Abstract
Objective
To assess the role of differentially expressed proteins as a resource for potential biomarker identification of infertility, as male infertility is of rising concern in reproductive medicine and evidence pertaining to its aetiology at a molecular level particularly proteomic as spermatozoa lack transcription and translation. Proteomics is considered as a major field in molecular biology to validate the target proteins in a pathophysiological state. Differential expression analysis of sperm proteins in infertile men and bioinformatics analysis offer information about their involvement in biological pathways.
Materials and methods
Literature search was performed on PubMed, Medline, and Science Direct databases using the keywords ‘sperm proteomics’ and ‘male infertility’. We also reviewed the relevant cross references of retrieved articles and included them in the review process. Articles written in any language other than English were excluded.
Results
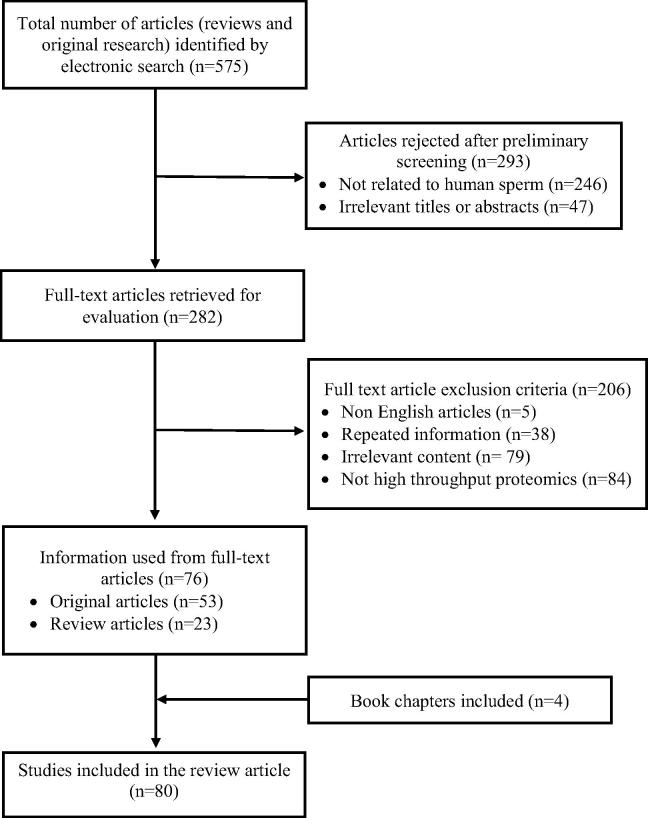
Of 575 articles identified, preliminary screening for relevant studies eliminated 293 articles. At the next level of selection, from 282 studies only 80 articles related to male infertility condition met the selection criteria and were included in this review.
Conclusion
In this molecular era, sperm proteomics has created a platform for enhanced understanding of male reproductive physiology as a potential tool for identification of novel protein biomarkers related to sperm function in infertile men. Therefore, it is believed that proteomic biomarkers can overcome the gaps in information from conventional semen analysis that are of limited clinical utility.
Keywords: Sperm proteomics, Male infertility, Spermatozoa, Varicocele, Biomarkers
Introduction
Currently, infertility is one of the most addressed issues related to male reproductive dysfunction. Amongst the 9% of the world’s infertility cases, ∼20% is contributed by the male population alone [1]. There are multiple factors that govern and regulate male factor infertility, although most of these cases remain idiopathic. Andrology laboratories rely mainly on semen analysis to evaluate male infertility in patients with poor semen quality. Conventional tests such as basic semen analysis to determine sperm concentration, motility, vitality, and morphology are used for diagnosing male infertility based on reference values established by the WHO [2]. However, advanced laboratory tests such as quantification of reactive oxygen species (ROS)1 and antioxidants in semen by chemiluminescence assay [3], oxidation–reduction potential in semen by Male Infertility Oxidative System (MiOXSYS®; Aytu BioScience Inc., Englewood, CO USA) [4], and sperm DNA fragmentation assessment by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assay [5], are clinically used to identify the specific cause of infertility for further utilisation in assisted reproductive technology. However, the aetiological changes at the subcellular level of the spermatozoa remain unknown.
New generation techniques, such as proteomics, are poised to help researchers identify the molecular aspects of spermatozoa that are affected in infertility conditions. The majority of protein biomolecules are involved in cell signalling pathways. Generally, spermatozoa are transcriptionally and translationally silent, so they depend on their proteins to carry out their biological functions [6]. Proteomics is an emerging tool that could potentially help identify protein alterations in spermatozoa and seminal plasma of male infertile patients [7]. Differential expression of sperm proteins in fertility compromised patients is an indicator of defective spermatogenesis, motility, capacitation, hyperactivation, acrosome reaction, and fertilisation processes at a molecular level.
Aberrant expression of proteins in spermatozoa of men causes changes in physiological functions due to post-translational modifications, such as phosphorylation, glycosylation, proteolytic cleavage, and mutations [8], [9]. Therefore, it is important to understand the changes in the proteins and cellular pathways affected in infertile patients for better diagnosis in a clinical perspective.
Materials and methods
An extensive search of studies published until October 2017 was performed using PubMed, MedLine, and Science Direct databases. The search was limited to full articles published in the English language and studies on human semen were only included. ‘Sperm proteomics’ and ‘male infertility’ were the two primary keywords used to retrieve articles from different databases. Combination of the following keywords relevant to infertility and proteomics was used to extract the articles: ‘spermatozoa’, ‘sperm proteomics’, ‘varicocele proteomics’, ‘proteome’, ‘oxidative stress and proteomics’, ‘2D-PAGE, mass spectrometry’. Search terms such as ‘azoospermia’, ‘asthenozoospermia’, ‘mitochondrial dysfunction’, ‘testicular cancer and proteomics’ were also used. Cross referencing was also referred to and used in the review process.
Results
Comprehensive literature collection via electronic search resulted in a total of 575 review and original research articles. Preliminary screening resulted in 282 articles that included different proteomic studies from human sperm (Fig. 1). In the subsequent screening, 206 studies were rejected as many (n = 84) were not related to high-throughput proteomics. Finally, 80 full-text articles (review, original research, and book chapters) met the inclusion criteria and were found to be eligible for the review.
Fig. 1.
Flow diagram illustrating the study selection criteria.
Proteomic analysis
Proteins are the functional biomolecules of the cell. Alteration in their structure, composition, and interaction with other proteins, and post-translational modification affect the normal physiological processes of a cell [10], [11]. Understanding the physiological functions of all the proteins and polypeptides is required to delineate their involvement in biological and molecular pathways in a particular type of cell. Proteomic-based studies on spermatozoa will explain the functional role of fertility-related proteins and the factors affecting their normal expression. Proteomic studies in earlier days were confined to checking the expression of proteins in different pathological conditions, but in the current proteomics era, sperm proteomics has been explored by various researchers to understand cellular pathways affecting male infertility [11].
Advanced proteomic methodology and tools
Previously, identification and quantification of multiple proteins at a single time were a challenging task. Development and introduction of new advanced tools and techniques have revolutionised the field of proteomics. In general, the protein techniques are classified into separation and identification techniques. Conventional separation techniques include separation of proteins in a given sample by sodium dodecyl sulphate (SDS)–PAGE based on their molecular weight. Similarly, two dimensional (2D)-gel electrophoresis is widely used for quantitative and qualitative proteins in a much more efficient manner based on the isoelectric focusing point and molecular weight of proteins. However, this technique is unable to generate a complete profile of all the proteins and is not sensitive enough to detect low-abundance proteins.
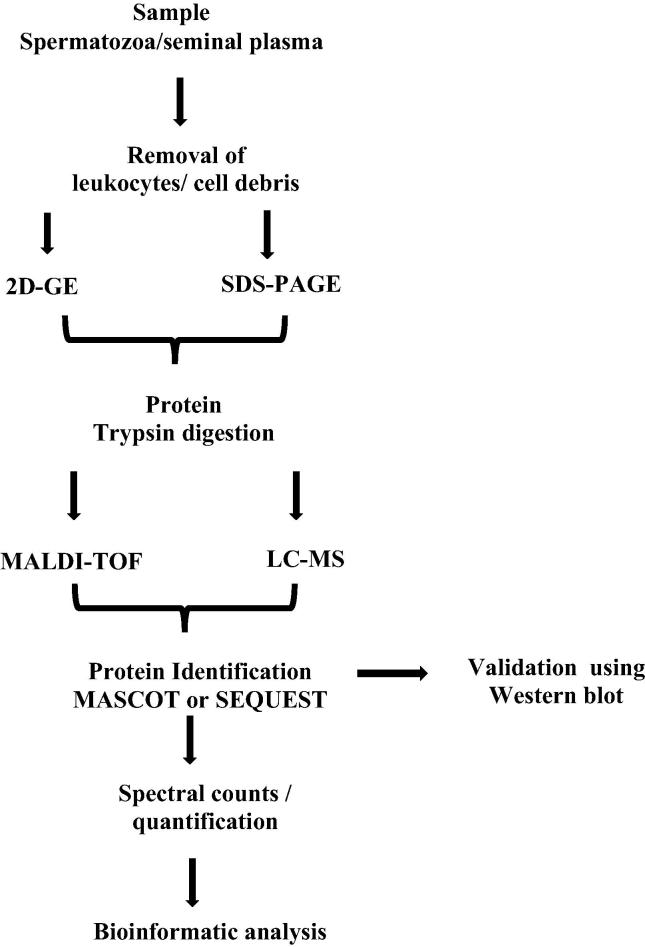
Current proteomic approaches are aimed at protein profiling, differential expression of proteins, localisation, and identification of post-translational modification, analysis of protein–protein interactions, and protein involvement in regulating biological and molecular pathways. Mass spectrometry (MS) techniques are able to overcome the omission in protein and peptide identification by measuring the m/z ratio. Initially the complexity of the proteins is broken down by 2D-gel electrophoresis and further MS characterises the proteins. It is an excellent technique to monitor and analyse thousands of peptides and proteins in a short time (Fig. 2) [12]. Current methodological advances in sperm proteomics using matrix-assisted laser desorption ionisation time-of-flight (MALDI-TOF) and liquid chromatography (LC)–MS/MS techniques have led to increased throughput study of the sperm proteome [13].
Fig. 2.
Flow diagram for the processing of semen samples using proteomic tools and bioinformatics analysis.
Quantitative proteomics
The relative quantity of the proteins is determined by comparing the number of spectra, termed spectral counts, used to identify each protein. The total numbers of mass spectra that match peptides to a particular protein (spectral counts or SpCs) are used to measure the abundance of proteins in the complex mixture. Normalisation of SpCs using the normalised spectral abundance factor (NSAF) approach is applied prior to relative protein quantification. Differentially expressed proteins (DEPs) are obtained by applying different constraints for significance tests and/or fold change thresholds based on the average SpC of the protein from multiple runs, as accurate quantification and determination of real biological change are a function of the absolute number of SpCs. The abundance of proteins can be classified as ‘High’, ‘Medium’, ‘Low’, or ‘Very Low’ based on their average SpCs. Different constraints for significance tests (P value) and/or fold change thresholds (or NSAF ratio) are applied for better statistical analysis [14].
Bioinformatics analysis of proteomics data
Bioinformatics is a fusion of biological science with software-based computer science. It is mainly involved in the analysis of molecular scientific data using advanced software tools. Raw data obtained from the MALDI-TOF and LC–MS/MS techniques are subjected to bioinformatics analysis to distil the huge amount of data generated during proteomic studies into a more presentable form of results [15]. Each protein analysis software works on specific algorithms and further analysis generates a list of proteins. Initially, MS/MS collision-induced dissociation (CID) spectra are analysed using data searching tools, such as Mascot, SEQUEST and X!Tandem to determine the SpCs. The proteins and peptides are then further matched with protein sequences, expressed sequence tags (EST) and DNA sequences that are available on global databases.
Gene names corresponding to the identified proteins are provided for functional annotations, whilst gene prediction programs are used to identify the proteins unavailable in the database based on their functional group [16]. The next step is gene ontology (GO) analysis for the identified list of proteins using databases such as GO Term Finder and GO Term Mapper, which provide information for the proteins based on their function, localisation, structure, and biological function in cellular pathways. The genes/proteins are basically classified into cellular components, biological processes, and molecular functions. Interaction network and pathway analysis for the group of proteins is performed using proprietary software packages such as Ingenuity Pathway Analysis (IPA) and Metacore™ to identify the pathways, interactions, and cellular distribution of the proteins. Another online tool, Search Tool for the Retrieval of Interacting Genes/Proteins (STRING), displays the functional link between the protein–protein interactions amongst the proteins [17].
Semen proteome
Semen is the most accepted and suitable sample for proteomic studies, as it contains thousands of proteins. Semen is composed of spermatozoa and protein-rich seminal fluid. Characterisation of proteins in spermatozoa and seminal plasma provides the function of fertility-related specific proteins [18]. The male gamete with specialised functions is produced by a complex process known as spermatogenesis. Protein–protein interaction regulates the developmental process of spermatozoa [19]. Using 1D-SDS–PAGE combined with the in-gel digestion coupled with MS analysis (GeLC–MS/MS) technique, Johnston et al. [7] mapped 1760 proteins in human spermatozoa and also reported the abundance of 27 proteins present in the 26S proteasome complex of spermatozoa. Characterisation by the 2D-gel MALDI-TOF proteomic approach elucidated that proteins in the spermatozoa are involved in vital functions such as: energy production, apoptosis and oxidative stress (OS), cytoskeleton, flagella and cell movement, protein transport, and folding [20]. A review by Amaral et al. [21] using 30 different studies on sperm proteomics identified a total of 6198 proteins that constitute the sperm proteome, including protein characterisation studies from sperm head, tail, and membrane separately. Of these proteins, 898 were present in the head, 984 in the tail, and 532 in both locations [22]. Functional analysis predicted sperm proteins were found to regulate important pathways such as: energy metabolism, protein metabolism, post-translational modifications, membrane trafficking, OS, DNA damage, and apoptosis [21], [23].
Seminal plasma is heterogeneous as it is composed of secretions from the testis and accessory glands (including the prostate, seminal vesicles, epididymis, and Cowper’s gland), which provide a favourable environment for the spermatozoa [24]. It has a rich protein concentration (35–55 g/L) and most seminal plasma proteins originate from accessory sex glands. Seminal plasma proteins are responsible for the coagulation–liquefaction process, making it complex for proteomic studies. Pilch and Mann [25] reported the expression of 923 proteins in the seminal plasma and 70% of these proteins were present in spermatozoa [23]. Jodar et al. [22] identified 284 proteins including: TGF β1 (TGFB1), TGF β3 (TGFB3), antimicrobial peptide 1 (AMP1), serpin family A member 7 (SERPINA7), low-density lipoprotein receptor (LDLR), dystroglycan 1 (DAG1), disintegrin and metalloproteinase domain 10 (ADAM10), vitronectin (VTN), platelet-derived growth factor subunit A (PDGFA) and IGF-binding protein 2 (IGFBP2), which were specific only to seminal plasma. The same research group reviewed nine studies and reported 2064 proteins in the seminal plasma. Semenogelins (SEMG1 and SEMG2) were the two most abundant proteins (80%), whereas 10% of the proteins were from seminal extracellular vesicles, including epididymosomes and prostasomes [22].
The testicular proteome too reflects the function of sperm, as spermatogenesis takes place in the testis. Guo et al. [26] reported that the human testis expressed >1400 proteins and that 39 testis-specific proteins are involved in testicular function. Integrative analysis of the testis, sperm, and seminal plasma proteome reflected that 3901 sperm proteins are also present in the testes and 1213 proteins were associated with the acrosome and proteasome complex. Proteins belonging to molecular processes such as cell motility and peptidase activity that are essential for sperm motility and capacitation are transported from the seminal plasma to spermatozoa [22].
Protein profiling and male infertility
In 2004, Pixton et al. [68] reported the expression of at least 20 proteins that were altered in an infertile patient compared to fertile donors. Later, several proteomic studies were conducted to decipher the role of differentially expressed proteins in different infertility conditions related to sperm defects and abnormalities, i.e. varicocele, OS, mitochondrial dysfunction, sperm DNA damage, and testicular cancer. Ultimately, a set of potential protein biomarkers was proposed to distinguish infertility conditions as a cause of subcellular changes.
Semen abnormalities and proteomics
Azoospermia is associated with the absence of spermatozoa in the semen ejaculate and is the most discussed male infertility condition [27]. Obstructive azoospermia (OA) is caused by physical obstruction or blockage in the male reproductive tract, whereas non-obstructive azoospermia (NOA) is mainly due to the arrest of the spermatogenesis process. Proteomic analysis of seminal plasma has shown the absence of certain proteins responsible for sperm function. Prostatic acid phosphatase/prostatic-specific acid phosphatase (ACPP) and kallikrein 3 (KLK3) proteins were absent in azoospermic patients [28], and other proteins such as clusterin (CLU), zinc α2-glycoprotein 1 (AZGP1) and progestogen-associated endometrial protein/glycodelin S (PAEP) were also reported to be absent [29]. Different proteomic studies were conducted to determine the differential expression of proteins in azoospermia [24], [29], [30], [31]. Testis-expressed protein 101 (TEX101) is characterised as the biomarker for azoospermia and extracellular matrix protein 1 (ECM1) was able to differentiate NOA and post-vasectomy men with a threshold value of 2.3 μg/mL [32].
Asthenozoospermia patients are characterised by reduced progressive motility of spermatozoa (<32%). Proteins related to motility and energy metabolism are altered especially in spermatozoa. A similar proteome profile was exhibited by both asthenozoospermia and immature sperm [33], and the DEPs were associated with energetic metabolism, protein folding/degradation, vesicle trafficking, and cytoskeleton. Dysregulation of protein tyrosine phosphatase, non-receptor type 14 (PTPN14), a tyrosine phosphatase protein involved in the regulation of sperm motility is seen in asthenozoospermic patients. Recently, Hashemitabar et al. [34] identified 14 DEPs as potential protein biomarkers that are associated with vital sperm function. A similar study by Martinez-Heredia et al. [35] also identified 17 DEPs. Alteration in the expression of the phosphorylated proteins such as heat shock proteins (HSPs) due to post-translational modification also had a negative impact on sperm motility [36].
Globozoospermia, known as round-headed sperm syndrome, is associated with sperm abnormalities. Key proteins involved in spermatogenesis, sperm motility, and cytoskeleton organisation are differentially expressed in globozoospermia [37]. Proteins related to acrosome formation were also altered, especially the proteins present in the perinuclear theca region [38].
Another important and common sperm abnormality, oligoasthenozoospermia (OAT), is a male infertility factor with mitochondrial and chromosomal abnormalities. Proteomic profiling of patients with OAT identified a total of 2489 proteins from seminal plasma and their participation in the glycerolipid metabolism pathway [39]. A study by Sharma et al. [40], [41] reported the down-regulation of cystatin 3 (CST3) and up-regulation of KLK3 and SEMG1 sperm proteins in OAT semen samples. Another similar study also determined the down-regulation of epididymal secretory protein E1 (NPC2), galectin-3 binding protein (LGALS3BP), lipocalin 1 (LCN1), and prolactin induced protein (PIP) in oligoasthenozoospermia compared with normozoospermia [42] (Table 1 [29], [30], [31], [34], [35], [39], [41], [79].
Table 1.
Identification of DEPs involved in semen abnormalities using high-throughput techniques.
| Condition | Sample | DEPs | References |
|---|---|---|---|
| Azoospermia | Seminal plasma | CLU, AZGP1, PAEP, APCS, NPC2, CRISP1 and SOD1 | [29] |
| Seminal plasma | STAB2, CP135, GNRP, PIP, NPC2 | [30] | |
| Seminal plasma | LDHC, SPAG11B, MUC15, TEX101 and CEL | [31] | |
| Seminal plasma | COL6A2, GGT7, SORD, PGK2, LDHC, ZPBP2 and ELSPBP1 | [79] | |
| Asthenozoospermia | Spermatozoa | COX6B, HIST1H2BA and HSPA2 | [35] |
| Spermatozoa | COX6B and HSPA2 | [34] | |
| Oligoasthenozoospermia | Seminal plasma | TBCB, AACT and ALDR | [39] |
| Seminal plasma | CST3, AZGP1, TIMP1, SEMG1, and KLK3 | [41] |
AACT, α1-antichymotrypsin; ALDR, aldose reductase; APCS, amyloid P component, serum; CEL, carboxyl ester lipase; CP135, centrosomal protein 135; COL6A2, collagen type VI α2 chain; COX6B, cytochrome C oxidase subunit 6B; ELSPBP1, epididymal sperm-binding protein 1; GGT7, γ-glutamyltransferase 7; GNRP, guanine nucleotide-releasing protein; HIST1H2BA, histone cluster 1 H2B family member A; LDHC, lactate dehydrogenase C; MUC15, mucin 15, cell surface associated; SORD, sorbitol dehydrogenase; SPAG11B, sperm-associated antigen 11B; STAB2, stabilin 2; TBCB, tubulin-folding cofactor B; TIMP1, tissue inhibitor of metalloproteinase 1.
Male reproductive disorders and dysfunction
Varicocele is the abnormal dilation of the pampiniform venous plexus surrounding the spermatic cord involving the back flow of blood from the abdomen into the testis [43]. It affects 35% and 81% of the primary and secondary infertile men, respectively [44]. Even though patients with varicocele have normal semen parameters, their fertility status is likely to be compromised. This is due to disturbance in the spermatozoa at a molecular level. Nitric oxide metabolism involved in the generation of ROS was activated in patients with varicocele [45], whereas varicocelectomy increased the expression of HSP family A (HSP70) member 5 (HSPA5), superoxide dismutase 1 (SOD1) and ATP synthase, H+ transporting, mitochondrial F1 complex, delta subunit (ATP5D) in spermatozoa [46]. Seminal plasma proteins [calcium binding protein (CAB45) and cysteine-rich secretory protein 3 (CRISP3)] also showed altered expression in patients with varicocele [47].
Extensive research has been carried out at our laboratory to decipher the role of DEPs in patients with both unilateral and bilateral varicocele. All these proteins were involved in spermatogenesis, mitochondrial dysfunction, energy metabolism, and acrosome reaction [48], [49], [50], [51]. Comparative proteomic analysis identified 58 DEPs in bilateral varicocele [48] and 38 proteins were unique in patients with unilateral varicocele [50]. Another study comparing patients with unilateral and bilateral varicocele predicted glutathione S-transferase mu 3 (GSTM3), sperm protein associated with the nucleus, X chromosome, family member B1 (SPANXB1), Parkinson disease protein 7 (PARK7), proteasome subunit α8 (PSMA8), dihydrolipoamide dehydrogenase (DLD), SEMG1, and SEMG2 as potential biomarkers to differentiate unilateral from bilateral varicocele [48]. Network and pathway analysis was able to show that 87% of the DEPs involved in major energy metabolism were associated with mitochondrial dysfunction [48]. A list of potential biomarkers in different varicocele conditions is provided in Table 2 [13], [41], [48], [49], [66], [80].
Table 2.
Identification of DEPs involved in male reproductive disorders and dysfunction using high-throughput techniques.
| Condition | Sample | DEPs | References |
|---|---|---|---|
| Varicocele | Spermatozoa | TEKT3 and TCP11 | [48] |
| HSPA2, ODF2, CCT6B | [49] | ||
| OS | Spermatozoa | HIST1H2BA, MDH2, TGM4, GPX4, GLUL, HSP90B1, HSPA5 | [80] |
| Spermatozoa | CLGN, TPPII, DNAI2, EEA1, HSPA4L, SERPINA5 | [13] | |
| Seminal plasma | PIP, SEMG2, ACPP, CLU, AZGP1, KLK3, CST4, ALB, LTF, FN1, MIF, LGALS3BP | [41] | |
| Mitochondrial dysfunction | Seminal plasma | ANXA7 | [66] |
| Spermatozoa | NDUFS1, UQCRC2, ACO2, NAD, IDH3B, OGDH | [49] |
ACO2, aconitate hydratase; ALB, albumin; CCT6B, chaperonin containing t-complex protein 1 subunit 6; CLGN, calmegin; CST4, cystatin S-type 4; DNAI2, dynein axonemal intermediate chain 2; EEA1, early endosome antigen 1; GLUL, glutamate-ammonia ligase; GPX4, glutathione peroxidase 4; HSPA4L, HSP Family A (Hsp70) Member 4 Like; HSP90B1, HSP 90 β Family Member 1; IDH3B, isocitrate dehydrogenase (NAD) subunit β; LTF, lactotransferrin; MDH2, malate dehydrogenase 2; NAD, nicotinamide adenine dinucleotide; NDUFS1, NADH:ubiquinone oxidoreductase core subunit S1; OGDH, 2-oxoglutarate dehydrogenase; SERPINA5, serpin family A member 5; TCP 11, T-complex protein 11; TEKT3, tektin 3; TGM4, transglutaminase 4; TPPII, tripeptidyl peptidase 2; UQCRC2, complex-III cytochrome b-c1 complex subunit 2.
OS due to increased production of ROS in semen is the most cited cause of infertility [52], [53], [54]. Excessive production of ROS including the hydroxyl radical, peroxyl radicals, nitrous oxide, and nitroxyl anions results in prolonged OS, which can result in DNA damage [55], [56], [57], [58], [59], [60]. High levels of oxidants also negatively impact sperm motility, count, morphology, and viability [4], [61]. OS affects the stress responses and regulatory pathways in seminal plasma, as well as metabolic and stress responses in spermatozoa [17]. Sharma et al. [40] conducted proteomic studies on ROS-positive and -negative patients and proposed several potential biomarkers in both spermatozoa and seminal plasma (Table 2). Important proteins such as fibronectin 1 (FN1), macrophage migration inhibitory factor (MIF) and LGALS3BP were absent in the ROS-positive group but present in the ROS-negative group. However, in the seminal plasma, membrane metalloendopeptidase (MME) protein was absent in the ROS-negative group. A study by Intasqui et al. [62] suggested mucin 5B, oligomeric mucus/gel-forming (MUC5B) as a potential biomarker in patients with OS with high lipid peroxidation.
Nuclear DNA damage to the spermatozoa affects the fertilisation process due to alteration in the sperm proteome. Intasqui et al. [63] reported 23 and 71 overexpressed proteins in spermatozoa with high and low DNA damage, respectively. Overexpression of proteins had an impact on the triacylglycerol metabolism, energy production, protein folding, response to unfolded proteins, and cellular detoxification process. Certain proteins such as solute carrier family 2 member 14 (SLC2A14), phosphoglycerate kinase 2 (PGK2), outer dense fibre of sperm tails 1 (ODF1), CLU, voltage-dependent anion channel 2 (VDAC2), VDAC3, zona pellucida binding protein 2 (ZPBP2), and gastricsin (PGC) were identified as potential biomarkers. However, only nine and 21 proteins in the seminal plasma were differentially expressed in samples with low and high sperm DNA fragmentation, respectively [64]. Cysteine-rich secretory protein LCCL domain-containing 1 (CRISPLD1), CRISPLD2, and retinoic acid receptor responder protein 1 (RARRES1) were identified as seminal plasma biomarkers and proteasome subunit α type A signalling was hyper-regulated affecting sperm motility, acrosome reaction, and capacitation [65], [66].
Mitochondrial dysfunction is a result of the disturbance in the electron transport chain and energy metabolism process of the spermatozoa. Mitochondrial proteins play a major role in maintaining energy homeostasis of spermatozoa and its activity level determines normal sperm function. Annexin A7 (ANXA7) is a proposed biomarker for sperm mitochondrial dysfunction disorder [66]. Mitochondrial dysfunction of sperm is more prominent in varicocele and it is implicated by alteration in the proteins involved in mitochondrial electron transport complex, energy metabolism, and sperm motility [48].
Miscellaneous causes include those related to androgen deficiency, in which AZGP1, ACPP and PIP proteins involved in the catalytic and binding activities were found to be absent in androgen-deficient patients [67]. A study by Pixton et al. [68] identified that PIP and ODF2 proteins were overexpressed in spermatozoa that were unable to fertilise an oocyte. Whereas an inflammatory condition of epididymis known as epididymitis affected the expression of 35 sperm proteins [69].
A recent in vitro study conducted on spermatozoa to check the effect of harmful xenoestrogen bisphenol-A revealed a change in the sperm proteome. A set of 24 proteins were differentially expressed on exposure to bisphenol-A and were involved in the activation of several kinase pathways in spermatozoa [70].
Male reproductive cancer
Fertility preservation has become an essential process in patients being treated for cancer. Generally, sperm concentration is low in ∼50% of patients with testicular cancer and 40% of patients with Hodgkin’s disease. Infertility is one of the noted side-effects of cancer treatment. Cancer treatment causes severe damage to the gonads and DNA of germ cells, thus affecting the fertilisation process. Cancers related to the reproductive system not only decrease the immunity of the system, but also have harmful effects on spermatogenesis [71]. Analysis of human testicular tissue using 2D-high-performance liquid chromatography–MS/MS detected that out of 7346 proteins, transmembrane protease, serine 12 (TMPRSS12); tubulin polymerisation promoting protein family member 2 (TPPP2); protease, serine 55 (PRSS55); doublesex and mab-3 related transcription factor 1 (DMRT1); piwi-like RNA-mediated gene silencing 1 (PIWIL1), and hemogen (HEMGN) were associated with cancer [72]. Our laboratory was the first to identify 398 DEPs [including the overexpression of PSA, prostatic acid phosphatase (PAcP), zinc α2-glycoprotein (ZAG), and SEMG1 and SEMG2, as well as under expression of A-kinase anchoring protein 4 (AKAP4) and dynein axonemal heavy chain 17 (DNAH17)] in patients with testicular cancer using a global proteomic approach. Mitochondrial dysfunction, oxidative phosphorylation, and tricarboxylic acid cycle were the major pathways dysregulated in the spermatozoa of patients with testicular cancer [73].
Biomarker identification and diagnostic approach
Biomarker screening and identification is a laborious process and the most important task that needs to be accomplished for the development of novel diagnostics or therapeutics. Biomarkers are biomolecules present in body fluids and alteration in their concentration or expression is indicative of a pathophysiological state. It is used as a tool for predicting prognosis, diagnosis, and treatment outcomes [74]. In the past two decades, molecular biomarkers have gained importance in the field of medicine as diagnostic molecules due to their specificity, detectability, and availability for identification in the early stages of cellular or tissue damage [75]. The translational research platform has improved much with the use of biomarkers in clinical diagnosis and treatment.
The main challenge faced in the field of male infertility is to understand the subcellular changes or causes that affect physiological function of spermatozoa due to its complex biological system. Proteomics has proven to be a promising tool for discovering biomarkers related to male infertility. Analysis of proteomic data using bioinformatics tools provides functional information about the proteins regulating biological pathways related to reproductive function [76]. Clinical sperm proteomics serves as a useful platform and scientists have discovered various potential molecular biomarkers associated with various disorders of male infertility.
Future of proteomics in male infertility
The future of proteomics in male infertility depends on overcoming the limitation in analysing semen samples. High-throughput sperm proteomic studies have certain limitations due to the complexity of the sample. It is well explained by the presence of highly abundant proteins such as SEMGs, particularly in the seminal plasma [45], [64]. These highly abundant peptides mask the detection of other proteins analysed by MS. Therefore, a rapid increase in sensitivity and resolution of analytical techniques is required [77], [78]. Integration of the other ‘omics’ (transcriptomics and metabolomics) with proteomics should interlink the pathways affected in spermatozoa and provide unbiased results. Apart from the discoveries in basic research, its purpose is fulfilled by translating these findings into a clinical setting.
Conclusion
Proteomic studies on the highly differentiated spermatozoa have evaluated the proteins involved in infertility conditions. Most of the DEPs in infertile patients alter normal sperm function at a molecular level. Validation and screening of the identified potential protein biomarker using Western blot and ELISA will further strengthen the biomarker confirmation for a specific infertility condition. Furthermore, it will benefit infertile couples with personalised treatment by biomarker screening in patients. Finally, the use of all omic-based platforms in diagnosing male infertility will have a maximum clinical impact.
Conflict of interest
None.
Source of funding
None.
Diagnosis
Footnotes
Peer review under responsibility of Arab Association of Urology.
Abbreviations: ACPP, prostatic acid phosphatase/prostatic-specific acid phosphatase; ANXA7, annexin A7; AZGP1, zinc α2-glycoprotein 1; CLU, clusterin; CRISP(1)(3),cysteine-rich secretory protein (1) (3); CRISPLD(1)(2), CRISP LCCL domain-containing (1) (2); CST3, cystatin 3; 2D, two dimensional; DEP, differentially expressed protein; FN1, fibronectin 1; GO, gene ontology; HIST1H2BA, histone cluster 1 H2B family member A; HSP, heat shock protein; HSPA(2)(5), HSP family A (HSP70) member (2) (5); KLK3, kallikrein 3; LC–(MS), liquid chromatography–(mass spectrometry); LGALS3BP, galectin-3 binding protein; MALDI-TOF, matrix-assisted laser desorption ionisation time-of-flight; MIF, macrophage migration inhibitory factor; NSAF, normalised spectral abundance factor; (N)OA, (non-) obstructive azoospermia; OAT, oligoasthenozoospermia; ODF(1), outer dense fibre of sperm tails (1) (2); OS, oxidative stress; PAEP, progestogen-associated endometrial protein/glycodelin S; PGK2, phosphoglycerate kinase 2; PIP, prolactin induced protein; ROS, reactive oxygen species; SDS, sodium dodecyl sulphate; SEMG(1)(2), semenogelin (1) (2); SOD1, superoxide dismutase 1; SpC, spectral count; TEX101, testis-expressed protein 101; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling; VDAC(2)(3), voltage-dependent anion channel (2) (3); ZPBP2, zona pellucida binding protein 2.
References
- 1.Agarwal A., Samanta L., Bertolla R.P., Durairajanayagam D., Intasqui P. Springer; New York: 2016. Springer briefs in reproductive biology: proteomics in human reproduction: biomarkers for millennials. [Google Scholar]
- 2.World Health Organization . 5th ed. WHO press; Switzerland: 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 3.Agarwal A., Gupta S., Sharma R. Reactive oxygen species (ROS) measurement. In: Agarwal A., Gupta S., Sharma R., editors. Andrological evaluation of male infertility: a laboratory guide. Springer International Publishing; New York: 2016. pp. 155–163. [Google Scholar]
- 4.Agarwal A., Sharma R., Roychoudhury S., Du Plessis S., Sabanegh E. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril. 2016;106:566–573. doi: 10.1016/j.fertnstert.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A., Gupta S., Sharma R. Measurement of DNA fragmentation in spermatozoa by TUNEL assay using bench top flow cytometer. In: Agarwal A., Gupta S., Sharma R., editors. Andrological evaluation of male infertility: a laboratory guide. Springer International Publishing; New York: 2016. pp. 181–203. [Google Scholar]
- 6.Baker M.A., Nixon B., Naumovski N., Aitken R.J. Proteomic insights into the maturation and capacitation of mammalian spermatozoa. Syst Biol Reprod Med. 2012;58:211–217. doi: 10.3109/19396368.2011.639844. [DOI] [PubMed] [Google Scholar]
- 7.Johnston D.S., Wooters J., Kopf G.S., Qiu Y., Roberts K.P. Analysis of the human sperm proteome. Ann N Y Acad Sci. 2005;1061:190–202. doi: 10.1196/annals.1336.021. [DOI] [PubMed] [Google Scholar]
- 8.Baker M.A., Aitken R.J. Proteomic insights into spermatozoa: critiques, comments and concerns. Expert Rev Proteomics. 2009;6:691–705. doi: 10.1586/epr.09.76. [DOI] [PubMed] [Google Scholar]
- 9.Baker M.A., Witherdin R., Hetherington L., Cunningham-Smith K., Aitken R.J. Identification of post-translational modifications that occur during sperm maturation using difference in two-dimensional gel electrophoresis. Proteomics. 2005;5:1003–1012. doi: 10.1002/pmic.200401100. [DOI] [PubMed] [Google Scholar]
- 10.Mann M., Jensen O.N. Proteomic analysis of post-translational modifications. Nat Biotechnol. 2003;21:255–261. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- 11.du Plessis S.S., Kashou A.H., Benjamin D.J., Yadav S.P., Agarwal A. Proteomics: a subcellular look at spermatozoa. Reprod Biol Endocrinol. 2011;9:36. doi: 10.1186/1477-7827-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnum K.E., Frappier S.L., Caprioli R.M. Matrix-assisted laser desorption/ionization imaging mass spectrometry for the investigation of proteins and peptides. Annu Rev Anal Chem. 2008;1:689–705. doi: 10.1146/annurev.anchem.1.031207.112841. [DOI] [PubMed] [Google Scholar]
- 13.Oliva R., De Mateo S., Castillo J., Azpiazu R., Oriola J., Ballescà J.L. Methodological advances in sperm proteomics. Hum Fertil (Camb) 2010;13:263–267. doi: 10.3109/14647273.2010.516877. [DOI] [PubMed] [Google Scholar]
- 14.Ayaz A., Agarwal A., Sharma R., Arafa M., Elbardisi H., Cui Z. Impact of precise modulation of reactive oxygen species levels on spermatozoa proteins in infertile men. Clin Proteomics. 2015;12:4. doi: 10.1186/1559-0275-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lan N., Montelione G.T., Gerstein M. Ontologies for proteomics: towards a systematic definition of structure and function that scales to the genome level. Curr Opin Chem Biol. 2003;7:44–54. doi: 10.1016/s1367-5931(02)00020-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhou T., Zhou Z.M., Guo X.J. Bioinformatics for spermatogenesis: annotation of male reproduction based on proteomics. Asian J Androl. 2013;15:594–602. doi: 10.1038/aja.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal A., Durairajanayagam D., Halabi J., Peng J., Vazquez-Levin M. Proteomics, oxidative stress and male infertility. Reprod Biomed Online. 2014;29:32–58. doi: 10.1016/j.rbmo.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Duncan M.W., Thompson H.S. Proteomics of semen and its constituents. Proteomics Clin Appl. 2007;1:861–875. doi: 10.1002/prca.200700228. [DOI] [PubMed] [Google Scholar]
- 19.Sharma R., Agarwal A. Spermatogenesis: an overview. In: Zini A., Agarwal A., editors. Sperm chromatin: biological and clinical applications in male infertility and assisted reproduction. Springer; New York: 2011. pp. 19–44. [Google Scholar]
- 20.Martínez-Heredia J., Estanyol J.M., Ballescà J.L., Oliva R. Proteomic identification of human sperm proteins. Proteomics. 2006;6:4356–4369. doi: 10.1002/pmic.200600094. [DOI] [PubMed] [Google Scholar]
- 21.Amaral A., Castillo J., Ramalho-Santos J., Oliva R. The combined human sperm proteome: cellular pathways and implications for basic and clinical science. Hum Reprod Update. 2013;20:40–62. doi: 10.1093/humupd/dmt046. [DOI] [PubMed] [Google Scholar]
- 22.Jodar M., Soler-Ventura A., Oliva R. Molecular Biology of Reproduction and Development Research Group2. Semen proteomics and male infertility. J Proteomics. 2017;162:125–134. doi: 10.1016/j.jprot.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Jodar M., Sendler E., Krawetz S.A. The protein and transcript profiles of human semen. Cell Tissue Res. 2016;363:85–96. doi: 10.1007/s00441-015-2237-1. [DOI] [PubMed] [Google Scholar]
- 24.Batruch I., Lecker I., Kagedan D., Smith C.R., Mullen B.J., Grober E. Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J Proteome Res. 2011;10:941–953. doi: 10.1021/pr100745u. [DOI] [PubMed] [Google Scholar]
- 25.Pilch B., Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo X., Zhang P., Huo R., Zhou Z., Sha J. Analysis of the human testis proteome by mass spectrometry and bioinformatics. Proteomics Clin Appl. 2008;2:1651–1657. doi: 10.1002/prca.200780120. [DOI] [PubMed] [Google Scholar]
- 27.Hamada A., Sharma R., du Plessis S.S., Willard B., Yadav S.P., Sabanegh E. Two-dimensional differential in-gel electrophoresis–based proteomics of male gametes in relation to oxidative stress. Fertil Steril. 2013;99:e2. doi: 10.1016/j.fertnstert.2012.11.046. 1216-26.e2. [DOI] [PubMed] [Google Scholar]
- 28.Starita-Geribaldi M., Poggioli S., Zucchini M., Garin J., Chevallier D., Fenichel P. Mapping of seminal plasma proteins by two-dimensional gel electrophoresis in men with normal and impaired spermatogenesis. Mol Hum Reprod. 2001;7:715–722. doi: 10.1093/molehr/7.8.715. [DOI] [PubMed] [Google Scholar]
- 29.Starita-Geribaldi M., Roux F., Garin J., Chevallier D., Fénichel P., Pointis G. Development of narrow immobilized pH gradients covering one pH unit for human seminal plasma proteomic analysis. Proteomics. 2003;3:1611–1619. doi: 10.1002/pmic.200300493. [DOI] [PubMed] [Google Scholar]
- 30.Yamakawa K., Yoshida K., Nishikawa H., Kato T., Iwamoto T. Comparative analysis of interindividual variations in the seminal plasma proteome of fertile men with identification of potential markers for azoospermia in infertile patients. J Androl. 2007;28:858–865. doi: 10.2164/jandrol.107.002824. [DOI] [PubMed] [Google Scholar]
- 31.Drabovich A.P., Jarvi K., Diamandis E.P. Verification of male infertility biomarkers in seminal plasma by multiplex selected reaction monitoring assay. Mol Cell Proteomics. 2011;10(M110):004127. doi: 10.1074/mcp.M110.004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drabovich A.P., Dimitromanolakis A., Saraon P., Soosaipillai A., Batruch I., Mullen B. Differential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasma. Sci Transl Med. 2013;5:212ra160. doi: 10.1126/scitranslmed.3006260. [DOI] [PubMed] [Google Scholar]
- 33.Amaral A., Paiva C., Attardo Parrinello C., Estanyol J.M., Ballescà J.L., Ramalho-Santos J. Identification of proteins involved in human sperm motility using high-throughput differential proteomics. J Proteome Res. 2014;13:5670–5684. doi: 10.1021/pr500652y. [DOI] [PubMed] [Google Scholar]
- 34.Hashemitabar M., Sabbagh S., Orazizadeh M., Ghadiri A., Bahmanzadeh M. A proteomic analysis on human sperm tail: comparison between normozoospermia and asthenozoospermia. J Assist Reprod Genet. 2015;32:853–863. doi: 10.1007/s10815-015-0465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martínez-Heredia J., de Mateo S., Vidal-Taboada J.M., Ballescà J.L., Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod. 2008;23:783–791. doi: 10.1093/humrep/den024. [DOI] [PubMed] [Google Scholar]
- 36.Parte P.P., Rao P., Redij S., Lobo V., D'Souza S.J., Gajbhiye R. Sperm phosphoproteome profiling by ultra-performance liquid chromatography followed by data independent analysis (LC–MS E) reveals altered proteomic signatures in asthenozoospermia. J Proteomics. 2012;75:5861–5871. doi: 10.1016/j.jprot.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Liao T.T., Xiang Z., Zhu W.B., Fan L.Q. Proteome analysis of round-headed and normal spermatozoa by 2-D fluorescence difference gel electrophoresis and mass spectrometry. Asian J Androl. 2009;11:683–693. doi: 10.1038/aja.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez Sedó C., Rawe V.Y., Chemes H.E. Acrosomal biogenesis in human globozoospermia: immunocytochemical, ultrastructural and proteomic studies. Hum Reprod. 2012;27:1912–1921. doi: 10.1093/humrep/des126. [DOI] [PubMed] [Google Scholar]
- 39.Herwig R., Knoll C., Planyavsky M., Pourbiabany A., Greilberger J., Bennett K.L. Proteomic analysis of seminal plasma from infertile patients with oligoasthenoteratozoospermia due to oxidative stress and comparison with fertile volunteers. Fertil Steril. 2013;100:e2. doi: 10.1016/j.fertnstert.2013.03.048. 355-66 e2. [DOI] [PubMed] [Google Scholar]
- 40.Sharma R., Agarwal A., Mohanty G., Du Plessis S.S., Gopalan B., Willard B. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol. 2013;11:85. doi: 10.1186/1477-7827-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma R., Agarwal A., Mohanty G., Jesudasan R., Gopalan B., Willard B. Functional proteomic analysis of seminal plasma proteins in men with various semen parameters. Reprod Biol Endocrinol. 2013;11:38. doi: 10.1186/1477-7827-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giacomini E., Ura B., Giolo E., Luppi S., Martinelli M., Garcia R.C. Comparative analysis of the seminal plasma proteomes of oligoasthenozoospermic and normozoospermic men. Reprod Biomed Online. 2015;30:522–531. doi: 10.1016/j.rbmo.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal A., Hamada A., Esteves S.C. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9:678–690. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 44.Esteves S.C., Agarwal A. Afterword to varicocele and male infertility: current concepts and future perspectives. Asian J Androl. 2016;18:319–322. doi: 10.4103/1008-682X.172820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camargo M., Intasqui Lopes P., Del Giudice P.T., Carvalho V.M., Cardozo K.H., Andreoni C. Unbiased label-free quantitative proteomic profiling and enriched proteomic pathways in seminal plasma of adult men before and after varicocelectomy. Hum Reprod. 2013;28:33–46. doi: 10.1093/humrep/des357. [DOI] [PubMed] [Google Scholar]
- 46.Hosseinifar H., Sabbaghian M., Nasrabadi D., Modarresi T., Dizaj A.V., Gourabi H. Study of the effect of varicocelectomy on sperm proteins expression in patients with varicocele and poor sperm quality by using two-dimensional gel electrophoresis. J Assist Reprod Genet. 2014;31:725–729. doi: 10.1007/s10815-014-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Giudice P., Belardin L.B., Camargo M., Zylbersztejn D.S., Carvalho V.M., Cardozo K.H. Determination of testicular function in adolescents with varicocoele–a proteomics approach. Andrology. 2016;4:447–455. doi: 10.1111/andr.12174. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal A., Sharma R., Durairajanayagam D., Cui Z., Ayaz A., Gupta S. Spermatozoa protein alterations in infertile men with bilateral varicocele. Asian J Androl. 2016;18:43–53. doi: 10.4103/1008-682X.153848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agarwal A., Sharma R., Samanta L., Durairajanayagam D., Sabanegh E. Proteomic signatures of infertile men with clinical varicocele and their validation studies reveal mitochondrial dysfunction leading to infertility. Asian J Androl. 2016;18:282–291. doi: 10.4103/1008-682X.170445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal A., Sharma R., Durairajanayagam D., Cui Z., Ayaz A., Gupta S. Differential proteomic profiling of spermatozoal proteins of infertile men with unilateral or bilateral varicocele. Urology. 2015;85:580–588. doi: 10.1016/j.urology.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 51.Agarwal A., Sharma R., Durairajanayagam D., Ayaz A., Cui Z., Willard B. Major protein alterations in spermatozoa from infertile men with unilateral varicocele. Reprod Biol Endocrinol. 2015;13:8. doi: 10.1186/s12958-015-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aitken R.J., Clarkson J.S., Hargreave T.B., Irvine D.S., Wu F.C. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J Androl. 1989;10:214–220. doi: 10.1002/j.1939-4640.1989.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 53.Sharma R.K., Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–850. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 54.Agarwal A., Sharma R.K., Nallella K.P., Thomas A.J., Jr, Alvarez J.G., Sikka S.C. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–885. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal A., Saleh R.A., Bedaiwy M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 56.Aitken R., De Iuliis G. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2009;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 57.Aitken R.J., Koppers A.J. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novotny J., Aziz N., Rybar R., Brezinova J., Kopecka V., Filipcikova R. Relationship between reactive oxygen species production in human semen and sperm DNA damage assessed by Sperm Chromatin Structure Assay. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:383–386. doi: 10.5507/bp.2013.065. [DOI] [PubMed] [Google Scholar]
- 59.Dorostghoal M., Kazeminejad S.R., Shahbazian N., Pourmehdi M., Jabbari A. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia. 2017;49:e12762. doi: 10.1111/and.12762. [DOI] [PubMed] [Google Scholar]
- 60.Wang X., Sharma R.K., Sikka S.C., Thomas A.J., Jr, Falcone T., Agarwal A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil Steril. 2003;80:531–535. doi: 10.1016/s0015-0282(03)00756-8. [DOI] [PubMed] [Google Scholar]
- 61.Agarwal A., Wang S.M. Clinical relevance of oxidation-reduction potential in the evaluation of male infertility. Urology. 2017;104:84–89. doi: 10.1016/j.urology.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 62.Intasqui P., Antoniassi M.P., Camargo M., Nichi M., Carvalho V.M., Cardozo K.H. Differences in the seminal plasma proteome are associated with oxidative stress levels in men with normal semen parameters. Fertil Steril. 2015;104:292–301. doi: 10.1016/j.fertnstert.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 63.Intasqui P., Camargo M., Del Giudice P.T., Spaine D.M., Carvalho V.M., Cardozo K.H. Unraveling the sperm proteome and post-genomic pathways associated with sperm nuclear DNA fragmentation. J Assist Reprod Genet. 2013;30:1187–1202. doi: 10.1007/s10815-013-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Intasqui P., Camargo M., Del Giudice P.T., Spaine D.M., Carvalho V.M., Cardozo K.H. Sperm nuclear DNA fragmentation rate is associated with differential protein expression and enriched functions in human seminal plasma. BJU Int. 2013;112:835–843. doi: 10.1111/bju.12233. [DOI] [PubMed] [Google Scholar]
- 65.Antoniassi M.P., Intasqui P., Camargo M., Zylbersztejn D.S., Carvalho V.M., Cardozo K.H. Analysis of the functional aspects and seminal plasma proteomic profile of sperm from smokers. BJU Int. 2016;118:814–822. doi: 10.1111/bju.13539. [DOI] [PubMed] [Google Scholar]
- 66.Intasqui P., Camargo M., Antoniassi M.P., Cedenho A.P., Carvalho V.M., Cardozo K.H. Association between the seminal plasma proteome and sperm functional traits. Fertil Steril. 2016;105:617–628. doi: 10.1016/j.fertnstert.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Milardi D., Grande G., Vincenzoni F., Giampietro A., Messana I., Castagnola M. Novel biomarkers of androgen deficiency from seminal plasma profiling using high-resolution mass spectrometry. J Clin Endocrinol Metab. 2014;99:2813–2820. doi: 10.1210/jc.2013-4148. [DOI] [PubMed] [Google Scholar]
- 68.Pixton K.L., Deeks E.D., Flesch F.M., Moseley F.L., Björndahl L., Ashton P.R. Sperm proteome mapping of a patient who experienced failed fertilization at IVF reveals altered expression of at least 20 proteins compared with fertile donors: case report. Hum Reprod. 2004;19:1438–1447. doi: 10.1093/humrep/deh224. [DOI] [PubMed] [Google Scholar]
- 69.Pilatz A., Lochnit G., Karnati S., Paradowska-Dogan A., Lang T., Schultheiss D. Acute epididymitis induces alterations in sperm protein composition. Fertil Steril. 2014;101 doi: 10.1016/j.fertnstert.2014.03.011. 1609–17.e1-5. [DOI] [PubMed] [Google Scholar]
- 70.Rahman M.S., Kwon W.S., Lee J.S., Yoon S.J., Ryu B.Y., Pang M.G. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci Rep. 2015;5:9169. doi: 10.1038/srep09169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yokonishi T., Ogawa T. Cryopreservation of testis tissues and in vitro spermatogenesis. Reprod Med Biol. 2016;15:21–28. doi: 10.1007/s12522-015-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu M., Hu Z., Qi L., Wang J., Zhou T., Guo Y. Scanning of novel cancer/testis proteins by human testis proteomic analysis. Proteomics. 2013;13:1200–1210. doi: 10.1002/pmic.201200489. [DOI] [PubMed] [Google Scholar]
- 73.Agarwal A., Tvrda E., Sharma R., Gupta S., Ahmad G., Sabanegh E.S. Spermatozoa protein profiles in cryobanked semen samples from testicular cancer patients before treatment. Fertil Steril. 2015;104(Suppl.):260. [Google Scholar]
- 74.Mischak H., Allmaier G., Apweiler R., Attwood T., Baumann M., Benigni A. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med. 2010;2:46ps42. doi: 10.1126/scitranslmed.3001249. [DOI] [PubMed] [Google Scholar]
- 75.Kovac J.R., Pastuszak A.W., Lamb D.J. The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil Steril. 2013;99:998–1007. doi: 10.1016/j.fertnstert.2013.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Y., Wu Y., Jin K., Lu H., Liu F., Guo Y. Differential proteomic profiling in human spermatozoa that did or did not result in pregnancy via IVF and AID. Proteomics Clin Appl. 2013;7:850–858. doi: 10.1002/prca.201200078. [DOI] [PubMed] [Google Scholar]
- 77.Wang K., Huang C., Nice E. Recent advances in proteomics: towards the human proteome. Biomed Chromatogr. 2014;28:848–857. doi: 10.1002/bmc.3157. [DOI] [PubMed] [Google Scholar]
- 78.Fuhrer T., Zamboni N. High-throughput discovery metabolomics. Curr Opin Biotechnol. 2015;31:73–78. doi: 10.1016/j.copbio.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Batruch I., Smith C.R., Mullen B.J., Grober E., Lo K.C., Diamandis E.P. Analysis of seminal plasma from patients with non-obstructive azoospermia and identification of candidate biomarkers of male infertility. J Proteome Res. 2012;11:1503–1511. doi: 10.1021/pr200812p. [DOI] [PubMed] [Google Scholar]
- 80.Sharma R., Agarwal A., Mohanty G., Hamada A.J., Gopalan B., Willard B. Proteomic analysis of human spermatozoa proteins with oxidative stress. Reprod Biol Endocrinol. 2013;11:48. doi: 10.1186/1477-7827-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]