Abstract
The primary cilium is a mechanosensor in a variety of mammalian cell types, initiating and directing intracellular signaling cascades in response to external stimuli. When primary cilia formation is disrupted, cells have diminished mechanosensitivity and an abrogated response to mechanical stimulation. Due to this important role, we hypothesized that increasing primary cilia length would enhance the downstream response, and therefore, mechanosensitivity. To test this hypothesis, we increased osteocyte primary cilia length with fenoldopam and lithium and found that cells with longer primary cilia are more mechanosensitive. Furthermore, fenoldopam treatment potentiated adenylyl cyclase activity and was able to recover primary cilia form and sensitivity in cells with impaired cilia. This work demonstrates that modulating the structure of the primary cilium directly impacts cellular mechanosensitivity. Our results implicate cilium length as a potential therapeutic target for combating numerous conditions characterized by impaired cilia function.
Keywords: Primary cilia, mechanosensitivity, fenoldopam, osteocyte
Introduction
Mechanotransduction is a critical cellular process in a variety of tissues. Endothelial cells sense blood flow and transduce the mechanical stimuli into biochemical responses to adjust blood vessel diameter (Ku, 1997). Kidney epithelial cells in the collecting duct similarly sense and respond to varying rates of urine flow (Liu et al., 2003). Bone maintenance requires mechanical stimulation to maintain balanced formation and resorption (You et al., 2008). Understanding how cells sense mechanical cues and transduce them into biochemical responses is a critical impediment to developing novel treatments for a wide variety of diseases of structural tissues.
Cell mechanotransduction has been studied through several different mechanisms. Altering cytoskeletal mechanics by disruption of actin polymerization with cytochalasin D inhibits flow-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells (Arnsdorf et al., 2009). Similar experiments in osteoblasts treated with cytochalasin D and exposed to fluid flow demonstrated increased cellular mechanosensitivity, as measured by prostaglandin E2 release (Malone et al., 2007; Norvell et al., 2004). Integrins couple the extracellular matrix with the cytoskeleton through focal adhesions comprised of actin-associated proteins such as talin and vinculin, and inhibition of focal adhesion dynamics impairs mechanosensitivity in response to fluid shear (Castillo et al., 2012; Geiger et al., 2001). Mathematical models of osteocytes in vivo estimate that osteocyte dendritic processes may experience higher strain than the cell body (Vaughan et al., 2014). In a separate study, a transwell filter system was used to differentiate the osteocyte cell body and dendritic processes, and each were mechanically loaded separately (Burra et al., 2010). It was found that the glycocalyx of the dendritic processes is critical in forming integrin attachments that initiate a mechanotransduction pathway that results in the opening of hemichannels on the cell body. Gap junctions between adjacent cells are thought to contribute to mechanotransduction by mediating calcium, ATP, and prostaglandin E2 intercellular signaling (Plotkin et al., 2015). Membrane deformations also play a significant role in cell mechanosensing where stretch-activated channels, such as polycystin-2 (PC2) and transient receptor potential vanilloid 4 (TRPV4), mediate calcium influx to initiate mechanotransduction (Lee et al., 2015b; Phan et al., 2009; Piperi, 2015).
In this work we focus on primary cilia, single immotile organelles extending from the surface of nearly all mammalian cells, which have been implicated as mechanosensors in a variety of cell types. Praetorius and Spring first demonstrated that kidney epithelial cells respond to fluid flow, and specifically, that this mechanical stimulation causes primary cilia deflection (Praetorius and Spring, 2001). Furthermore, fluid flow initiates an intracellular calcium increase that is diminished when cilia are removed (Praetorius and Spring, 2003). Primary cilia have since been identified as mechanosensing organelles in a variety of cell types, including bone (Malone et al., 2007).
Osteocytes are mechanosensitive cells within bone, and previously, we have demonstrated that the primary cilium functions as a mechanosensor in this context (Malone et al., 2007). Fluid flow mechanical stimulation of osteocytes in vitro enhances expression of the osteogenic genes cyclooxygenase-2, COX-2, and osteopontin, OPN. COX-2 synthesizes prostaglandin E2, and OPN is a critical extracellular matrix protein. Increases in the production of both are indicative of osteogenesis (Ehrlich and Lanyon, 2002; Fujihara et al., 2006; Klein-Nulend et al., 1997; Raisz, 1999). When osteocyte primary cilia formation is inhibited, the cells display an abrogated osteogenic response to flow, implicating the cilium as a critical mechanosensor in osteocytes (Malone et al., 2007). Furthermore, we have previously reported that adenylyl cyclases, specifically AC6, play a significant role in osteocyte mechanosensitivity (Kwon et al., 2010). Adenylyl cyclases convert ATP to the ubiquitous second messenger cAMP, a process which can be specifically stimulated by forskolin.
Despite prior in vitro work, the role of osteocyte primary cilia in vivo is yet unclear. The presence of primary cilia within mineralized bone has been addressed with conflicting reports. Tonna and Lampen found that less than 4% of osteocytes possessed primary cilia, while Uzbekov et al. reported greater than 94% incidence (Tonna and Lampen, 1972; Uzbekov et al., 2012). Furthermore, the lacunar space within which osteocytes reside suggests that primary cilia are less than 1 μm long, as opposed to in vitro lengths which can be 4 μm (Malone et al., 2007; McNamara et al., 2009). A recent fluid-structure interface model was developed to estimate how primary cilia may deform in vivo (Vaughan et al., 2014). It was concluded that a short cilium, approximately 0.2 μm, was not long enough to be a mechanosensor, but that an elongated cilium spanning the full pericellular space could be. We have previously utilized a mouse model where primary cilia were deleted from osteocytes and osteoblasts, resulting in impaired mechanotransduction and abrogated load-induced bone formation (Temiyasathit et al., 2012). While the specific orientation, and even incidence, of osteocyte primary cilia in vivo remains unclear, evidence suggests that the cilium may yet play a key role in bone mechanosensing, and that modifying cilium structure may alter mechanotransduction.
While several small molecules exist to increase cilia length, in this work we utilize two drugs that are both clinically approved and have distinct mechanisms of action — fenoldopam, used to treat hypertension, and lithium, a treatment for bipolar disorder — to increase primary cilia length. Fenoldopam is a dopamine D1-like receptor agonist, and has previously been used to increase primary cilia length in endothelial cells and kidney epithelial cells, potentially through an adenylyl cyclase-cAMP mechanism (Kathem et al., 2014; Upadhyay et al., 2014). In the context of bone, MC3T3 osteoblasts express dopamine receptors 1-5, yet the role of specific dopamine receptors and the effects of fenoldopam treatment is unknown, and has never been examined in MLOY4 osteocytes (Lee et al., 2015a). Analysis of gene expression patterns after tibial loading in adult rats reveals a wide array of upregulated genes, including dopamine D1 receptor mRNA (Mantila Roosa et al., 2011). Interestingly, transgenic mice with a global deletion of this receptor have no apparent difference in bone architecture or calcification compared to controls (Drago et al., 1994). Mice with a homozygous deletion of the dopamine transporter have diminished bone mass, but the role of specific dopamine receptors was not examined (Bliziotes et al., 2000). Lithium is regularly used as an agonist of the Wnt signaling pathway in various cell types including MLO-Y4 osteocytes, and has been shown to increase primary cilia length in cultured fibroblasts and neurons through a yet incompletely characterized mechanism (Bivi et al., 2013; Miyoshi et al., 2009). In Lrp5 knockout mice, lithium treatment restores bone metabolism and bone mass, and activates Wnt signaling in isolated calvarial osteoblasts (Clément-Lacroix et al., 2005). Lithium and fenoldopam have distinct mechanisms of action and both increase primary cilia length in a variety of cell types, but their effects on primary cilia-mediated mechanotransduction in osteocytes has not been studied.
Due to the significance of primary cilia in cellular mechanotransduction, we hypothesized that increasing their length would enhance mechanosensitivity. Here, we treat osteocytes with lithium and fenoldopam to increase primary cilia length, and then mechanically stimulate the cells. We then examine the potential of targeting primary cilia length to recover impaired primary cilia-mediated mechanotransduction using models of impaired cilia and ciliary proteins. Our results highlight the importance of cilium length in cellular mechanosensitivity, and that this is a process that can be modulated by pharmacologic intervention.
Materials and Methods
Cell culture and drug treatments
MLO-Y4 osteocytes were cultured on collagen I-coated (Corning) dishes in MEMα (Life Technologies) supplemented with 5% fetal bovine serum, 5% calf serum, and 1% penicillin/streptomycin at 37°C and 5% CO2. Fenoldopam mesylate (Sigma) was used at 10 μM diluted in DMSO, dimethyl sulfoxide, (Sigma) and normal culture media, as previously described (Kathem et al., 2014; Upadhyay et al., 2014). Lithium chloride (Sigma) was used at 500 μM diluted in normal culture media – a dose response from 50 μM to 10 mM was examined with 500 μM being the lowest dose to increase length significantly, data not shown. These agents, or their vehicle control, were applied to cells for 16 hours prior to experimentation. MTT, methylthiazolyldiphenyl-tetrazolium bromide, assay (Sigma) was performed according to manufacturer’s protocol to assess cell viability with drug treatments. Phase contrast microscopy with an Olympus CKX41 inverted microscope and 40× objective was used to assess cell morphology.
Immunocytochemistry
For primary cilia imaging and analysis, cells cultured on collagen I-coated glass were fixed in 10% formalin and treated with anti-acetylated α-tubulin primary antibody, 1:1, from a C3B9 hybridoma cell line (Sigma). Cilia were visualized with Alexa-Fluor 488 secondary antibody, 1:1000, (Life Technologies), and imaged with a 100× oil objective on an Olympus Fluoview FV1000 confocal microscope. Nuclei were stained with DAPI (Life Technologies). Cilia lengths were analyzed using Image J.
Oscillatory fluid flow
Cells were exposed to oscillatory fluid flow as a mechanical stimulus. Cells were seeded on collagen I-coated glass slides at ~ 2800 cells/cm2 and cultured for 72 hours before application of flow. Drug treatments were applied 16 hours prior to experimentation. Slides were loaded into parallel plate flow chambers (dimensions: 75 × 38 × 0.28 mm) that we have previously described and allowed to incubate at 37°C for 30 minutes prior to initiation of stimulation (Kwon et al., 2010; Lee et al., 2014; Malone et al., 2007). Flow was applied for 1 hour at 1 Hz with a peak flow rate of 18.8 ml/min, providing 1 Pa peak wall shear stress.
mRNA expression
Immediately after flow, cells were washed with PBS and total mRNA was isolated using TriReagent (Sigma). Total mRNA was converted to cDNA by TaqMan reverse transcriptase (Applied Biosystems). Gene expression was analyzed by quantitative real-time PCR using primers and probes (Life Technologies) for analysis of cyclooxygenase-2, COX-2 (Mm00478374_m1); osteopontin, OPN (Mm00436767_m1); adenylyl cyclase 6, AC6 (Mm00475772_m1); intraflagellar transport 88, IFT88 (Mm00493675_m1); and GAPDH (4351309). Samples and standards were run in triplicate, and all gene expression was normalized to GAPDH endogenous control, as previously performed (Kwon et al., 2010; Lee et al., 2014).
RNA interference
Gene silencing was performed by siRNA mediated knockdown, and compared to scramble siRNA control (Life Technologies). For primary cilia disruption, cells were transfected with 20 μM IFT88 siRNA (5′-CCAGAAACAGATGAGGACGACCTTT-3′), AC6 siRNA (5′-CCTGCCACCTACAACAGCTCAATTA-3′). or scrambled siRNA control using Lipofectamine 2000 (Life Technologies) as previously described (Kwon et al., 2010).
Adenylyl cyclase activity
Adenylyl cyclase activity was quantified by cAMP ELISA (Enzo). Cells were cultured as previously described and treated with 10 μM fenoldopam for 16 hours. Cells were stimulated by 10 μM forskolin (Sigma) or DMSO vehicle control for 20 minutes prior to lysis with 0.1 M HCl. Cell lysate was analyzed according to manufacturer’s protocol, and normalized to total protein quantified by BCA (Thermo Fisher). All samples and standards were run in duplicate.
Analysis
All data were analyzed with one-way ANOVA followed by Bonferroni post-hoc correction. Values are reported as mean ± SEM, with p < 0.05 considered statistically significant. Sample size, n, represents biological replicates.
Results
To test our hypothesis that cilium length directly affects mechanosensitivity, we first verified that we could modulate primary cilia length. We cultured MLO-Y4 osteocytes and treated them with two distinct small molecules to increase cilia length. Cells were cultured in media supplemented with fenoldopam, lithium, or vehicle control for 16 hours. Immunocytochemistry was used to image primary cilia and assess changes in cilia length. Both fenoldopam and lithium treatments induced significant increases in cilia length by 26% ± 7% and 46% ± 5%, respectively, compared to vehicle control (Fig. 1A, B). Cell viability was assessed with MTT assay and found no change effect of the drug treatments (Fig. 1C). Additionally, no gross morphological changes resulted from fenoldopam or lithium treatment (Fig. 1D, E).
Figure 1.
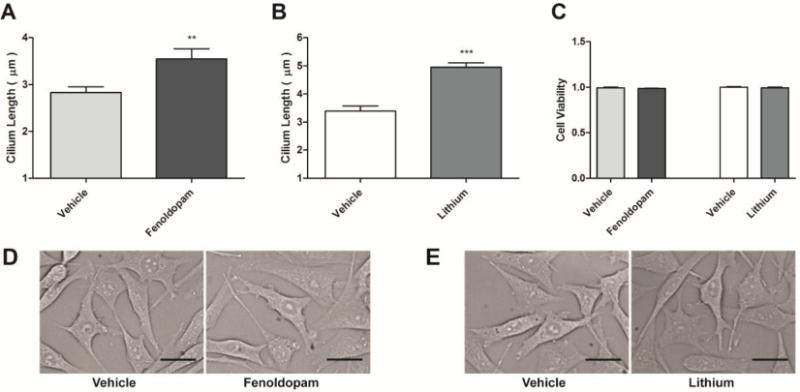
Small molecule treatments increase primary cilia length. 10 μM fenoldopam (A) and 500 μM Lithium (B) treatment for 16 hours significantly increases primary cilia length compared to vehicle control, with no changes in cellular morphology. Drug treatments elicited no change in cell viability, as assessed by MTT assay (C), and no gross morphological differences were exhibited (D, E). Mean ± SEM; n > 25 cilia for each group, n = 4 for MTT assay; **p < 0.01, ***p < 0.001; scale bars = 20 μm.
Next, we examined the effect of elongating cilia on cellular mechanosensitivity by mechanically stimulating cells with longer cilia and analyzing their osteogenic response. Osteocytes were treated with fenoldopam, lithium, or vehicle control, and exposed to oscillatory fluid flow for 1 hr. As a control, samples were simultaneously loaded into flow chambers, but not subjected to flow. Mechanosensitivity was then quantified at the mRNA level with analysis of COX-2 and OPN expression, and presented as the fold change of flow over no flow control (Fig. 2). Cells with cilia lengthened by fenoldopam were more responsive, exhibiting elevated mRNA expression of 124% ± 27% and 48% ± 8% of COX-2 and OPN respectively compared to unlengthened controls. Lithium resulted in a more modest, but still significant increase in response of 61% ± 13% and 34% ± 8% for COX-2 and OPN. This flow-induced enhanced osteogenic response was observed in cells with elongated primary cilia, regardless of the means of lengthening, suggesting that the effect is due to lengthening and not an unanticipated effect of the agents utilized.
Figure 2.
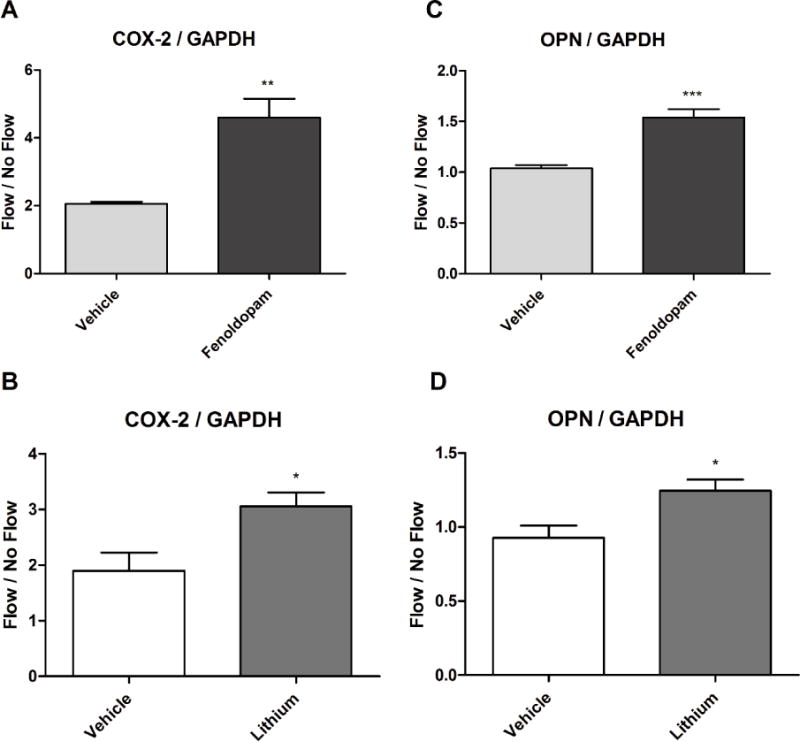
Cells with longer cilia are more mechanosensitive. Cells were subjected to fluid flow for 1 hour, and the fold change of flow vs no flow control groups was compared. Cells expressed significant increases in COX-2 (A, B) and OPN (C, D) mRNA relative to GAPDH endogenous control when treated with either fenoldopam (A, C) or LiCl (B, D) for 16 hours. Mean ± SEM; n ≥ 5 for each group; *p < 0.05, **p < 0.01, ***p < 0.001.
We next sought to examine the potential of targeting primary cilia length to recover impaired cilia function. IFT88 inhibition was employed as a model of dysfunctional cilia, and has previously been used to mimic the effects of polycystic kidney disease (Lehman et al., 2008). IFT88 is a critical component of intraflagellar transport and is necessary for proper primary cilia formation (Pazour et al., 2000; Yoder et al., 2002). Cells treated with IFT88 siRNA displayed decreased primary cilia length and incidence compared to scramble control (Fig. 3A, B). IFT88 siRNA treated cells were then treated with fenoldopam, and cilia length and incidence were noticeably recovered (Fig. 3C). Upon analysis, cilia of IFT88 siRNA treated cells were significantly shorter (Fig. 4A) and were present with lower incidence (Fig. 4C) than scramble control groups, with fenoldopam treatment significantly recovering cilia incidence. We then mechanically stimulated these cells to examine whether ciliogenesis recovery restored mechanosensitivity. Fluid flow was applied for 1 hr and cells with impaired primary cilia formation displayed significantly decreased flow-induced OPN mRNA expression by 43% ± 2%, compared to scramble control (Fig. 4B). Fenoldopam treatment was then able to recover this OPN response by 52% ± 1%, compared to IFT88 siRNA and vehicle treated cells. This further suggests that the length of primary cilia is critical to their function as a mechanosensor, and that as cilia formation was restored, so too was mechanosensitivity. We then confirmed that fenoldopam treatment of healthy, ciliated cells had no significant effect on cilia incidence (Fig. 4D) or IFT88 mRNA expression (Fig. 4E).
Figure 3.
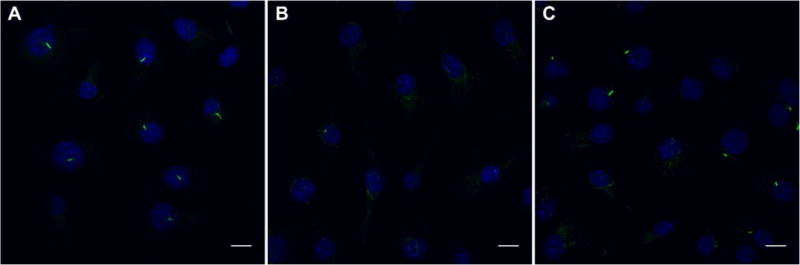
Treatment with IFT88 siRNA disrupts primary cilia formation. Overlays of primary cilia (green) and nuclei (blue) illustrate primary cilia incidence. Scramble control siRNA treatment for 48 hours does not disrupt primary cilia formation (A). IFT88 siRNA treatment results in decreased cilia length and incidence (B). Fenoldopam treatment recovers primary cilia formation in IFT88 siRNA treated cells (C). Scale bars = 10 μm.
Figure 4.
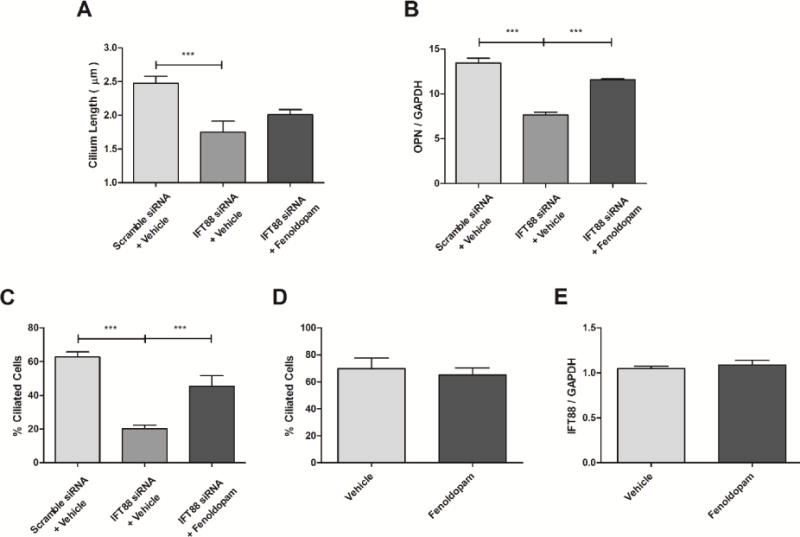
Fenoldopam rescues ciliogenesis and mechanosensing. Cells treated with IFT88 siRNA had decreased cilia length compared to scramble control, while fenoldopam treatment appears to recover cilia length (n ≥ 25 for scramble and fenoldopam treated, n = 15 for IFT88 siRNA alone) (A). Oscillatory fluid flow was applied to cells treated with IFT88 or Scramble control siRNA. Impaired cilia displayed a decreased OPN response to fluid flow, while fenoldopam treatment was able to recover flow stimulated OPN expression (B). Treatment with IFT88 siRNA decreases cilia incidence, but is recovered with fenoldopam treatment; n ≥ 8 fields of view (C). Fenoldopam treatment on untransfected cells has no effect on cilia incidence; n ≥ 8 fields of view (D). Fenoldopam also does not alter IFT88 mRNA expression in untransfected cells (E). Mean ± SEM; n ≥ 4; ***p < 0.001.
Finally, we examined a potential molecular pathway through which fenoldopam increases primary cilia length. cAMP has been previously shown to be involved in ciliogenesis and primary cilia-mediated mechanotransduction, so we quantified adenylyl cyclase activity by measuring stimulated cAMP production (Besschetnova et al., 2010; Kwon et al., 2010). We increased primary cilia length by fenoldopam treatment, and then briefly stimulated the cells with the adenylyl cyclase agonist, forskolin (Fig. 5A). Fenoldopam treatment significantly enhanced the forskolin stimulated cAMP response by 130% ± 25% compared to vehicle control. Additionally, fenoldopam treatment stimulated a 20% ± 8% increase in AC6 mRNA expression (Fig. 5B). Because of the significant role of AC6 in primary cilia-mediated mechanotransduction, we used inhibition of AC6 as an alternative model of impaired cell mechanosensitivity. Treatment of osteocytes with AC6 siRNA resulted in a small but significant decrease in cilia length by 10% ± 3% (Fig. 5C), while AC6 inhibition had no effect on cilia incidence (Fig. 5D). Fenoldopam had no effect on recovering cilia length or incidence in AC6 siRNA treated cells. When cells with diminished AC6 were mechanically stimulated, AC6 knockdown cells displayed decreased AC6 and flow-induced OPN expression by 50% ± 3% and 30% ± 3% respectively, which was not recovered with fenoldopam treatment (Fig. 5E, F).
Figure 5.
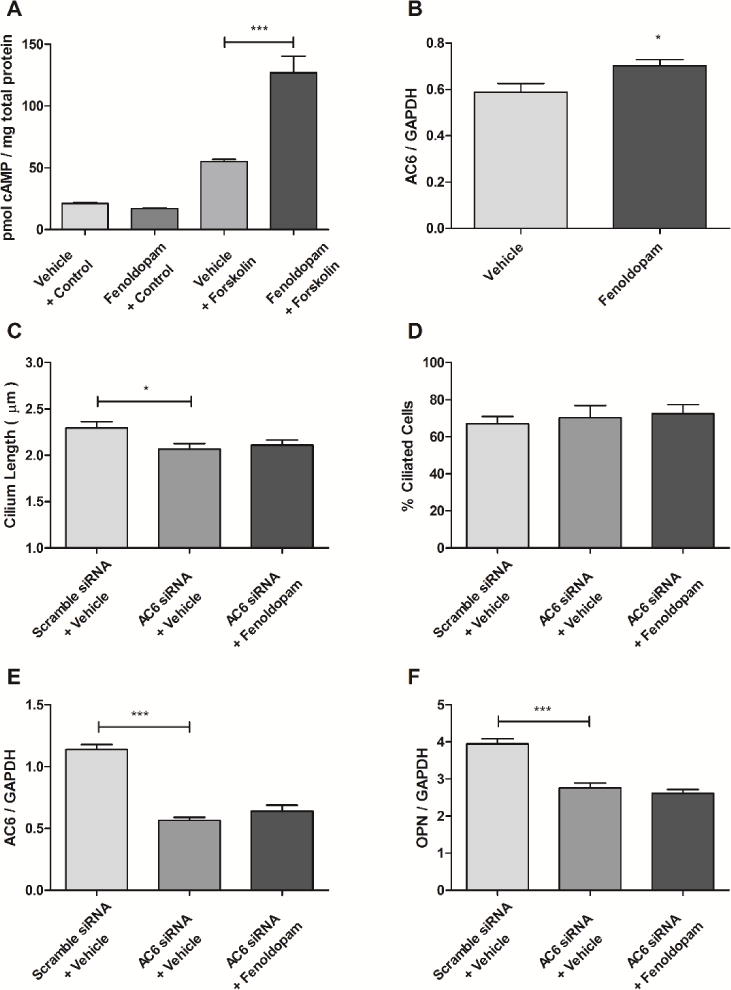
Fenoldopam enhances adenylyl cyclase production and activity. Cells were treated with fenoldopam or vehicle control for 16 hours, and then forskolin stimulated for 20 minutes. Fenoldopam treatment significantly increased the cAMP response to forskolin stimulation (A). Fenoldopam treatment significantly increased AC6 mRNA expression (B). AC6 knockdown decreases cilia length, but is not recovered with fenoldopam treatment (C). Neither AC6 siRNA nor fenoldopam alter cilia incidence; n ≥ 8 fields of view (D). Oscillatory flow applied to AC6 siRNA treated cells elicits a decrease in AC6 and OPN mRNA expression, and is not recovered with fenoldopam treatment (E, F). Mean ± SEM; n ≥ 4 for each group; *p < 0.05, ***p < 0.001.
Discussion
Our results dem1onstrate that primary cilia length plays a significant role in cell mechanosensitivity. We employed two distinct, clinically utilized, small molecules to increase cilia length, and both resulted in enhanced mechanosensitivity. Cells with impaired ciliogenesis have impaired mechanosensing, but this may be recovered with fenoldopam treatment. Finally, we show that fenoldopam modulates osteocyte mechanosensitivity through a mechanism involving AC6, and cells with diminished AC6 have shorter cilia and impaired mechanosensing.
Based on clinical and biochemical considerations, fenoldopam was a more suitable candidate for further study than lithium, and thus was only used in studying the effects of IFT88 and AC6 knockdowns. Fenoldopam is a dopamine D1-like receptor agonist clinically used as a vasodilator in cases of extreme hypertension (Murphy et al., 2001; Post and Frishman, 1998). Lithium has a much less defined function and is clinically used to treat a wide range of mental disorders, including bipolar disorder (Marmol, 2008). Furthermore, lithium is an inhibitor of GSK-3β and can have downstream effects on various signaling pathways including Wnt and Hedgehog. While lithium has been used to increase cilia length in a variety of cell types, other GSK-3β inhibitors have no effect on cilia length (Jope, 2003; Ou et al., 2009).
Fenoldopam treatment increased cilia length, but also plays a role in adenylyl cyclase activity. The increase in forskolin stimulated adenylyl cyclase activity with fenoldopam treatment implicates two potential mechanisms. First, it is possible that fenoldopam sensitizes adenylyl cyclases, resulting in an increased cAMP response to forskolin. Alternatively, fenoldopam may increase production of adenylyl cyclases, augmenting forskolin stimulated cAMP production. This second notion is consistent with previous work indicating that fenoldopam treatment upregulates AC6, a specific adenylyl cyclase isoform, production in kidney cells (Yu et al., 2014). Our results support this possible molecular pathway by demonstrating an increase in AC6 mRNA expression in response to fenoldopam stimulation. Previously, we have demonstrated that AC6 localizes to the osteocyte primary cilium and is critical for primary cilia-mediated mechanotransduction (Kwon et al., 2010). Besschetnova et al, reported that stimulating the cAMP signaling pathway results in increased cilia length (Besschetnova et al., 2010). Using an siRNA mediated knockdown, they then showed that AC6 has a functional role in mediating primary cilia elongation. Together, these findings indicate that AC6-cAMP dynamics are critical to both primary cilia length and mechanotransduction, and that fenoldopam treatment stimulates this pathway. Furthermore, these results suggest that fenoldopam treatment enhances ciliary protein production to promote cilium elongation.
Adenylyl cyclases and cAMP contribute to recovering and elongating primary cilia by stimulating IFT particle transport. It has previously been reported that stimulation of the adenylyl cyclase-cAMP-PKA signaling pathway augments anterograde transport of IFT particles to promote cilia elongation (Besschetnova et al., 2010). Because fenoldopam enhances adenylyl cyclase production, this suggests that fenoldopam treatment is potentiating adenylyl cyclase activity and IFT particle transport. Our model of impaired cilia utilized an IFT88 knockdown, not a complete knockout of the gene, so it is possible that fenoldopam was able to enhance remaining IFT88 function and promote cilia elongation and rescue cilia incidence. Furthermore, this presupposes that even though the IFT88 knockdown is satisfactory to impair cilia formation and function, sufficient IFT88 remains to elongate cilia. Our data show no change in IFT88 mRNA expression elicited by fenoldopam treatment suggesting that fenoldopam stimulated the remaining IFT88, rather than promoting production of new IFT88. This does not, however, discount the notion that fenoldopam treatment may instead prevent IFT88 knockdown driven disassembly of the cilium.
The specific means by which cells with longer cilia are more mechanosensitive remains elusive, but there are two potential mechanisms of how this may occur. Schwartz et al, developed one of the first models of primary cilia deflection under fluid flow and hypothesized that longer cilia would experience greater membrane strain to increase opening of stretch-activated ion channels on the ciliary membrane (Schwartz et al., 1997). Alternatively, longer cilia may simply allow for the presence of more cilia-specific proteins and signaling molecules within this microdomain (Breslow et al., 2013; Kee et al., 2012). Increasing the total amount of ciliary protein could enhance signal transduction within the ciliary compartment, modifying primary cilia-mediated mechanosensitivity. In fact, fenoldopam treated cells exposed to fluid flow have increased ciliary influx of calcium, which has been identified as one initiator of the mechanotransduction signaling cascade (Jin et al., 2014; Yuan et al., 2015). It is also possible that cilium-lengthening agents actually enhance ciliary protein production and trafficking to promote cilium elongation.
The correlation between cilia length and critical ciliary proteins involved in mechanosensing was examined with AC6 siRNA treatment. This knockdown of AC6 decreased flow-induced osteogenic gene expression, yet fenoldopam treatment was not sufficient to rescue AC6 expression, or OPN expression as was demonstrated in the IFT88 knockdown model. Because fenoldopam treatment was not able to recover AC6 or OPN mRNA expression in AC6 knockdown cells, this may suggest that the ability of fenoldopam to enhance AC6 activity is critical to recovering cellular mechanosensing. However, AC6 knockdown also decreased primary cilia length, which was not recovered with fenoldopam, and did not alter cilia incidence. Altogether, these data suggest that both cilia length and protein production may be critical in primary cilia-mediated mechanosensing.
Cells with longer cilia are more mechanosensitive, but primary cilia cannot be elongated indefinitely. Longer cilia are exposed to greater drag force, and are more likely to be sheared off (Hierck et al., 2008). For example, endothelial cell primary cilia are flow sensors in regions of low shear, specifically, because they are cleaved off as shear stresses increase (Van der Heiden et al., 2008). Interestingly, electron microscopy has shown that primary cilia structure is not constant along the ciliary axoneme and becomes increasingly disorganized and asymmetric at the distal tip (Odor and Blandau, 1985; Yamamoto and Kataoka, 1986). This loss of microtubule symmetry reduces the bending stiffness of the cilium at the distal end, making drastically elongated cilia more susceptible to removal by fluid shear (Rydholm et al., 2010).
While osteocyte primary cilia are free-standing flow sensors in vitro, their mechanosensing function may differ in vivo. It has been estimated that the lacunar space in which osteocytes reside in vivo allows for only a 1 μm long cilium (McNamara et al., 2009; Uzbekov et al., 2012). Due to the spatial limitations within the lacuna, the potential effect of pharmacologically enhancing osteocyte cilia length in vivo is unclear. In fact, these spatial constraints may point to the cilium not being a free-flowing mechanosensor at all. Rather, osteocyte cilia may anchor to the lacunar wall, similarly to chondrocyte primary cilia which form integrin attachments with the surrounding extracellular matrix, ECM (McGlashan et al., 2006). A computational model by Vaughan et al, simulated osteocytes exposed to fluid flow within the lacunar-canalicular network (Vaughan et al., 2014). The authors modeled a free-standing cilium, 0.5 μm long, within a lacuna and calculated the resulting strain at the base of the cilium. Their model suggests that a cilium in this configuration does not experience a great enough strain to function as a flow sensor, but a cilium directly attached to the ECM does. The authors, however, do not account for the amount of membrane strain necessary to stimulate stretch-activated ion channels on the ciliary membrane, and may have overestimated the required strain for cilium stimulation. Fenoldopam treatment not only increases length, but may also enhance mechanosensitive protein levels, such as adenylyl cyclases and ion channels, within the cilium. Regardless of cilium length, this enriched protein trafficking to the cilium would increase chemical kinetics within the ciliary microdomain to modify cellular mechanosensitivity.
Our results certainly suggest fenoldopam treatment, or similar agents which may increase cilia length, can be a potent method to enhance cell mechanosensitivity. However, the effect of fenoldopam on whole bone has not been examined, so the potential skeletal and systemic consequences in translating this work to in vivo models is still unknown. Statistical analysis of patients treated with various antidepressants, such as selective serotonin reuptake inhibitors which can block dopamine D2 receptors, revealed that these patients have increased risk of hip and femur fracture (Van Den Brand et al., 2009; Smith et al., 2009). Furthermore, many antidepressants and antipsychotics that block dopamine D2 receptors can cause decreased estrogen and testosterone levels leading to significantly reduced bone mass (Phillip and Lazar, 2003). All of these studies examine treatments which may affect dopamine D2 receptors, but at present there are no examinations of the impact of dopamine D1 receptor agonists on bone formation. As such, no connection between fenoldopam treatment and skeletal health has been established. Because of this lack of direct evidence, such drugs have the potential for a wide range of unknown side effects. Further work needs to be performed before any drugs altering dopamine signaling are used to treat bone disease.
Targeting primary cilia-mediated mechanotransduction has widespread applications in preventative medicine that reach far beyond osteocytes. Numerous diseases are characterized by impaired primary cilia function. Mutations of PC2, polycystin 2, are attributed to polycystic kidney disease and skeletal deformations. Bardet-Biedl syndrome is characterized by malfunctioning BBS proteins at the base of the primary cilium, causing retinopathy, polydactyly, and renal failure (Loktev et al., 2008; Mochizuki et al., 1996; Xiao et al., 2011). Recently, primary cilia have even been implicated in tumor development. Primary cilia help regulate Wnt signaling, changes in which have been correlated with cancer cell progression (Lancaster et al., 2011). Furthermore, some cancer cell types lose their primary cilia, which potentially contributes to their insensitivity to repressive signals (Plotnikova et al., 2008). Additionally, atherosclerotic plaques form in areas of low and disturbed arterial fluid flow, regions that interestingly have an increased incidence of primary cilia. This suggests that these cells are compensating, increasing their sensitivity to low fluid flow in order to promote an adequate cellular response (Van der Heiden et al., 2008; Warboys et al., 2011). Within bone, osteocytes utilize primary cilia to sense and respond to mechanical cues. In vitro and in vivo studies demonstrate that when these cilia are removed there is a decreased bone formation response to loading (Kwon et al., 2010; Malone et al., 2007; Temiyasathit et al., 2012). Fenoldopam is already an FDA approved drug, and our results point to it being an attractive candidate for study in numerous in vitro and in vivo applications to treat such a myriad of conditions.
Conclusion
We have demonstrated that cells with longer primary cilia are more mechanosensitive, and we present a simple, yet robust, method to enhance primary cilia-mediated mechanotransduction. Fenoldopam treatment not only increased primary cilia length, protein production, and mechanosensitivity, but also rescued cilia form and function in cells with impaired cilia. Although fenoldopam and lithium are likely not ideal treatments for all cilia-related conditions, the strategy of modulating primary cilia sensitivity may aid in combating the phenotypes displayed by various ciliopathies, maintain sensitivity of cancer cells so that they respond to repressive signals, cue cellular responses to slow atherosclerotic plaque formation, and stimulate load-induced bone formation.
Acknowledgments
We would like to acknowledge Emily Moore and Michael Duffy for their valuable discussions and input in regards to this work. This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institute of Health (NIAMS/NIH) grant AR062177.
References
- Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation–the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122:546–53. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol. 2010;20:182–7. doi: 10.1016/j.cub.2009.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivi N, Pacheco-Costa R, Brun LR, Murphy TR, Farlow NR, Robling AG, Bellido T, Plotkin LI. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J Orthop Res. 2013;31:1075–1081. doi: 10.1002/jor.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliziotes M, McLoughlin S, Gunness M, Fumagalli F, Jones SR, Caron MG. Bone histomorphometric and biomechanical abnormalities in mice homozygous for deletion of the dopamine transporter gene. Bone. 2000;26:15–19. doi: 10.1016/S8756-3282(99)00232-X. [DOI] [PubMed] [Google Scholar]
- Van Den Brand MWM, Samson MM, Pouwels S, Van Staa TP, Thio B, Cooper C, Leufkens HGM, Egberts ACG, Verhaar HJJ, De Vries F. Use of anti-depressants and the risk of fracture of the hip or femur. Osteoporos Int. 2009;20:1705–1713. doi: 10.1007/s00198-009-0849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow DK, Koslover EF, Seydel F, Spakowitz AJ, Nachury MV. An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J Cell Biol. 2013;203:129–47. doi: 10.1083/jcb.201212024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, Jiang JX. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A. 2010;107:13648–53. doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo AB, Blundo JT, Chen JC, Lee KL, Yereddi NR, Jang E, Kumar S, Tang WJ, Zarrin S, Kim JB, Jacobs CR. Focal Adhesion Kinase Plays a Role in Osteoblast Mechanotransduction In Vitro but Does Not Affect Load-Induced Bone Formation In Vivo. PLoS One. 2012;7:1–11. doi: 10.1371/journal.pone.0043291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssière B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102:17406–11. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci U S A. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P, Lanyon L. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002:688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- Fujihara S, Yokozeki M, Oba Y, Higashibata Y, Nomura S, Moriyama K. Function and regulation of osteopontin in response to mechanical stress. J Bone Min Res. 2006;21:956–964. doi: 10.1359/jbmr.060315. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Van der Heiden K, Hierck BP, Krams R, de Crom R, Cheng C, Baiker M, Pourquie MJBM, Alkemade FE, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2008;196:542–50. doi: 10.1016/j.atherosclerosis.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, Poelmann RE. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- Jin X, Mohieldin AM, Muntean BS, Green Ja, Shah JV, Mykytyn K, Nauli SM. Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol Life Sci. 2014;71:2165–78. doi: 10.1007/s00018-013-1483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Lithium and GSK-3: One inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- Kathem SH, Mohieldin AM, Abdul-Majeed S, Ismail SH, Altaei QH, Alshimmari IK, Alsaidi MM, Khammas H, Nauli AM, Joe B, Nauli SM. Ciliotherapy: a novel intervention in polycystic kidney disease. J Geriatr Cardiol. 2014;11:63–73. doi: 10.3969/j.issn.1671-5411.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee HL, Dishinger JF, Lynne Blasius T, Liu C-J, Margolis B, Verhey KJ. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol. 2012 doi: 10.1038/ncb2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Nulend J, Burger EH, Semeins CM, Raisz LG, Pilbeam CC. Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J Bone Miner Res. 1997;12:45–51. doi: 10.1359/jbmr.1997.12.1.45. [DOI] [PubMed] [Google Scholar]
- DN Ku. Blood Flow in Arteries. Annu Rev Fluid Mech. 1997;29:399–434. doi: 10.1146/annurev.fluid.29.1.399. [DOI] [Google Scholar]
- Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. FASEB J. 2010;24:2859–68. doi: 10.1096/fj.09-148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster Ma, Schroth J, Gleeson JG. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat Cell Biol. 2011;13:700–7. doi: 10.1038/ncb2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Tseng HC, Wong SW, Wang Z, Deng M, Ko C-C. Dopaminergic effects on in vitro osteogenesis. Bone Res. 2015a;3:15020. doi: 10.1038/boneres.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Hoey DA, Spasic M, Tang T, Hammond HK, Jacobs CR. Adenylyl cyclase 6 mediates loading-induced bone adaptation in vivo. FASEB J. 2014;28:1157–1165. doi: 10.1096/fj.13-240432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Guevarra MD, Nguyen AM, Chua MC, Wang Y, Jacobs CR. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015b;4:7. doi: 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JM, Michaud EJ, Schoeb TR, Aydin-Son Y, Miller M, Yoder BK. The Oak Ridge Polycystic Kidney mouse: Modeling ciliopathies of mice and men. Dev Dyn. 2008;237:1960–1971. doi: 10.1002/dvdy.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol. 2003;285:F998–F1012. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, Bazan JF, Slusarski DC, Sheffield VC, Jackson PK, Nachury MV. BBSome Subunit A Links Ciliogenesis, Microtubule Stability, and Acetylation. Dev Cell. 2008;15:854–865. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Malone AMD, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantila Roosa SM, Liu Y, Turner CH. Gene expression patterns in bone following mechanical loading. J Bone Miner Res. 2011;26:100–112. doi: 10.1002/jbmr.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmol F. Lithium: Bipolar disorder and neurodegenerative diseases Possible cellular mechanisms of the therapeutic effects of lithium. Prog Neuro-Psychopharmacology Biol Psychiatry. 2008;32:1761–1771. doi: 10.1016/j.pnpbp.2008.08.012. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. Anat Rec. 2009;292:355–363. doi: 10.1002/ar.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem Biophys Res Commun. 2009;388:757–62. doi: 10.1016/j.bbrc.2009.08.099. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- Murphy MB, Murray C, Shorten GD. Fenoldopam: a selective peripheral dopamine-receptor agonist for the treatment of severe hypertension. N Engl J Med. 2001;345:1548–1557. doi: 10.1056/NEJMra010253. [DOI] [PubMed] [Google Scholar]
- Norvell SM, Ponik SM, Bowen DK, Gerard R, Pavalko FM. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J Appl Physiol. 2004;96:957–966. doi: 10.1152/japplphysiol.00869.2003. [DOI] [PubMed] [Google Scholar]
- Odor DL, Blandau RJ. Observations on the solitary cilium of rabbit oviductal epithelium: its motility and ultrastructure. Am J Anat. 1985;174:437–453. doi: 10.1002/aja.1001740407. [DOI] [PubMed] [Google Scholar]
- Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoornc Fa. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res. 2009;315:2802–2817. doi: 10.1016/j.yexcr.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene Tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan MN, Leddy Ha, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Suk HL, Liedtke W, Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillip M, Lazar L. The Regulatory Effect of Hormones and Growth Factors on the Pubertal Growth Spurt. Endocrinologist. 2003;13:465–469. doi: 10.1097/01.ten.0000098609.68863.ab. [DOI] [Google Scholar]
- Piperi C. Polycystins and mechanotransduction: From physiology to disease. World J Exp Med. 2015;5:200. doi: 10.5493/wjem.v5.i4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Speacht TL, Donahue HJ. Cx43 and mechanotransduction in bone. Curr Osteoporos Rep. 2015;13:67–72. doi: 10.1007/s11914-015-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Golemis Ea, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68:2058–61. doi: 10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post JB, Frishman WH. Fenoldopam: a new dopamine agonist for the treatment of hypertensive urgencies and emergencies. J Clin Pharmacol. 1998;38:2–13. doi: 10.1002/j.1552-4604.1998.tb04369.x. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- Raisz LG. Prostaglandins and bone: Physiology and pathophysiology. Osteoarthr Cartil. 1999;7:419–421. doi: 10.1053/joca.1998.0230. [DOI] [PubMed] [Google Scholar]
- Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H. Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol. 2010;298:F1096–102. doi: 10.1152/ajprenal.00657.2009. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272:F132–8. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- Smith GS, Ma Y, Dhawan V, Chaly T, Eidelberg D. Selective serotonin reuptake inhibitor (SSRI) modulation of striatal dopamine measured with [ 11 C]-raclopride and positron emission tomography. Synapse. 2009;63:1–6. doi: 10.1002/syn.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temiyasathit S, Tang WJ, Leucht P, Anderson CT, Monica SD, Castillo AB, Helms JA, Stearns T, Jacobs CR. Mechanosensing by the primary cilium: Deletion of Kif3a reduces bone formation due to loading. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonna EA, Lampen NM. Electron microscopy of aging skeletal cells. I. Centrioles and solitary cilia. J Gerontol. 1972;27:316–324. doi: 10.1093/geronj/27.3.316. [DOI] [PubMed] [Google Scholar]
- Upadhyay VS, Muntean BS, Kathem SH, Hwang JJ, Aboualaiwi Wa, Nauli SM. Roles of dopamine receptor on chemosensory and mechanosensory primary cilia in renal epithelial cells. Front Physiol. 2014;5:72. doi: 10.3389/fphys.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzbekov RE, Maurel DB, Aveline PC, Pallu S, Benhamou CL, Rochefort GY. Centrosome fine ultrastructure of the osteocyte mechanosensitive primary cilium. Microsc Microanal. 2012;18:1430–41. doi: 10.1017/S1431927612013281. [DOI] [PubMed] [Google Scholar]
- Vaughan TJ, Mullen Ca, Verbruggen SW, McNamara LM. Bone cell mechanosensation of fluid flow stimulation: a fluid-structure interaction model characterising the role integrin attachments and primary cilia. Biomech Model Mechanobiol. 2014;2 doi: 10.1007/s10237-014-0631-3. [DOI] [PubMed] [Google Scholar]
- Warboys CM, Amini N, de Luca A, Evans PC. The role of blood flow in determining the sites of atherosclerotic plaques. F1000 Med Rep. 2011;3:5. doi: 10.3410/M3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Dallas M, Qiu N, Nicolella D, Cao L, Johnson M, Bonewald L, Quarles LD. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 2011;25:2418–2432. doi: 10.1096/fj.10-180299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Kataoka K. Electron microscopic observation of the primary cilium in the pancreatic islets. Arch Histol Jpn Nippon soshikigaku kiroku. 1986;49:449–457. doi: 10.1679/aohc.49.449. [DOI] [PubMed] [Google Scholar]
- Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol. 2002;282:F541–F552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone. 2008;42:172–179. doi: 10.1016/j.bone.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Sun M, Villar VAM, Zhang Y, Weinman EJ, Felder Ra, Jose Pa. Differential dopamine receptor subtype regulation of adenylyl cyclases in lipid rafts in human embryonic kidney and renal proximal tubule cells. Cell Signal. 2014;26:2521–2529. doi: 10.1016/j.cellsig.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Zhao L, Brueckner M, Sun Z. Intraciliary Calcium Oscillations Initiate Vertebrate Left-Right Asymmetry. Curr Biol. 2015;25:556–567. doi: 10.1016/j.cub.2014.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]


