium, lead, iron, and copper), pesticides, phthalate, and pollution can lead to sperm damage [7]. Multiple studies link industrial exposure with decreased sperm function and increased DNA damage, but not all of these effects can be attributed solely to ROS. Pesticides and phthalates not only induce oxidative stress, but can also disrupt the hypothalamic–pituitary–gonadal axis in animal models [17]. Inhibition of the GnRH inhibits the release of LH and FSH. This results in inhibition of gametogenesis and steroidogenesis, leading to deleterious effects on sperm function as well as testicular atrophy.
Smoking has also been associated with decreased spermatic function. Cigarette smoking is associated with significant decreases in sperm density, total sperm count, sperm motility, and sperm morphology [18]. Tobacco use has also been correlated with higher levels of sperm DNA damage [5]. Smoking incites local inflammation and is associated with a 48% increase in seminal leucocyte levels and a 107% increase in seminal ROS levels [19].
Excessive alcohol intake leads to increased acetaldehyde, a by-product of ethanol metabolism, which interacts with proteins and lipids generating ROS [20]. Excessive ethanol intake is also associated with morphologically abnormal spermatozoa, reductions in spermatogenesis, decreased seminal fluid volume, low LH, FSH, and testosterone, and increased oxidative stress [21].
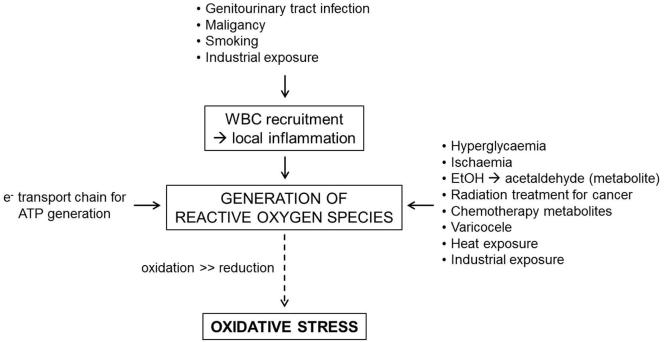
Patients with a history of malignancy are at a higher risk of oxidative stress due to exposure to radiation and chemotherapy. Men who are exposed to gonadotoxic chemotherapy, such as cisplatin, doxorubicin, or cyclophosphamide undergo increased oxidative stress, possibly mediated by heat shock proteins [22]. Radiation exposure causes damage by ROS-triggered lipid peroxidation [23]. Low level radiation also increases ROS by increasing NADH oxidase leading to increased sperm death [24]. A summary of factors affecting the production of ROS is provided in Fig. 2.
Fig. 2.
The physiological and pathological aetiologies of ROS generation. WBC, white blood cell.
Physiological role of ROS on sperm
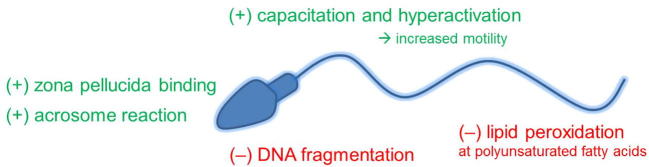
For optimal sperm function and for fertilisation to occur, there must be a balance of ROS and antioxidants (Fig. 3). ROS induces cyclic adenosine monophosphate (cAMP) in spermatozoa that inhibits tyrosine phosphatase leading to tyrosine phosphorylation [25]. In particular, H2O2 stimulates capacitation via tyrosine phosphorylation triggering a cell signalling cascade [26]. Capacitation not only requires ROS, but it can be inhibited by catalase (CAT) [26]. It has been described that high levels of ROS promote the acrosome reaction, whereas the presence of CAT or superoxide dismutase (SOD) inhibits the acrosome reaction [27]. The mechanism of inducing the acrosome reaction appears to be ROS-modulated tyrosine phosphorylation [26]. Motility can also be affected by ROS. Hyperactivation is increased when spermatozoa are exposed to ROS [28]. ROS-mediated tyrosine phosphorylation in the flagellum causes hyperactivation [13], [25]. Tyrosine phosphorylation also augments sperm membrane binding to the zona pellucida ZP-3 protein, promoting sperm–oocyte fusion [29].
Fig. 3.
The physiological (+) and pathological (−) roles of oxidation in spermatozoa.
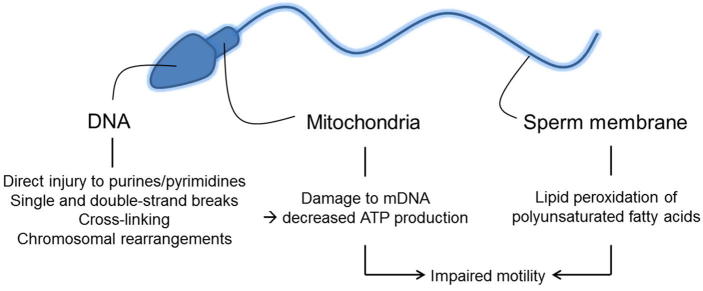
Pathological effects on spermatogenesis and function (Fig. 4)
Fig. 4.
Oxidative stress and injury to DNA, mitochondria, and plasma membrane of spermatozoa.
Although ROS are necessary for normal physiological function of sperm, excessive oxidative stress can cause increased susceptibility of DNA damage, potentially leading not only to infertility, but also recurrent pregnancy loss or genetically heritable mutations causing paediatric diseases [30]. Due to the high susceptibility of unsaturated fatty acid content of sperm to oxidative stress, extensive membrane damage can occur. Once lipid oxidisation has been initiated, the double bonds of an unsaturated fatty acid are attacked by a free radical to create a lipid peroxide radical. The lipid peroxide radical reacts with the neighbouring lipid molecule triggering a chain reaction that can lead to >50% oxidation of the sperm plasma membrane [31]. Eventually lipid peroxidation stops once two lipid radicals react with each other thereby reaching stability [31]. By-products of lipid oxidisation include mutagenic and genotoxic molecules malondialdehyde (MDA) and 4 hydroxy-nonenal (4-HNE) leading indirectly to DNA damage [5].
Free radicals have the ability to directly damage sperm DNA by attacking the purine and pyrimidine bases [32]. ROS cause damage via single and double strand DNA breaks, cross links, and chromosomal rearrangements [5]. Most of the sperm genome (∼85%) is bound to central nucleoprotamines that protect it from free radical attack [5]. Infertile men often have deficient protamination, which may make their sperm DNA more vulnerable to ROS damage [33]. Another mechanism of spermatic DNA damage is free radical-initiated apoptosis, leading to caspase-mediated destruction of DNA [7]. Spermatozoa have a limited ability to repair DNA and can only perform repairs during specific stages of spermatogenesis [34]. Repair mechanisms in haploid spermatozoa are necessary to allow chromatin remodelling [34]. Interactions of sperm with an oocyte may allow for some repairs of DNA, which can affect fertilisation and possible pregnancy [13].
Decreased motility has been shown to be due to ROS-induced, primarily H2O2-mediated, peroxidation of lipids in the sperm membrane decreasing flexibility and by inhibition of motility mechanisms [35]. The reduction in sperm motility is proportional to the amount of lipid peroxidation [35]. ROS-induced damage of mitochondrial DNA leads to decreased ATP and energy availability, impeding sperm motility [36].
Studies have shown higher levels of ROS in patients with oligospermia [37]. High levels of ROS disrupt mitochondrial membranes, leading to activation of caspases and ultimately apoptosis [38]. Cytochrome c release during the apoptotic pathway further increases levels of ROS, promoting DNA damage and fragmentation, and potentially augmenting the apoptotic cycle [39]. Sperm with higher levels of oxidative stress, especially after freeze/thaw cycles, have higher levels of caspase activation that can trigger apoptosis [40].
Physiological role of antioxidants
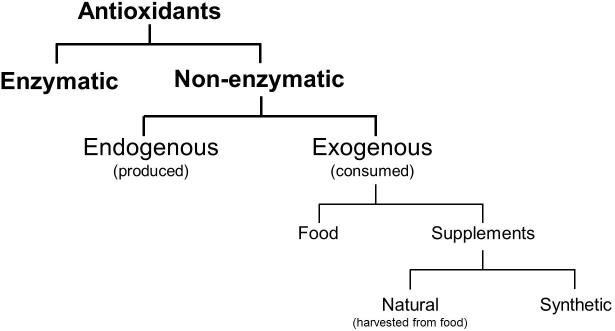
To maintain physiological oxidative mechanisms whilst minimising the risk of cellular injury, a balance between ROS and antioxidants is necessary. Antioxidants are reducing agents that react with and buffer ROS and are considered a form of defence against oxidative stress [41]. They are categorised as enzymatic or non-enzymatic agents [41], with non-enzymatic agents subdivided into those endogenously produced or those consumed through food or supplements (Fig. 5). The use of antioxidants as a form of infertility treatment will be discussed in a separate review.
Fig. 5.
Categorisation of antioxidants.
During spermatogenesis, sperm shed cytoplasm. By limiting intracellular antioxidants, this necessitates extracellular antioxidants to counteract oxidative stress. Seminal plasma secreted from the seminal vesicles, prostate, and bulbourethral glands provides optimal pH, viscosity, nutrition, and a significant portion of antioxidants within the semen to support spermatozoa [42]. The activity levels of enzymatic antioxidants in the ejaculate of fertile men and men that had previously undergone vasectomy were similar [42], suggesting that antioxidant activity required for fertility is produced and secreted in seminal plasma. In vitro incubation of sperm in the absence of seminal plasma shows a significant increase in markers for oxidative stress and a corresponding reduction in motility after 2 h [43]. Seminal plasma in men with idiopathic infertility has been found to have a lower antioxidant carrying capacity compared to fertile men [44].
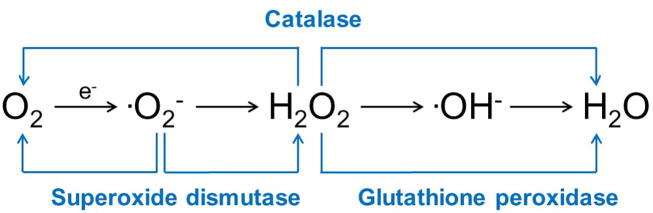
Important enzymatic antioxidants in infertility include SOD, glutathione peroxidase (GPX), and CAT (Fig. 6). SOD scavenges superoxide anion and catalyses its conversion to H2O2 and O2. High levels of SOD have been found in Sertoli cells, whilst germ cells have high activity levels that are maintained during spermatogenesis in developing and maturing spermatozoa [45]. SOD has also been identified throughout the epididymis and in seminal plasma [46]. This enzyme decreases markers for oxidative stress [43], protects sperm against lipid peroxidation [47], decreases DNA damage [46], and is associated with motility in human spermatozoa [43].
Fig. 6.
Enzymatic antioxidants that react with and buffer free radicals.
GPX uses glutathione as an electron donor to catalyse H2O2 and superoxide anion [47]. High levels of GPX are present in Sertoli cells [45]. GPX is expressed and secreted from the head of the epididymis and is found in semen [46]. GPX primarily protects against lipid peroxidation of the plasma membrane of spermatozoa [47].
CAT is an enzyme found in peroxisomes that converts H2O2 to H2O and O2. Although minimal CAT is present in developing sperm, there is a constant low level of activity in the testicle [45]. Men with asthenozoospermia have lower levels of CAT in their semen compared to normospermic men [48], potentially demonstrating the importance of seminal plasma levels of CAT [46], [48].
Non-enzymatic antioxidants are endogenously produced or consumed from food or supplements. Glutathione is a free radical scavenger found in high levels of developing spermatocytes and spermatids [45] that also acts as a coenzyme for GPX. Ascorbic acid (vitamin C) is a water-soluble vitamin that neutralises ROS and may protect against DNA damage in sperm [49]. Tocopherol (vitamin E) is a fat-soluble vitamin that reduces lipid peroxidation in spermatozoa [49]. Selenium and zinc are trace elements. Selenium serves as a cofactor to certain isoforms of GPX [5] and zinc may provide protection against lipid oxidation [50]. Lycopene, a carotenoid, is localised to the prostate and testicles and may protect against ischaemia–reperfusion tissue injury [51]. However, lycopene has not been detected in semen [49]. Ubiquinol, found within seminal plasma, is the reduced form of coenzyme Q10 and has been associated with lower levels of ROS and a corresponding increase in sperm count and motility [52].
Pathological effects of antioxidants
Dosing and antioxidant supplementation regimens are not currently regulated. Despite the expectation that antioxidants would prevent or potentially repair oxidative damage, unregulated supplementation can lead to a pathological abundance of antioxidants causing an imbalance between oxidation and reduction. The antioxidant paradox describes how antioxidants can exacerbate injury through a loss of oxidative mechanisms, promotion of tissue injury, and a paradoxical increase in ROS [53]. As previously discussed, oxidation plays a necessary role in male reproduction and unregulated antioxidant supplementation can inhibit these mechanisms. Surplus CAT can prevent capacitation by inhibiting local oxidation [26]. Similarly, excess SOD and CAT can prevent the acrosome reaction from occurring [27].
Testing
Assessing ROS levels in infertile men could allow for identifying those who might benefit from antioxidant treatment given the detrimental effects of ROS on semen and sperm. There are various tests that can be used to assess ROS that are divided into direct tests and indirect tests (Table 1).
Table 1.
Direct and indirect tests used to measure oxidation. A. Direct tests measure ROS production and sperm cell oxidation. B. Indirect tests evaluate oxidation by measuring the downstream markers of injury from ROS.
| Test | Function | Method of measurement |
|---|---|---|
| A. Direct tests | ||
| Chemiluminescence assay | Detection of oxidation or reduction through generation of light (by-product of reaction) | Uncharged and charged probes undergo either oxidation or reduction to generate light as a by-product |
| Flow cytometry | Measurement of ROS | Incubation with dye emits fluorescence when excitated by a light source at specific wavelengths |
| Electron spin resonance (ESR) | Direct detection of free radicals (but not at low concentrations or short half-lives) | ESR obtains absorption spectra of the spin energy of unpaired electrons in an applied magnetic field |
| Cytochrome c reduction | Measurement of ROS on the cell membrane | Superoxide radicals being reduced to ferricytochrome c are identified |
| Nitroblue tetrazolium tests | Localisation of reactions between superoxide ions and sperm cells or leucocytes | Nitroblue tetrazolium reacts with superoxide ions and turns from yellow to purple/blue when ROS are present |
| B. Indirect tests | ||
| Myeloperoxidase test | Detection of granulocytes in semen | Benzidine is used to buffer and assess samples for peroxidase positivity through staining |
| Lipid peroxidation level | Detection of lipid peroxidation through identification of by-products | Levels of mutagenic MDA and toxic 4-HNE by-products of lipid peroxidation are measured using thiobarbituric acid assays and colorimetric reactions |
| MiOxsys | Measurement of oxidation–reduction potential | A galvanostat-based system is used to measure the transfer of electrons from reductant to an oxidant in millivolts |
| Total antioxidant capacity | Evaluation of antioxidant status of a biological sample | The addition of an enhanced chemo-luminescent assay to seminal plasma measures suppression of chemiluminescence and time to recovery |
Indications to test
Currently there is controversy over which patients should be tested for oxidative stress, as well as which test to perform. Although routine semen analyses parameters are reduced in the setting of oxidative stress, asthenozoospermia has been proposed as the best marker of ROS seen in a standard infertility evaluation [20]. Viscosity has also been suggested as a surrogate measure for oxidative stress, as hyperviscosity is associated with increased MDA levels [20]. High levels of leucocytes, seen as round cells in semen analysis, are also associated with ROS and may be an indication for ROS testing. However, as round cells are a nonspecific finding, further testing is often required to differentiate immature sperm from leucocytes [20]. Abnormal sperm morphology related to residual cytoplasm correlates to high levels of ROS and may be another finding that should lead to further evaluation for ROS [9], [20]. The hypo-osmotic swelling test shows sperm membrane damage, which correlates with lipid peroxidation and may imply the presence of high levels of ROS [20]. Any of the above findings on fertility evaluation may be an indication to pursue further testing for ROS. Some recommend testing for ROS in patients in which no one cause can be found for infertility [54]. However, currently no infertility guidelines recommend routine ROS testing.
Conclusion
The impact of ROS on spermatic function has been well-established. Current research has elucidated several mechanisms of generation of ROS, the physiological role of ROS, and various effects of antioxidants to counteract oxidative stress. There are many different laboratory tests available that have the ability to screen for and quantify ROS in semen samples. This information will probably impact diagnosis and treatment of male infertility. However, despite improvements in knowledge, there is still debate over how to apply this to clinical practice. ROS have been shown to cause abnormalities in semen analysis, sperm concentration, and DNA, but the effects of ROS on fertilisation and pregnancy remains controversial. At this time, there are no guidelines that incorporate testing of ROS and treatment with antioxidants for male infertility.
Conflict of interest
None.
Source of funding
None.
Etiology
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Male Infertility Best Practice Committee of the American Urological Association. Practice Committee of the American Society for Reproductive Medicine Report on optimal evaluation of the infertile male. Fertil Steril. 2006;86:S202–9. doi: 10.1016/j.fertnstert.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 2.Jarow J.P., Sharlip I.D., Belker A.M., Lipshultz L.I., Sigman M., Thomas A.J. Best practice policies for male infertility. J Urol. 2002;167:2138–2144. [PubMed] [Google Scholar]
- 3.MacLeod J. The role of oxygen in metabolism and motility of human spermatozoa. Am J Physiol. 1943;138:512–518. [Google Scholar]
- 4.Tosic J., Walton A. Formation of hydrogen peroxide by spermatozoa and its inhibitory effect of respiration. Nature. 1946;158:485. doi: 10.1038/158485a0. [DOI] [PubMed] [Google Scholar]
- 5.Bisht S., Faiq M., Tolahunase M., Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14:470–485. doi: 10.1038/nrurol.2017.69. [DOI] [PubMed] [Google Scholar]
- 6.Henkel R.R. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl. 2011;13:43–52. doi: 10.1038/aja.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremellen K. Oxidative stress and male infertility – a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 8.Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez E., Buckingham D.W., Brindle J., Lanzafame F., Irvine D.S., Aitken R.J. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl. 1996;17:276–287. [PubMed] [Google Scholar]
- 10.Plante M., de Lamirande E., Gagnon C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil Steril. 1994;62:387–393. doi: 10.1016/s0015-0282(16)56895-2. [DOI] [PubMed] [Google Scholar]
- 11.Trum J.W., Mol B.W., Pannekoek Y., Spanjaard L., Wertheim P., Bleker O.P. Value of detecting leukocytospermia in the diagnosis of genital tract infection in subfertile men. Fertil Steril. 1998;70:315–319. doi: 10.1016/s0015-0282(98)00163-0. [DOI] [PubMed] [Google Scholar]
- 12.Depuydt C.E., Bosmans E., Zalata A., Schoonjans F., Comhaire F.H. The relation between reactive oxygen species and cytokines in andrological patients with or without male accessory gland infection. J Androl. 1996;17:699–707. [PubMed] [Google Scholar]
- 13.Wright C., Milne S., Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 2014;28:684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Ni K., Steger K., Yang H., Wang H., Hu K., Zhang T. A comprehensive investigation of sperm DNA damage and oxidative stress injury in infertile patients with subclinical, normozoospermic, and astheno/oligozoospermic clinical varicocele. Andrology. 2016;4:816–824. doi: 10.1111/andr.12210. [DOI] [PubMed] [Google Scholar]
- 15.Rao M., Zhao X.L., Yang J., Hu S.F., Lei H., Xia W. Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. Asian J Androl. 2015;17:668–675. doi: 10.4103/1008-682X.146967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira P.F., Tomas G.D., Dias T.R., Martins A.D., Rato L., Alves M.G. White tea consumption restores sperm quality in prediabetic rats preventing testicular oxidative damage. Reprod Biomed Online. 2015;31:544–556. doi: 10.1016/j.rbmo.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Queiroz E.K., Waissmann W. Occupational exposure and effects on the male reproductive system. Cad Saúde Pública. 2006;22:485–493. doi: 10.1590/s0102-311x2006000300003. [DOI] [PubMed] [Google Scholar]
- 18.Sharma R., Harlev A., Agarwal A., Esteves S.C. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur Urol. 2016;70:635–645. doi: 10.1016/j.eururo.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Saleh R.A., Agarwal A., Kandirali E., Sharma R.K., Thomas A.J., Nada E.A. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril. 2002;78:1215–1224. doi: 10.1016/s0015-0282(02)04237-1. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal A., Virk G., Ong C., du Plessis S.S. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.La Vignera S., Condorelli R.A., Balercia G., Vicari E., Calogero A.E. Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl. 2013;15:221–225. doi: 10.1038/aja.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiligada E. Chemotherapy: induction of stress responses. Endocr Relat Cancer. 2006;13(Suppl. 1):S115–24. doi: 10.1677/erc.1.01272. [DOI] [PubMed] [Google Scholar]
- 23.Manda K., Ueno M., Moritake T., Anzai K. Α-lipoic acid attenuates X-irradiation-induced oxidative stress in mice. Cell Biol Toxicol. 2007;23:129–137. doi: 10.1007/s10565-006-0137-6. [DOI] [PubMed] [Google Scholar]
- 24.Desai N.R., Kesari K.K., Agarwal A. Pathophysiology of cell phone radiation: oxidative stress and carcinogenesis with focus on male reproductive system. Reprod Biol Endocrinol. 2009;7:114. doi: 10.1186/1477-7827-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Flaherty C., de Lamirande E., Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radical Biol Med. 2006;41:528–540. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Aitken R., Paterson M., Fisher H., Buckingham D., Van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995;108:2017–2025. doi: 10.1242/jcs.108.5.2017. [DOI] [PubMed] [Google Scholar]
- 27.Lamirande E., Tsai C., Harakata A., Gagnon C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J Androl. 1998;19:585–594. [PubMed] [Google Scholar]
- 28.Lamirande E., Gagnon C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int J Androl. 1993;16:21–25. doi: 10.1111/j.1365-2605.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 29.Aitken R.J. Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev. 1995;7:659–668. doi: 10.1071/rd9950659. [DOI] [PubMed] [Google Scholar]
- 30.Dinesh V., Shamsi M., Dada R. Supraphysiological free radical levels and their pathogenesis in male infertility. Reprod Sys Sex Disord. 2012;1:2. [Google Scholar]
- 31.Kothari S., Thompson A., Agarwal A., du Plessis S.S. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol. 2010;48:425–435. [PubMed] [Google Scholar]
- 32.Geva E., Lessing J.B., Lerner-Geva L., Amit A. Free radicals, antioxidants and human spermatozoa: clinical implications. Hum Reprod. 1998;13:1422–1424. doi: 10.1093/oxfordjournals.humrep.a019709. [DOI] [PubMed] [Google Scholar]
- 33.Oliva R. Protamines and male infertility. Hum Reprod Update. 2006;12:417–435. doi: 10.1093/humupd/dml009. [DOI] [PubMed] [Google Scholar]
- 34.Marcon L., Boissonneault G. Transient DNA strand breaks during mouse and human spermiogenesis: new insights in stage specificity and link to chromatin remodeling1. Biol Reprod. 2004;70:910–918. doi: 10.1095/biolreprod.103.022541. [DOI] [PubMed] [Google Scholar]
- 35.Morielli T., O'Flaherty C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction. 2015;149:113–123. doi: 10.1530/REP-14-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamirande E., Gagnon C. Reactive oxygen species and human spermatozoa. J Androl. 1992;13:368–378. [PubMed] [Google Scholar]
- 37.Agarwal A., Mulgund A., Sharma R., Sabanegh E. Mechanisms of oligozoospermia: an oxidative stress perspective. Syst Biol Reprod Med. 2014;60:206–216. doi: 10.3109/19396368.2014.918675. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal A., Makker K., Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 39.Thomson L.K., Fleming S.D., Aitken R.J., De Iuliis G.N., Zieschang J.A., Clark A.M. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod. 2009;24:2061–2070. doi: 10.1093/humrep/dep214. [DOI] [PubMed] [Google Scholar]
- 40.Paasch U., Grunewald S., Agarwal A., Glandera H.J. Activation pattern of caspases in human spermatozoa. Fertil Steril. 2004;81:802–809. doi: 10.1016/j.fertnstert.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 41.Sies H. Strategies of antioxidant defense. FEBS J. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 42.Yeung C., Cooper T., De Geyter M., De Geyter C., Rolf C., Kamischke A. Studies on the origin of redox enzymes in seminal plasma and their relationship with results of in-vitro fertilization. Mol Hum Reprod. 1998;4:835–839. doi: 10.1093/molehr/4.9.835. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi T., Miyazaki T., Natori M., Nozawa S. Protective role of superoxide dismutase in human sperm motility: superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum Reprod. 1991;6:987–991. doi: 10.1093/oxfordjournals.humrep.a137474. [DOI] [PubMed] [Google Scholar]
- 44.Alkan I., Simsek F., Haklar G., Kervancioğlu E., Ozveri H., Yalçin S. Reactive oxygen species production by the spermatozoa of patients with idiopathic infertility: relationship to seminal plasma antioxidants. J Urol. 1997;157:140–143. [PubMed] [Google Scholar]
- 45.Bauché F., Fouchard M.-H., Jégou B. Antioxidant system in rat testicular cells. FEBS Lett. 1994;349:392–396. doi: 10.1016/0014-5793(94)00709-8. [DOI] [PubMed] [Google Scholar]
- 46.Chen H., Chow P.H., Cheng S.K., Cheung A.L., Cheng L.Y. Male genital tract antioxidant enzymes: their source, function in the female, and ability to preserve sperm DNA integrity in the golden hamster. J Androl. 2003;24:704–711. doi: 10.1002/j.1939-4640.2003.tb02730.x. [DOI] [PubMed] [Google Scholar]
- 47.Tavilani H., Goodarzi M.T., Doosti M., Vaisi-Raygani A., Hassanzadeh T., Salimi S. Relationship between seminal antioxidant enzymes and the phospholipid and fatty acid composition of spermatozoa. Reprod Biomed Online. 2008;16:649–656. doi: 10.1016/s1472-6483(10)60478-6. [DOI] [PubMed] [Google Scholar]
- 48.Jeulin C., Soufir J., Weber P., Laval-Martin D., Calvayrac R. Catalase activity in human spermatozoa and seminal plasma. Gamete Res. 1989;24:185–196. doi: 10.1002/mrd.1120240206. [DOI] [PubMed] [Google Scholar]
- 49.Lewis S.E., Sterling E.S., Young I.S., Thompson W. Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil Steril. 1997;67:142–147. doi: 10.1016/s0015-0282(97)81871-7. [DOI] [PubMed] [Google Scholar]
- 50.Zago M.P., Oteiza P.I. The antioxidant properties of zinc: interactions with iron and antioxidants. Free Radical Biol Med. 2001;31:266–274. doi: 10.1016/s0891-5849(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 51.Hekimoglu A., Kurcer Z., Aral F., Baba F., Sahna E., Atessahin A. Lycopene, an antioxidant carotenoid, attenuates testicular injury caused by ischemia/reperfusion in rats. Tohoku J Exp Med. 2009;218:141–147. doi: 10.1620/tjem.218.141. [DOI] [PubMed] [Google Scholar]
- 52.Alleva R., Scararmucci A., Mantero F., Bompadre S., Leoni L., Littarru G. The protective role of ubiquinol-10 against formation of lipid hydroperoxides in human seminal fluid. Mol Aspects Med. 1997;18(Suppl.):221–228. doi: 10.1016/s0098-2997(97)00040-x. [DOI] [PubMed] [Google Scholar]
- 53.Halliwell B. The antioxidant paradox. Lancet. 2000;355:1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 54.Mayorga-Torres B.J., Camargo M., Cadavid A.P., Plessis S.S., Cardona Maya W.D. Are oxidative stress markers associated with unexplained male infertility? Andrologia. 2017:49. doi: 10.1111/and.12659. Epub 2016 Aug 10. [DOI] [PubMed] [Google Scholar]