Abstract
Background
First deliveries in women older than 35, 40, or 45 are at increased risk for adverse pregnancy outcomes compared with those in younger women. However, specific relationships between each additional year of maternal age and pregnancy risks remain unclear, and absolute risks at each maternal age are not known.
Methods
Using a population-based cohort of nulliparous women in British Columbia, Canada, from 2004–2014 (n=203,414), We examined relationships between maternal age (modeled flexibly to allow curvilinear shapes) and pregnancy outcomes using logistic regression. We plotted absolute predicted risks to display curves from age 20–50 estimated for two risk profiles: 1) population average values of all risk factors, 2) a low-risk profile without preexisting diabetes/hypertension, smoking, prior spontaneous/therapeutic abortion, diagnosed infertility, inadequate prenatal care, low income, rural residence, or obesity.
Results
Risks of hypertensive disorders increased gradually until age 35, then accelerated. Risk of multiple gestations, major congenital anomalies, and maternal mortality or severe morbidity increased slowly until age 30, then accelerated. Cesarean delivery and gestational diabetes risks increased linearly with age. While indicated preterm delivery increased rapidly with maternal age, spontaneous preterm delivery did not. Stillbirth, neonatal mortality, and infant mortality had j-shaped relationships with maternal age, with nadirs near 30. Despite age-related increases, risks of severe outcomes remained low for women 35 and 40: <1%–2% for severe maternal morbidity and 5%–7% for fetal–infant composite.
Conclusions
This study provides risks for specific maternal ages to inform clinical counseling and public health messaging regarding the potential implications of delayed childbearing.
Keywords: advanced maternal age, delayed childbearing, perinatal outcomes, maternal outcomes, pregnancy complications
Background
Delayed childbearing is increasingly common in the industrialized world, largely attributed to changing family planning and social structure trends.1,2 In 1988, 8% of babies in the United States were born to women older than 35 and 1% to women older than 40.1 Births to older mothers have increased dramatically over the past 3 decades, with 15.7% of babies born to women older than 35 in 2015, and 7.9% to women older than 40.3 Previous studies have found that deliveries to women older than 35, 40, or 45 are at increased risk for many adverse pregnancy and birth outcomes compared with deliveries to younger women, though thresholds used to define advanced maternal age and younger referent groups have varied substantially.1,4–10 In particular, advanced maternal age has been linked with increased risks of cardiometabolic dysfunction11 and aneuploidy due to aging oocytes.12,13 Cardiometabolic dysfunction manifests as hypertensive disorders of pregnancy or gestational diabetes during pregnancy, following the hypothesis that pregnancy serves as a stress test for underlying cardiovascular risk.11
Categorization of maternal age implies a biologically implausible threshold effect, in which pregnancy risks are minimal until age 35, then increase abruptly after age 35, 40, or 45. This approach obscures trends within age categories and may both underestimate age-related risks for women in younger age groups and overestimate risks for mothers in older age groups. Age 35 arose as the threshold used to define advanced maternal age from prenatal genetic screening work in 1970 as the age at which the risk of pregnancy loss due to amniocentesis was equal to the risk of Down syndrome.14 Subsequent literature has elucidated year-by-year risks of Down syndrome.15 However, this granular approach has not been applied to other important pregnancy and birth outcomes for which age 35 may not be particularly meaningful. Further, although analyses in perinatal epidemiology are often conducted in cohort studies, in which absolute measures of effect are estimable, studies of maternal age at pregnancy have generally presented odds ratios even for common outcomes. Odds ratios are less useful for clinical decisionmaking and for public health policies than absolute risks and may be particularly unclear in this area due to varying exposure and referent group definitions. Analytical shortcomings of available literature make it difficult for clinicians and childbearing families to understand year-by-year differences in pregnancy and birth risks on the absolute scale.
The objective of this study was to estimate absolute risks of pregnancy and birth outcomes according to 1-year intervals of maternal age at first birth among nulliparous women, treating age at first birth as a continuous variable with a flexible, non-linear approach. To examine the sensitivity of associations between maternal age and adverse pregnancy and birth outcomes to advanced maternal age and referent group definition thresholds, we also compared risk ratios for each outcome based on different exposure and referent group definitions.
Materials and Methods
Study population
This population-based cohort was drawn from all births in British Columbia, Canada, from April 1, 2004 to March 31, 2014, using the British Columbia Perinatal Data Registry (BCPDR), a high-quality birth registry maintained by Perinatal Services BC.16 This database contains data abstracted from obstetric and neonatal medical records on nearly 100% of births in the province of British Columbia from over 60 acute-care facilities as well as births occurring at home attended by registered midwives. The BCPDR includes pregnancies ending in a live or stillbirth of at least 20 weeks gestation or 500 grams birth weight and also collects data on maternal postpartum readmissions up to 42 days post-delivery and baby transfers and readmissions up to 28 days after birth. Health Information Management professionals abstract information from provincially standardized antenatal, labor and delivery, and newborn forms into the BCPDR. Diagnoses and procedures are coded using the International Classification of Diseases, 10th Revision, Canada (ICD-10-CA) and Canadian Classification of Intervention (CCI) coding systems, respectively. A recent validation study found that most core perinatal variables have excellent validity, with positive predictive values for variables in this study ranging from 67.3% for in vitro fertilization (IVF) to 100% for maternal age.17 Population Data BC linked the BCPDR data were with additional population level health and demographic data. The Medical Services Plan Payment Information File (physician billing)18 and PharmaNet outpatient prescription database19 were used to identify women with diagnosed infertility, hospital discharge data20 were used to identify severe maternal morbidity, and census data provided 3-digit postal code income level21 and rural residence22 information. Neonatal, infant, and maternal deaths were identified using Vital Statistics data.23
To examine age at first birth, we restricted this study to nulliparous women. Our data set includes multiple pregnancies, anomalies and defects, and terminations after 20 weeks. The University of British Columbia and Children’s and Women’s Heath Centre of British Columbia Research Ethics Board (#H15-01208) granted ethics approval.
Variable definitions
Maternal date of birth is linked to each woman’s provincially issued personal health number and is used throughout prenatal care for identification purposes. Maternal age at first birth is, thus, reported reliably and rarely missing.17 The individual outcomes we examined included 1) gestational diabetes mellitus; 2) hypertensive disorders of pregnancy; 3) cesarean delivery; 4) preterm delivery: delivery of a live infant before 37+0 weeks of gestation, using the Perinatal Services BC gestational age algorithm; 5) spontaneous preterm delivery (after the spontaneous onset of labor or spontaneous membrane rupture) and 6) indicated preterm delivery (those that did not meet the criteria for a spontaneous preterm delivery); 7) multiple gestations; 8) major congenital anomalies documented on the newborn medical record at the time of hospital discharge, excluding chromosomal anomalies and minor external congenital anomalies;24 9) small-for-gestational-age: birth weight for sex and gestational age lower than the 10th percentile, using Canadian reference charts;25 10) neonatal intensive care unit (NICU) stay ≥48 hours: a threshold of 48 hours was used to capture admissions for serious and persistent health concerns; 11) stillbirth: antenatal or intrapartum fetal death at or after 20+0 weeks of gestation or ≥500g; 12) neonatal mortality: death within the first 28 days after birth; and 13) infant mortality: death within the first year after birth; 14) maternal mortality or near-miss morbidity: adapted from the five-factor coding system developed by Geller and colleagues,26 includes organ failure (acute renal failure, hepatic failure, heart failure), unanticipated postpartum maternal surgical intervention, maternal ventilation for ≥12 hours, maternal blood transfusion of >3 units, maternal admission to the intensive care unit, or maternal mortality. We also examined two composite outcome measures to summarize our findings: 1) healthy pregnancy and delivery composite outcome, defined as the absence of all adverse outcomes above (excluding multiple gestations and cesarean delivery); and 2) severe fetal–infant composite outcome, defined as any of the following: major congenital anomaly, stillbirth, very preterm delivery (<28 weeks), very low birth weight (<1500g), or infant death. Detailed definitions for all outcomes are provided in eFile 1.
Statistical Analysis
We used logistic regression models to examine the relationship between maternal age at birth and each outcome of interest. Maternal age was defined using exact age based on maternal and perinatal dates of birth and modeled using restricted cubic splines to allow the most flexible characterization of the relationship with each outcome.27 After considering between three and seven knots placed at the default percentiles recommended by Harrell,27 we selected the number of knots based on the structure that minimized Bayesian Information Criterion in an unadjusted model for each outcome.28 Twelve outcomes were modeled using three knots at the 10th, 50th, and 90th percentiles of maternal age at first birth in the study population (ages 21.4, 29.3, and 36.5), and four outcomes were modeled using four knots at the 5th, 35th, 65th, and 95th percentiles (ages 19.7, 27.1, 31.5, and 38.6).
For each outcome, we fit an unadjusted logistic regression model with only maternal age spline terms. We then fit a multivariable model for each outcome including terms all conditions associated with maternal age, which were identified on a priori grounds. The following conditions were included: chronic hypertension, type 1 or type 2 diabetes, body mass index, smoking, inadequate antenatal care (fewer than five visits), low income (postal code census tract indicating the lowest two deciles of income), rural residence (defined as community size <10,000 according to Statistics Canada census geographical areas), prior known spontaneous or therapeutic abortion, and diagnosed infertility (outpatient infertility medication prescriptions or infertility diagnosis billing codes prior to conception). We further adjusted for calendar year using indicator variables to account for secular trends, with 2014 as the reference year. Risks from multivariable models were estimated at the lowest-risk values of all covariates (no pre-pregnancy hypertension or diabetes, no smoking, adequate antenatal care, not low-income, non-rural residence, no prior known spontaneous or therapeutic abortion, no infertility diagnosis or treatment, and body mass index of 23.9), with year set to 2014. Gestational diabetes analyses were restricted to women without pre-pregnancy type 1 or type 2 diabetes. Small-for-gestational-age analyses were restricted to singletons born between 22 and 43 weeks. Our main analyses include both singleton and multiple gestations; we then repeated all analyses among singletons and multiple births separately. For models including multiple gestations, we used a robust (sandwich) variance estimator for correlated outcomes among multiple gestations.28
We computed unadjusted and adjusted odds of each outcome at each maternal age value30 and transformed predicted odds to find predicted absolute risks (probabilities). Probabilities were tabulated and presented graphically to illustrate age-outcome relationships visually.
Finally, we estimated risk ratios for each outcome using three definitions of advanced maternal age (≥35, ≥40, or ≥45), each compared with six referent group definitions (20–24, 20–29, 20–34, <35, <40, or <45) using logistic regression models and post-estimation procedures. We compared the resulting 15 risk ratios for each outcome to examine the degree of variability according to threshold choice.
We imputed missing data for number of antenatal visits (n=16,191; 8.0%) and maternal pre-pregnancy body mass index (n=53,828; 26.5%) using multiple imputation. Missing census tract deciles = (n=31,249; 15.4%) and missing community size data (n=28,300, 13.9%) for the year of birth were imputed using the closest year with available data. Those with no census tract data (n=5,590; 2.8%) or community size data (2,613, 1.3%) for any year were imputed using multiple imputation. Maternal age, all covariates from adjusted models and all outcomes were used to impute values for 30 imputations. Imputation was implemented using Stata’s mi impute command, which both imputes and combines parameter estimates according to Rubin’s formula.31 Estimated risks from the multiple imputation analysis did not differ materially from estimates from a complete case analysis.
All analyses were conducted using Stata 14.2.31
Sensitivity analyses
Definitions of gestational diabetes and NICU admission changed during our study period. The International Association of Diabetes in Pregnancy Study Group’s (IADPSG)33 diagnostic criteria for gestational diabetes were adopted in British Columbia in 2010. Before April 2010, infants who occupied a level II or III bed were coded as NICU admissions; after April 2010, infants who required level II or III care were coded as NICU admissions regardless of the actual bed they occupied. We conducted sensitivity analyses for each outcome examining cases before and after coding changes separately. In vitro fertilization (IVF) data became available in the BCPDR in April 2008. While we did have data on diagnosed infertility and non-IVF infertility treatment throughout the study period, IVF may be an additional indicator of underlying infertility. We conducted a sensitivity analysis for all outcomes after April 2008 to compare predicted risks from multivariable models that included and excluded adjustment for IVF as a covariate.
Results
Of 436,498 births in British Columbia during the study period (2004–2014), the population for this study was restricted to the 203,414 births to nulliparous women. Maternal age at first birth increased steadily throughout the study period, from a mean of 30.3 years in 2004 to 31.2 in 2014. Similarly, the proportion of births to women older than 35 increased from 15.4% in 2004 to 16.5% in 2014, the proportion of births to women older than 40 increased from 2.7% to 3.3%, and the proportion of births to women older than 45 increased from 0.1% to 0.3%.
Clinical and demographic characteristics of the study population are tabulated according to 5-year age categories in Table 1. Pre-pregnancy type 1 or type 2 diabetes and chronic hypertension increased with increasing maternal age, though the proportion of births to women with either condition remained low across age groups (at <3% among those older than 45). On the other hand, current smoking, inadequate prenatal care, low-income status, and rural residence decreased with increasing maternal age. Pre-pregnancy obesity remained relatively stable across age categories. The incidence of a prior spontaneous or therapeutic abortion, diagnosed infertility, in vitro fertilization, and multiple gestations increased sharply with increasing maternal age, particularly after age 35. Approximately half of women with a first birth at age 40 or older had a prior spontaneous or therapeutic abortion (50.7%) or had been diagnosed with infertility (47.1%). Likewise, more than half of women with a first birth at 45 or older had a prior spontaneous or therapeutic abortion (56.0%) and the majority (71.9%) had been diagnosed with infertility. The proportion of women meeting the lowest-risk profile decreased with increasing maternal age, largely due to infertility diagnosis/treatment and prior spontaneous or therapeutic abortion, though 14.0% of women older than age 35 still met the low-risk criteria.
Table 1.
Demographic and clinical characteristics of all births to nulliparous women in British Columbia (Canada), 2004–2014, N (%).
| All 203,414 |
<20 years 11,524 |
20–<25 years 36,510 |
25–<30 years 62,214 |
30–<35 years 60,616 |
35–<40 years 26,708 |
40–<45 years 5,426 |
≥45 years 416 |
|
|---|---|---|---|---|---|---|---|---|
| Pre-pregnancy type 1 or type 2 diabetes | 1,010 (1) | 26 (0) | 125 (0) | 283 (1) | 298 (1) | 214 (1) | 56 (1) | 8 (2) |
| Pre-pregnancy hypertension | 1,078 (1) | <5 (0) | 63 (0) | 2225 (0) | 371 (2) | 288 (1) | 114 (2) | 12 (3) |
| Current smoker | 18,389 (9) | 3,516 (31) | 6,669 (18) | 4,401 (7) | 2,563 (4) | 1,017 (4) | 216 (4) | 7 (2) |
| Obesea (BMI≥30) | 15,660 (11) | 491 (7) | 2,981 (12) | 5,054 (11) | 4,548 (10) | 2,137 (11) | 421 (11) | 28 (10) |
| Inadequate antenatal careb | 12,413 (7) | 1,426 (13) | 2,742 (8) | 3,377 (6) | 3,036 (5) | 1,503 (6) | 304 (6) | 25 (7) |
| Low income (≤2nd income decile)c | 43,820 (22) | 3,707 (32) | 9,421 (26) | 13,144 (21) | 11,586 (19) | 4,913 (18) | 979 (18) | 70 (17) |
| Rural residenced | 21,427 (11) | 2,604 (23) | 6,094 (17) | 6,129 (10) | 4,445 (7) | 7,798 (7) | 334 (6) | 23 (6) |
| Previous spontaneous or induced abortion | 59,516 (29) | 2,098 (18) | 9,546 (26) | 16,172 (26) | 18,285 (30) | 10,431 (39) | 2,751 (51) | 233 (56) |
| Diagnosed infertility | 25,961 (13) | 55 (1) | 1,137 (3) | 5,239 (8) | 9,177 (15) | 7,498 (28) | 2,556 (47) | 299 (72) |
| Non-IVF infertility treatment/evaluation | 1,283 (0) | 0 (0) | 17 (0) | 180 (0) | 465 (0) | 416 (1) | 181 (1) | 24 (2) |
| In vitro fertilizatione | 5,286 (4) | 29 (0) | 91 (0) | 613 (2) | 1,630 (4) | 1,922 (12) | 816 (23) | 185 (67) |
| Low risk profilef | 38,851 (19) | 1,450 (13) | 16.6 (17) | 13,760 (22) | 13,035 (22) | 4,039 (15) | 506 (9) | 10 (2) |
BMI indicates body mass index, IVF in vitro fertilization.
Pre-pregnancy weight or maternal height was missing for 53,828 (26.5%) observations.
Number of antenatal visits is missingin 16,191 (8.0%).
Missing census tract decile for the year of birth (n=31,249; 15.4%) was imputed using census tract decile from the closest year with available data. Those with no census tract data for any year (n=5,590; 2.8%) were imputed using multiple imputation.
Missing community size (used to define rural residence) for the year of birth (n=28,300, 13.9%) was imputed using community size data from the closest year with available data. Those with no community size data for any year (n=2,613; 1.3%) were imputed using multiple imputation. Infertility treatment or evaluation defined as outpatient prescription of clomid or letrazole, or billing codes for artificial insemination or post coital test.
In vitro fertilization was not collected until April 1, 2008; IVF data in this table are restricted to April 1, 2008 – March 31, 2014.
Low risk profile defined as the absence of all age-related risk factors: no pre-pregnancy diabetes or hypertension, not a current smoker, not obese, received adequate antenatal care, urban residence, no previous known spontaneous or induced abortion,
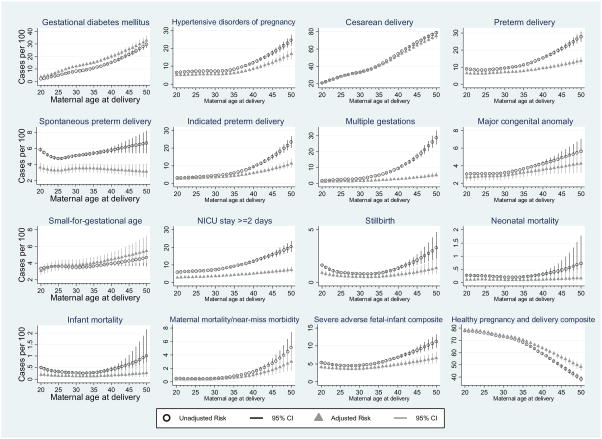
The Figure presents the unadjusted and adjusted predicted risks of each outcome at each maternal age from 20 to 50 years, with corresponding 95% confidence intervals. Though the forms of each curve differed, risks of all outcomes increased with increasing maternal age among women older than 30. Unadjusted curves show the population average risks for women at each age, while adjusted curves show risks for the lowest-risk profile (i.e., when age-related comorbidities and risk factors were absent). Predicted risks from multivariable models present a “best-case scenario” for low-risk women across the age continuum, with lower absolute risks compared with those from unadjusted models for most outcomes. Adjustment for infertility accounted for the greatest attenuation in risk observed between crude and adjusted estimates many outcomes, largely due to its strong association with multiple gestations. Curves for cesarean delivery, and the healthy pregnancy and delivery composite, were virtually unchanged by adjustment for covariates across the age continuum. Adjusted risks of gestational diabetes and small-for-gestational age were elevated compared with crude risks, largely due to low income status and calendar year. Predicted risks from the Figure are tabulated by 5-year increments of maternal age in Table 2, and by 1-year increments in eTable 1.
Figure 1.
Predicted absolute risks (with 95% confidence intervals) of adverse pregnancy and birth outcomes at specified values of maternal age at first birth in British Columbia (Canada), 2004–2014 (n=203,414). Unadjusted and adjusted risks according to maternal age at first birth: i) gestational diabetes mellitus; ii) hypertensive disorders of pregnancy; iii) cesarean delivery; iv) preterm delivery; v) spontaneous preterm delivery; vi) indicated preterm delivery; vii) multiple gestations; viii) major congenital anomaly; ix) small-for-gestational age; x) NICU stay >=2 days; xi) stillbirth; xii) neonatal mortality; xiii) infant mortality; xiv) maternal mortality or near-miss morbidity; xv) severe adverse fetal-infant composite; xvi) health pregnancy and delivery composite.
Models for gestational diabetes were restricted to those without pre-pregnancy type 1 or type 2 diabetes mellitus. Models for NICU stay ≥2 days restricted to births after April, 2008. Models for small-for-gestational age restricted to singleton pregnancies and gestational ages from 22 to 43 weeks. Adjusted models included pre-pregnancy diabetes, pre-pregnancy chronic hypertension, pre-pregnancy body mass index, smoking, indicator variables for calendar year, inadequate prenatal care, low income, rural residence, previous spontaneous or therapeutic abortion, diagnosed infertility, infertility treatment.
Maternal age at first birth was modeled using restricted cubic splines with 4 knots (at the 5th, 35th, 65th, and 95th percentiles; ages 19.7, 27.1, 31.5, and 38.6) for hypertensive disorders of pregnancy, gestational diabetes, cesarean delivery, spontaneous preterm delivery, and healthy preterm delivery and modeled using restricted cubic splines with 3 knots (at the 10th, 50th, and 90th percentiles; ages 21.4, 29.3, and 36.5) for all other outcomes.
Table 2.
Predicted absolute risks (95% confidence intervals) of adverse pregnancy and birth outcomes at specified values of maternal age among nulliparous women in British Columbia (Canada), 2004–2014 (n=203,414).
| 20 years | 25 years | 30 years | 35 years | 40 years | 45 years | 50 years | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Gestational diabetes mellitusa n=15,375 (7.6%) | |||||||
|
| |||||||
| Unadjusted | 2.1 (1.9, 2.2) | 5.0 (4.9, 5.2) | 8.0 (7.8, 8.2) | 11 (11, 11) | 15 (15, 16) | 21 (20, 23) | 29 (26, 31) |
| Adjusted | 3.6 (3.2, 4.1) | 7.9 (7.1, 8.8) | 12 (11, 13) | 15 (14, 17) | 20 (18, 22) | 25 (23, 28) | 32 (28, 36) |
|
| |||||||
| Hypertensive disorders of pregnancy n=15,708 (7.7%) | |||||||
|
| |||||||
| Unadjusted | 6.5 (6.2, 6.8) | 7.3 (7.1, 7.5) | 7.2 (7.0, 7.4) | 8.4 (8.2, 8.6) | 12 (11, 12) | 17 (16, 18) | 24 (21, 26) |
| Adjusted | 5.1 (4.5, 5.8) | 5.4 (4.8, 6.1) | 5.4 (4.8, 6.1) | 6.3 (5.5, 7.1) | 8.6 (7.5, 9.7) | 12 (10, 14) | 16 (14, 19) |
|
| |||||||
| Cesarean delivery n=68,050 (33.5%) | |||||||
|
| |||||||
| Unadjusted | 21 (20, 21) | 28 (28, 28) | 33 (33, 33) | 41 (41, 42) | 54 (54, 55) | 68 (66, 69) | 78 (77, 80) |
| Adjusted | 21 (20, 22) | 28 (27, 29) | 33 (32, 35) | 41 (39, 42) | 51 (50, 54) | 64 (62, 66) | 74 (72, 76) |
|
| |||||||
| Preterm delivery n= 20,028 (9.9%) | |||||||
|
| |||||||
| Unadjusted | 8.8 (8.5, 9.2) | 8.5 (8.3, 8.7) | 9.1 (8.9, 9.2) | 12 (11, 12) | 15 (15, 16) | 20 (19, 21) | 25 (24, 27) |
| Adjusted | 6.3 (5.6, 7.1) | 6.4 (5.7, 7.1) | 6.8 (6.1, 7.5) | 7.8 (7.0, 8.7) | 9.3 (8.3, 10) | 11 (10, 12) | 13 (11, 15) |
|
| |||||||
| Spontaneous preterm delivery n=10,667 (5.2%) | |||||||
|
| |||||||
| Unadjusted | 5.9 (5.6, 6.1) | 4.9 (4.7, 5.0) | 5.0 (4.9, 5.1) | 5.5 (5.3, 5.7) | 5.7 (5.4, 6.1) | 6.0 (5.3, 6.8) | 6.2 (5.2, 7.5) |
| Adjusted | 3.6 (3.1, 4.2) | 3.3 (2.8, 3.8) | 3.5 (3.0, 4.0) | 3.6 (3.1, 4.1) | 3.4 (2.9, 4.0) | 3.2 (2.5, 3.9) | 3.0 (2.3, 3.8) |
|
| |||||||
| Indicated preterm delivery n=9,364 (5.4) | |||||||
|
| |||||||
| Unadjusted | 3.1 (2.9, 3.3) | 3.4 (3.3, 3.5) | 4.2 (4.0, 4.3) | 6.0 (5.9, 6.2) | 9.2 (8.7, 9.6) | 14 (13, 15) | 20 (18, 22) |
| Adjusted | 2.6 (2.2, 3.1) | 2.9 (2.5, 3.4) | 3.4 (2.9, 4.0) | 4.4 (3.7, 5.1) | 5.8 (4.9, 6.7) | 7.6 (6.3, 9.0) | 9.9 (8.1, 12) |
|
| |||||||
| Multiple gestations n=7,188 (3.5%) | |||||||
|
| |||||||
| Unadjusted | 1.7 (1.5, 1.8) | 2.1 (2.0, 2.2) | 3.0 (2.9, 3.1) | 5.2 (5.0, 5.4) | 9.3 (8.8, 9.9) | 16 (15, 18) | 27 (24, 30) |
| Adjusted | 0.95 (0.72, 1.2) | 1.3 (1.0, 1.7) | 1.4 (1.1, 1.7) | 1.9 (1.5, 2.4) | 2.6 (2.1, 3.4) | 3.6 (2.8, 4.7) | 5.0 (3.7, 6.5) |
|
| |||||||
| Major congenital anomaly n=6,735 (3.3) | |||||||
|
| |||||||
| Unadjusted | 3.1 (2.9, 3.2) | 3.1 (3.0, 3.1) | 3.2 (3.1, 3.3) | 3.6 (3.5, 3.8) | 4.2 (4.0, 4.5) | 4.9 (4.4, 5.4) | 5.7 (4.9, 6.6) |
| Adjusted | 2.7 (2.2, 3.2) | 2.8 (2.3, 3.2) | 2.9 (2.5, 3.4) | 3.5 (3.0, 4.2) | 3.9 (3.2, 4.8) | 3.9 (3.1, 4.8) | 4.4 (3.5, 5.5) |
|
| |||||||
| Small-for-gestational agec n=18,816 (9.6) | |||||||
|
| |||||||
| Unadjusted | 8.7 (8.4, 9.0) | 9.3 (9.2, 9.5) | 9.8 (9.6, 1) | 10. (9.9, 10) | 10 (10, 11) | 11 (10, 11) | 11 (10, 12) |
| Adjusted | 8.5 (7.7, 9.4) | 9.8 (8.8, 11) | 11 (9.9, 12) | 11 (10, 13) | 12 (11, 13) | 12 (11, 14) | 13 (11, 14) |
|
| |||||||
| Neonatal intensive care unit stay 48 hoursb n=9,668 (7.7) | |||||||
|
| |||||||
| Unadjusted | 6.0 (5.7, 6.3) | 6.4 (6.2, 6.6) | 7.3 (7.1, 7.5) | 9.3 (9.0, 9.5) | 12 (12, 13) | 16 (14, 17) | 20 (18, 22) |
| Adjusted | 2.8 (2.4, 3.3) | 3.2 (2.7, 3.7) | 3.6 (3.1, 4.2) | 4.3 (3.7, 5.0) | 5.1 (4.4, 6.0) | 6.2 (5.2, 7.3) | 7.4 (6.1, 9.0) |
|
| |||||||
| Stillbirth n=2,064 (1.0) | |||||||
|
| |||||||
| Unadjusted | 1.6 (1.5, 1.8) | 0.94 (0.86, 1.0) | 0.81 (0.74, 0.88) | 0.88 (0.81, 0.97) | 1.4 (1.2, 1.5) | 2.1 (1.7, 2.7) | 3.3 (2.3, 4.8) |
| Adjusted | 0.90 (0.66, 1.2) | 0.61 (0.44, 0.84) | 0.52 (0.38, 0.71) | 0.54 (0.40, 0.75) | 0.73 (0.52, 1.0) | 1.0 (0.66, 1.5) | 1.36 (0.81, 2.3) |
|
| |||||||
| Neonatal mortality n=467 (0.2) | |||||||
|
| |||||||
| Unadjusted | 0.26 (0.21, 0.33) | 0.24 (0.20, 0.29) | 0.20 (0.17, 0.24) | 0.21 (0.17, 0.26) | 0.31 (0.24, 0.41) | 0.47 (0.26, 0.84) | 0.72 (0.29, 1.79) |
| Adjusted | 0.11 (0.05, 0.22) | 0.11 (0.06, 0.23) | 0.10 (0.05, 0.20) | 0.09 (0.04, 0.19) | 0.11 (0.05, 0.24) | 0.14 (0.05, 0.34) | 0.17 (0.05, 0.54) |
|
| |||||||
| Infant mortality n=662 (0.3) | |||||||
|
| |||||||
| Unadjusted | 0.50 (0.43, 0.57) | 0.32 (0.27, 0.37) | 0.26 (0.23, 0.31) | 0.28 (0.23, 0.33) | 0.42 (0.33, 0.53) | 0.65 (0.40, 1.1) | 1.0 (0.46, 2.2) |
| Adjusted | 0.19 (0.10, 0.37) | 0.15 (0.08, 0.28) | 0.13 (0.07, 0.25) | 0.13 (0.07, 0.24) | 0.16 (0.08, 0.31) | 0.21 (0.09, 0.46) | 0.27 (0.10, 0.74) |
|
| |||||||
| Maternal mortality or severe near-miss morbidity n=1,221 (0.6) | |||||||
|
| |||||||
| Unadjusted | 0.47 (0.40, 0.55) | 0.40 (0.34, 0.46) | 0.49 (0.43, 0.55) | 0.78 (0.70, 0.86) | 1.5 (1.3, 1.6) | 2.7 (2.1, 3.5) | 5.1 (3.4, 7.4) |
| Adjusted | 0.35 (0.22, 0.54) | 0.30 (0.19, 0.47) | 0.37 (0.24, 0.57) | 0.58 (0.37, 0.89) | 1.0 (0.64, 1.54) | 1.7 (1.1, 2.8) | 3.0 (1.7, 5.2) |
|
| |||||||
| Adverse fetal-infant composite outcome n=10,167 (5.0) | |||||||
|
| |||||||
| Unadjusted | 5.4 (5.2, 5.6) | 4.6 (4.5, 4.7) | 4.5 (4.4, 4.6) | 5.4 (5.2, 5.5) | 6.8 (6.5, 7.2) | 8.7 (8.0, 9.4) | 11 (9.8, 12) |
| Adjusted | 4.0 (3.5, 4.7) | 3.7 (3.2, 4.2) | 3.6 (3.2, 4.2) | 4.1 (3.6, 4.7) | 4.8 (4.1, 5.6) | 5.7 (4.8, 6.7) | 6.6 (5.5, 8.0) |
|
| |||||||
| Composite healthy pregnancy and birth outcome n=103,881 (51.1) | |||||||
|
| |||||||
| Unadjusted | 77 (77, 78) | 76 (76, 76) | 74 (73, 74) | 68 (68, 68) | 59 (59, 60) | 50 (49, 51) | 41 (39, 43) |
| Adjusted | 79 (77, 80) | 77 (75, 78) | 74 (73, 76) | 70 (68, 71) | 64 (62, 66) | 57 (55, 59) | 50 (47, 53) |
Maternal age at first birth was modeled using restricted cubic splines with 4 knots (at the 5th, 35th, 65th, and 95th percentiles; ages 19.7, 27.1, 31.5, and 38.6) for gestational diabetes, hypertensive disorders of pregnancy, cesarean delivery, spontaneous preterm delivery, and healthy preterm delivery and modeled using restricted cubic splines with 3 knots (at the 10th, 50th, and 90th percentiles; ages 21.4, 29.3, and 36.5) for all other outcomes.
Adjusted models included pre-pregnancy diabetes, pre-pregnancy chronic hypertension, pre-pregnancy body mass index, smoking, indicator variables for calendar year, inadequate prenatal care (yes/no, defined as <5 visits), low income (defined by <3rd decile postal code), rural residence (defined by community size <10,000), previous spontaneous or therapeutic abortion, diagnosed infertility (defined by ICD-9-CM code 628), infertility treatment.
Models for gestational diabetes were restricted to those without pre-pregnancy type 1 or type 2 diabetes mellitus.
Models for neonatal intensive care unit (NICU) stay ≥48 hours restricted to births after April 1, 2008, when coding criteria changed.
Models for small-for-gestational age restricted to singleton pregnancies and gestational ages between 22 and 43 weeks, in accordance with reference charts
Predicted risks stratified by multiple gestations are tabulated in eTables 3 and 4, and stratified curves are graphed in eFigure 1. Risks were higher for all outcomes among multiple gestations compared with singleton births, though curves were similarly shaped for many outcomes (gestational diabetes, hypertensive disorders, maternal mortality or near-miss morbidity, stillbirth, and severe adverse fetal-infant composite). Risk of neonatal or infant mortality were substantially elevated for multiple gestations to mothers younger than 35, though the rate of increase in risk to older mothers was similar among singleton and multiple gestations. Risks of cesarean delivery and preterm delivery were much higher for multiple gestations regardless of maternal age, yielding flatter curves compared with those for singleton births.
When we dichotomized maternal age to estimate risk ratios comparing each advanced maternal age definition to each referent group definition (eTable 2), we found sufficient variability in risk ratios to conclude that a more detailed approach than dichotomization of maternal age is recommended. However, the overall conclusions regarding the relationship between maternal age and pregnancy and birth risks were not qualitatively changed by exposure or referent group definitions.
Sensitivity analyses
Because of higher overall risk of NICU stay ≥48 hours before the change in coding, we restricted analyses for this outcome to after 1 April 2008, when the change took effect. Despite the change in diagnostic criteria for gestational diabetes during the study period, the incidence of gestational diabetes overall and according to maternal age at first pregnancy remained similar before and after the change in criteria. We therefore examined all cases of gestational diabetes together.
Multivariable models that included IVF (risks estimated in the absence of IVF) produced lower predicted risks for women older than 37, compared with multivariable models that excluded IVF (risks estimated at average IVF values). These differences were most pronounced for preterm delivery (both spontaneous and indicated), though estimates were somewhat lower for hypertensive disorders of pregnancy and severe fetal–infant summary outcome, and slightly lower for maternal mortality or near-miss morbidity, major congenital anomaly, and neonatal mortality. Analyses were not impacted at younger maternal ages, likely due to low use of IVF use among younger women. Predicted risks from adjusted models including and excluding IVF are juxtaposed in eFigure 2.
Discussion
This study found that risks for adverse pregnancy and birth outcomes increased steadily with increasing maternal age at first birth. The overall proportion of women in our study with each outcome according to age category was consistent with previous studies.2,4,8–10,34,35 Our findings provide baseline absolute risks of many important outcomes by age, which will be useful for patient counseling and decisionmaking, and the curves we present are accordant with most previous reports of risks increasing with maternal age.2,5–7,10,35–37
This paper complements previous work by presenting absolute risks and by displaying the form with which risks increase. While age 35, 40, and 45 have been used to define advanced, very advanced, and extremely advanced maternal age thresholds, clear inflection points at these ages were not evident for most outcomes. In fact, inflection points were not observed at age 40 or age 45 for any outcomes, despite clinical guideline cutoffs geared towards those older than 40 (e.g., induction at 40 weeks for women over 40).37 By examining absolute risks, we see that many pregnancies to mothers between age 35 and 40 result in healthy mothers and babies. Risks of less severe adverse outcomes (e.g., pregnancy complications, cesarean delivery) were fairly common among women older than age 35. However, absolute risks for the most severe outcomes (severe maternal morbidity and severe adverse fetal-infant composite) remained quite low (<1%–2% and 5%–7%, respectively) for women at age 35 and 40. By age 50, however, predicted absolute risks for even these outcomes were substantially higher.
Risks of hypertensive disorders of pregnancy were stable and low from age 20 until age 35, where there was a clear inflection point, after which risks increased rapidly (particularly among term deliveries). Despite previous reports of an association between “advanced maternal age” and increased risk of hypertensive disorders of pregnancy,39,40 this sharp change in risk at age 35 has not been previously reported. Further work in this area would be useful to elucidate whether the abrupt increase at age 35 is related to increased surveillance for women of “advanced maternal age” or reflecting true age-related pathophysiology. The quadratic increase in risk of major congenital anomalies with increasing maternal age may be a useful contribution to an area of confusion in the literature, with recent studies reporting conflicting relationships between non-chromosomal major congenital anomalies and maternal age.35,36,41 Curves for gestational diabetes and cesarean delivery showed linear increases in risk, and likelihood of a healthy pregnancy and delivery decreased linearly with increasing maternal age. Stillbirth, neonatal and infant mortality, and severe adverse fetal–infant composite curves were j-shaped, with elevated risks for mothers both younger and older mothers. Curves for indicated preterm delivery, multiple gestations, major congenital anomalies, NICU stay, and maternal mortality or near-miss morbidity showed increases in risk accelerating gradually with increasing maternal age, following a quadratic shape. In our study, the age-related indicated preterm delivery risk was attenuated by the null relationship between age and spontaneous preterm delivery when all preterm deliveries were examined together. Our findings suggest that previously mixed findings5,7,37 may be due to combining spontaneous and indicated preterm deliveries into a single outcome. Because the relationship between maternal age at first pregnancy and each outcome is different, examination of the risks for our two composite outcomes provides a vehicle for women and clinicians to summarize the overall role of maternal age in pregnancy and birth risks.
Chronologic age and biologic age are not always equal, with wide variability in the rate of reproductive aging.42 Examining both the average predicted risks (from unadjusted models) and the “best-case scenario” predicted risks from (multivariable models) provides a plausible range for the relationships between increasing maternal age at first birth on pregnancy and birth risks among healthy nulliparous women with access to care. The tables and figures can be used to facilitate family planning decision-making about risk differences associated with small differences in maternal age at first birth. Likewise, our findings can inform discussions about the importance of maintaining a low-risk profile for women who delay childbearing, by examining the slower increase in risks for women across the age spectrum when age-related risk factors are absent. It should be noted that our findings are meant to be interpreted as mean population parameters, rather than individual-level risk predictions for each outcome. Individual experiences will show more variability than the estimated population averages we report.
Several limitations to our study must be considered in interpreting our findings. Women who delay childbearing are less likely to become pregnant and more likely to experience spontaneous abortion than younger women.12,13,38 As our cohort was defined by women with a pregnancy of ≥20 weeks, we could not assess the risk of spontaneous abortion or inability to become pregnant in this study, which restricts interpretation of our findings to the unidentifiable group of women who will be able to become pregnant at each maternal age. Further, older parturients may undergo additional antenatal monitoring compared with younger women, which may lead to overestimation of risk for some outcomes for older women.
Our analyses compare outcomes from different women at each age, rather than changes in risk within women at each age. Thus, there may be confounding by unmeasured factors that differ between women. Of note, information on race is not collected in population-level data from BC. In the U.S., race is associated with age at first birth.43 The distribution of racial groups is different in BC from in the U.S., with a smaller black population and larger East and Southeast Asian population despite a similar proportion of white/non-visible minorities.44,45 Though universal health care in BC may attenuate the impact of race on health outcomes, failure to account for race in our multivariable models may result in unmeasured confounding. Further, if race modifies the relationship between maternal age and pregnancy and birth outcomes, our findings represent the population average for BC, which is different from the population averages in areas with different distributions of race/ethnicity. Future research exploring effect modification by race would be useful. In addition to race, marital status, educational attainment, and employment status would be of interest in describing our study population, and in defining our lowest-risk profile from the adjusted models. Unfortunately, these data are missing for a high fraction of pregnancies in the BCPDR and were thus excluded from our analysis. Although most IVF cases were identified by diagnosed infertility or other infertility treatment (86.7% sensitivity), IVF was not captured in our database until 2008. Thus, our multivariable models provide estimates at the population average values of IVF for each maternal age, which may lead to an overestimation of risk for older women who do not undergo IVF.
Despite these limitations, this population-based study provides a unique examination of the role of increasing maternal age in pregnancy and birth risks which complements previous work. We examined a wide range of maternal and perinatal outcomes using a high-quality, population-level, North American data set. By estimating absolute risks across the reproductive age spectrum, our results can be easily interpreted by childbearing women and clinicians, and may enable clinicians to approach discussions about advanced maternal age with more granularity.
Supplementary Material
Acknowledgments
Sources of Financial Support: Laura Schummers was supported by National Research Service Award 1F31HD086970-01A1 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, a Training Grant in Pharmacoepidemiology from the Harvard T. H. Chan School of Public Health, and received a CIHR-PHAC Family Planning Public Health Chair Seed Grant to support this project.
Dr. Hutcheon holds New Investigator awards from the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research.
The authors thank Monique Gagne, Population Data BC, for her assistance in compiling the study data. All inferences, opinions, and conclusions drawn in this publication are those of the authors, and do not reflect the opinions or policies of Population Data BC or the data stewards.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare
Description of process by which someone else could obtain data and computing code: The data used for this study are administered by Population Data BC. Population Data BC provides access to these data for research purposes, but does not allow the data to be shared publicly in order to maintain confidentiality and privacy of individual health information. Researchers who wish to access this data may submit a data request directly to Population Data BC.
References
- 1.Ventura SJ, Mosher WD, Curtin SC, Abma JC, Henshaw S. Trends in pregnancies and pregnancy rates by outcome: Estimates for the united states, 1976–96. Vital Health Stat. 2000;21(56):1–45. [PubMed] [Google Scholar]
- 2.Carolan M. Maternal age ≥45 years and maternal and perinatal outcomes: A review of the evidence. Midwifery. 2013;29:479–489. doi: 10.1016/j.midw.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: Final data for 2015. Nat Vit Stat Rep. 2017;66(1):1–70. [PubMed] [Google Scholar]
- 4.Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: A cohort study. Ultrasound Obstet Gynecol. 2013;42:634–643. doi: 10.1002/uog.12494. [DOI] [PubMed] [Google Scholar]
- 5.Cleary-Goldman J, Malone FD, Vidaver J, et al. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5):983–990. doi: 10.1097/01.AOG.0000158118.75532.51. [DOI] [PubMed] [Google Scholar]
- 6.Balayla J, Azoulay L, Assayag J, Benjamin A, Abenhaim HA. Effect of maternal age on the risk of stillbirth: A population-based cohort study on 37 million births in the united states. Am J Perinatol. 2011;28(8):643–650. doi: 10.1055/s-0031-1276739. [DOI] [PubMed] [Google Scholar]
- 7.Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–1418. doi: 10.1097/01.AOG.0000163256.83313.36. [DOI] [PubMed] [Google Scholar]
- 8.Yaniv SS, Levy A, Wiznitzer A, Holchberg G, Mazor M, Sheiner E. A significant linear association exists between advanced maternal age and adverse perinatal outcome. Arch Gynecol Obstet. 2011;283:755–759. doi: 10.1007/s00404-010-1459-4. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira FC, Costa ML, Cecatti JG, e Silva JLP, Surita FG. Maternal morbidity and near miss associated with maternal age: The innovative approach of the 2006 brazilian demographic health survey. Clinics. 2013;68(7):922–927. doi: 10.6061/clinics/2013(07)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laopaiboon M, Lumbiganon P, Intarut N, et al. Advanced maternal age and pregnancy outcomes: A multicountry assessment. Brit J Obstet Gynaec. 2014;121(Suppl 1):49–56. doi: 10.1111/1471-0528.12659. [DOI] [PubMed] [Google Scholar]
- 11.Ferranti EP, Jones EJ, Hernandez TL. Pregnancy reveals evolving risk for cardiometabolic disease in women. JOGNN. 2016;45(3):413–425. doi: 10.1016/j.jogn.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Fragouli E, Alfarawati S, Spath K, et al. The origin and impact of embryonic aneuploidy. Hum Genet. 2013;132(9):1001–1013. doi: 10.1007/s00439-013-1309-0. [DOI] [PubMed] [Google Scholar]
- 13.Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril. 2015;103(5):1136–1143. doi: 10.1016/j.fertnstert.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Littlefield JW. The pregnancy at risk for a genetic disorder. New Engl J Med. 1970;282(11):627–628. doi: 10.1056/NEJM197003122821115. [DOI] [PubMed] [Google Scholar]
- 15.Hook EB. Rates of chromosome abnormalities at different maternal ages. Obstet Gynecol. 1981;58(3):282–285. [PubMed] [Google Scholar]
- 16.Perinatal Services BC [creator] British Columbia Perinatal Data Registry. Population Data BC [publisher] Data Extract. PSBC (2017) 2015 http://www.perinatalservicesbc.ca/health-professionals/data-surveillance/perinatal-data-registry.
- 17.Frosst GO, Hutcheon JA, Joseph KS, Kinniburgh BA, Johnson C, Lee L. Validating the british columbia perinatal data registry: A chart re-abstraction study. BMC Preg Childbirth. 2015;15(123) doi: 10.1186/s12884-015-0563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.British Columbia Ministry of Health [creator] Medical services plan (MSP) payment information file. V2. Data Extract. Population Data BC [publisher] Data Extract. MOH (2016) 2015 http://www.popdata.bc.ca/data.
- 19.BC Ministry of Health [creator] PharmaNet. V2. BC Ministry of Health [publisher]. Data Extract. Data Stewardship Committee (2017) 2015 http://www.popdata.bc.ca/data.
- 20.Canadian Institute for Health Information [creator] Discharge abstract database (Hospital Separations). V2. Data Extract. Population Data BC [publisher] Data Extract. MOH. 2015 http://www.popdata.bc.ca/data.
- 21.Statistics Canada [creator] Statistics Canada Income Band Data. Catalogue number 13C0016. Population Data BC [publisher]. Data Extract. Population Data BC (2017) 2009 http://www.popdata.bc.ca/data.
- 22.BC Ministry of Health [creator] Consolidation file. V2. Population Data BC [publisher] Data Extract. MOH (2016) 2015 http://www.popdata.bc.ca/data.
- 23.BC Vital Statistics Agency [creator] Vital Statistics Deaths. V2. Population Data BC [publisher] Data Extract BC Vital Statistics Agency (2016) 2015 http://www.popdata.bc.ca/data.
- 24.WHO/CDC/ICBSR. Birth defects surveillance: A manual for programme managers. Geneva: World Health Organization; 2014. p. 124. [Google Scholar]
- 25.Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowics M, Blondel B, Breart G. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):e35–e42. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 26.Geller SE, Rosenberg D, Cox SM, Brown M, Simonson L, Kilpatrick S. A scoring system identified near-miss maternal morbidity during pregnancy. J Clin Epidemiol. 2004;57(7):716–7120. doi: 10.1016/j.jclinepi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer; 2001. [Google Scholar]
- 28.Kadane JB, Lazar NA. Methods and criteria for model selection. J Am Stat Ass. 2004;99(465):279–290. [Google Scholar]
- 29.Froot KA. Consistent covariance matrix estimation with cross-sectional dependence and heteroskedasticity in financial data. Journal of Financial and Quantitative Analysis. 1989;24(3):333–355. [Google Scholar]
- 30.Orsini N, Greenland S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J. 2011;11(1):1–29. [Google Scholar]
- 31.Carlin JB, Galati J, Royston P. A new framework for managing and analyzing multiply imputed data in stata. Stata J. 2008;8(1):49–67. [Google Scholar]
- 32.Stata Corp. Stata staistical software: Release 14. College Station, TX: Statacorp, LP; 2015. [Google Scholar]
- 33.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendation on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salihu H, Mbah AK, Alio AP, et al. Nulliparity and preterm birth in the era of obesity epidemic. J Matern-Fetal Neo M. 2010;23(12):1444–1450. doi: 10.3109/14767051003678044. [DOI] [PubMed] [Google Scholar]
- 35.Ciancimino L, Lagana AS, Chiofalo B, Granese R, Grasso R, Triolo O. Would it be too late? A retrospective case-control analysis to evaluate maternal-fetal outcomes in advanced maternal age. Arch Gynecol Obstet. 2014;290(6):1109–1114. doi: 10.1007/s00404-014-3367-5. [DOI] [PubMed] [Google Scholar]
- 36.Csermely G, Susansky E, Czeizel AE. Association of young and advanced age of pregnant women with the risk of isolated congenital abnormalities in hungary - a population-based case-matched control study. J Matern Fetal Neonatal Med. 2015;28(4):436–442. doi: 10.3109/14767058.2014.918946. [DOI] [PubMed] [Google Scholar]
- 37.Newburn-Cook CV, Onyskiw JE. Is older maternal age a risk factor for preterm birth and fetal growth restriction? A systematic review. Health Care Women Internat. 2005;26(9):852–875. doi: 10.1080/07399330500230912. [DOI] [PubMed] [Google Scholar]
- 38.Society of Obstetricians and Gynecologists. Committee opinion: Delayed childbearing. J Obstet Gynaecol Can. 2012;34(1):80–93. [Google Scholar]
- 39.Salihu HM, Shumpert MN, Slay M, Kirby RS, Alexander GR. Childbearing beyond maternal age 50 and fetal outcomes in the united states. Obstet Gynecol. 2003;5(1):1006–1014. doi: 10.1016/s0029-7844(03)00739-7. [DOI] [PubMed] [Google Scholar]
- 40.Usta IM, Nassar AH. Advanced maternal age. part 1: Obstetric complications. Am J Perinatol. 2008;25(8):521–534. doi: 10.1055/s-0028-1085620. [DOI] [PubMed] [Google Scholar]
- 41.Goetzinger KR, Shanks AL, Odibo AO, Macones GA, Cahill AG. Advanced maternal age and the risk of major congenital anomalies. Am J Perinatol. 2017;34(3):217–222. doi: 10.1055/s-0036-1585410. [DOI] [PubMed] [Google Scholar]
- 42.Alviggi C, Humaidan P, Howles C, Tredway D, Hillier SG. Biological versus chronological ovarian age: Implications for assisted reproductive technology. Reprod Biol Endocrinol. 2009;7(101) doi: 10.1186/1477-7827-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathews TJ, Hamilton BE. Delayed childbearing: More women are having their first child later in life. NCHS Data Brief. 2009:21. [PubMed] [Google Scholar]
- 44.BC Stats. 2006 census fast facts: Ethnicity and visible minority characteristics of BC’s population. 2008:2006–12. [Google Scholar]
- 45.United States Census Bureau, Population Estimates Program. [Accessed May 16, 2017];ACS demographic and housing estimates: 2011–2015 American community survey 5-year estimates. Updated 2017. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_15_5YR_DP05&src=pt.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.