Abstract
Context
Autopsy studies of the older population (≥65 years of age), and particularly of the “oldest-old” (≥85 years of age), have identified a significant proportion (~20%) of cognitively impaired patients in which hippocampal sclerosis is the major substrate of an amnestic syndrome. Hippocampal sclerosis may also be comorbid with frontotemporal lobar degeneration, Alzheimer disease, and Lewy body disease. Until recently, the terms hippocampal sclerosis of aging or hippocampal sclerosis dementia were applied in this context. Recent discoveries have prompted a conceptual expansion of hippocampal sclerosis of aging because (1) cellular inclusions of TAR DNA-binding protein 43 kDa (TDP-43) are frequent; (2) TDP-43 pathology may be found outside hippocampus; and (3) brain arteriolosclerosis is a common, possibly pathogenic, component.
Objective
To aid pathologists with recent recommendations for diagnoses of common neuropathologies in older persons, particularly hippocampal sclerosis, and highlight the recent shift in diagnostic terminology from HS-aging to cerebral age-related TDP-43 with sclerosis (CARTS).
Data Sources
Peer-reviewed literature and 5 autopsy examples that illustrate common age-related neuropathologies, including CARTS, and emphasize the importance of distinguishing CARTS from late-onset frontotemporal lobar degeneration with TDP-43 pathology and from advanced Alzheimer disease with TDP-43 pathology.
Conclusions
In advanced old age, the substrates of cognitive impairment are often multifactorial. This article demonstrates common and frequently comorbid neuropathologic substrates of cognitive impairment in the older population, including CARTS, to aid those practicing in this area of pathology.
Autopsy-based studies of cognitive impairment in the older US population (≥65 years of age1) show that most dementias arise in the setting of 2 or more different neuropathologies.2,3 Among the common dementia-related diseases, cerebrovascular,4 Alzheimer disease (AD), and Lewy body disease (LBD) pathologies exert well-known effects on cognitive function. However, pathologists working in the area of autopsy neuropathology must also recognize other recently described neuropathologies of aging, including cerebral age-related TDP-43 pathology with sclerosis (CARTS)2 and primary age-related tauopathy (PART).5 We emphasize here that these 2 pathologically defined conditions are quite common in elderly populations across the cognitive continuum, including patients without evident cognitive decline, those with mild cognitive impairment, and more commonly in those with overt dementia.
Alzheimer disease is the most prevalent dementia-related brain disease and the recent recommendations of the National Institute on Aging and the Alzheimer’s Association (NIA-AA) provide guidelines to all practicing pathologists regarding appropriate diagnostic terminology for this entity.6,7 These diagnostic guidelines provide 4 levels of “AD neuropathologic change,” based on the ordinal scale ranking of 3 characteristic AD-associated neuropathologies: neuritic plaque densities, neurofibrillary tangle (NFT) anatomic distribution, and the distribution of parenchymal deposits of β-amyloid peptides (Aβ).7 Based on these pathologies, it is possible to assign “not,” “low,” “intermediate,” or “high” scores of AD neuropathologic change for most subjects exhibiting these pathologic features.6,7
Per NIA-AA guidelines, AD neuropathologic change of an “intermediate” or “high” level is considered as sufficient explanation for dementia in the appropriate clinical setting.7 However, older patients with dementia often lack sufficiently abundant or extensive pathologic deposition of tau and Aβ to explain their clinical states. This diagnostic gap (ie, clinical dementia with “not” or “low” AD neuropathologic change) has implications for the patient’s family, who may have concerns about a clinical diagnosis that is not explainable according to the autopsy report. In older patients with dementia, non-AD entities assist in bridging this gap: LBD, vascular brain injury in the form of “vascular cognitive impairment” and “vascular dementia,” and PART. While LBD and vascular brain injuries are commonly recognized, PART, although described initially several decades ago, lacked consensus neuropathologic criteria for diagnosis until recently. PART is characterized by NFT pathology in structures of the mesial temporal lobe, basal forebrain, and brainstem (Braak stages ≤ IV) with minimal or no Aβ or neocortical neuritic plaque pathologies.5 PART may occur in older, cognitively impaired patients and was previously referred to as tangle-predominant senile dementia in that setting.8
Hippocampal sclerosis (HS) is a common, but underappreciated, pathologic finding associated with aging and dementia, also referred to as hippocampal sclerosis of aging (HS-aging), hippocampal sclerosis dementia,9–11 and hippocampal sclerosis of the elderly.12 Hippocampal sclerosis most frequently occurs in the “oldest old” (>85 years of age)1 with a prevalence of ~20% of all individuals in this age group in community-based autopsy studies.13 The postmortem assessment of HS-aging may be underestimated owing to inexact consensus-based diagnostic definition, distraction from concurrent AD pathologic features in mixed cases, and also, importantly, from unilateral sampling of the hippocampus at autopsy.9,14
Hippocampal sclerosis of aging has a clinical picture dominated by episodic memory impairment,13,15,16 which is often confused with the clinical phenotype of AD.11,16 Pathologically, HS-aging is defined by neuron loss and gliosis in the hippocampal sector CA1 and subiculum that is out of proportion to the burden of AD neuropathologies.7,13,17,18 This results in “sclerosis,” or hardening of the atrophic hippocampus, as well as severe astrogliosis and neuronal loss. Specific genetic risk factors have been linked to HS-aging pathology, including gene variants of progranulin (GRN),19 transmembrane protein 106B (TMEM106B),12 ATP-binding cassette, subfamily C member 9 (ABCC9),14 and potassium calcium-activated channel subfamily M regulatory β subunit 2 (KCNMB2).20 Apolipoprotein E (APOE) is not considered a genetic risk factor for HS-aging,13 indicating that HS-aging has a pathogenesis distinct from AD.
A critical diagnostic feature of HS-aging is the presence of TAR DNA-binding protein 43 kDa (TDP-43) pathology. TDP-43 is a nucleic acid–binding protein, which plays a role in transcriptional regulation and posttranscriptional processing.21 Under normal physiologic conditions, TDP-43 is predominantly localized in the cell nucleus, but in pathologic conditions, such as frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis, TDP-43 is cleaved, ubiquinated, phosphorylated and accumulates as cellular inclusions, most frequently in the cytoplasm of neurons and glia.22–24 TDP-43 pathology was previously recognized as a feature of hippocampal pathology in patients with HS-aging,25 but it is now recognized as extending outside of the hippocampus in HS-aging to involve other regions of the limbic system18,25 and basal forebrain.26 Hippocampal sclerosis of aging has also been associated with pancerebral arteriolosclerosis,27,28 consistent with earlier suggestions that vascular disease may be an important contributor to this condition.10 However, the current understanding is that HS is not simply a result of hypoxic/ischemic brain injury. Whereas HS-aging and hypoxic/ischemic injury may have a similar appearance by hematoxylin-eosin staining, hippocampal lesions in the latter typically are TDP-43 negative.29 Furthermore, neither gross nor microscopic brain infarcts have been shown to be associated with HS-aging,17 and ischemia-associated pathology is not necessarily associated with hippocampal atrophy pathologically or on volumetric magnetic resonance imaging (MRI) studies.15 Other studies taking the patient’s age into consideration have shown that coronary artery disease, angioplasty, and diabetes are not associated with HS pathology, suggesting that conventional vascular risk factors and subsequent cerebrovascular injury are distinct from the pathology seen in HS-aging.13
CARTS: A NEW TERM FOR HS-AGING AND HS-DEMENTIA
Recently it has been recognized that HS-aging, or HS dementia, is a frequent pathologic substrate of dementia in older persons. These patients have unique clinical profiles and disease-associated genetic risk factors, as well as extrahippocampal TDP-43 pathology and brain arteriolosclerosis. These findings have led to a recent reconsideration of HS-aging and its nosology under the new terminology cerebral age-related TDP-43 with sclerosis, or CARTS.2
In brief, the diagnosis of CARTS (see also Table) is considered appropriate in the following settings: the patient’s age at death is above 85 years, TDP-43 pathology is present in hippocampus and possibly extrahippocampal regions, AD pathologic changes are not “high” (ie, Braak NFT stages ≤ IV), and clinical features of frontotemporal dementia are not present.
Salient Features of Cerebral Age-Related TDP-43 With Sclerosis (CARTS)
Overlapping diagnostic terms:
|
Hippocampal sclerosis of the elderly Typical clinical associations:
|
Typical pathology:
|
Genetic risk factors:
|
NOT significantly associated with, but may be seen with
|
CARTS terminology should NOT be applied in these clinical scenarios:
|
Abbreviations: ALS, amyotrophic lateral sclerosis; FTD, frontotemporal dementia; SNPs, single nucleotide polymorphisms.
The following findings do not exclude the application of CARTS terminology for reasons that are further clarified below: the absence of morphologic HS (although frequently present), the absence of severe cerebral arteriolosclerosis (although frequently present), and/or the presence of other degenerative brain diseases, including α-synucleinopathies, and non-AD tauopathies, such as PART, progressive supranuclear palsy, and argyrophilic grain disease.
For the remainder of this article, the term “CARTS” is applied to this specific entity, and the term “HS” is used when the setting is less specific, for example, in the context of another degenerative disease process, such as FTLD or ischemic/hypoxic injury without TDP-43 inclusions. Comprehensive reviews of the clinical, genetic, neuroimaging, and pathophysiologic features of CARTS are available elsewhere.2,9
DIAGNOSTIC CRITERIA FOR CARTS: RATIONALE
Several questions may arise following a review of the preceding criteria for the application of CARTS terminology. Why is an age threshold necessary? Why is TDP-43 pathology a core diagnostic feature? Why is morphologic HS not always required? Why is “high” AD pathology with TDP-43 pathology excluded?
In regard to the first question, the cutoff age of 85 years is based on many studies that have shown that HS is most common in much older patients. A review of prior studies on this topic was published by Nelson et al.9 in 2013. For example, one study17 showed that the highest prevalence of HS-aging (here, CARTS) is after the age of 90 years (18% of autopsied patients) versus 9.2% of autopsied patients younger than 90 years. Importantly, the pathologic hallmarks of clinical AD (parenchymal tau and Aβ deposition) level off or decline in the “oldest old” even if the incidence of clinical dementia does not.9,30 In other words, in the “oldest old” population, additional substrates of cognitive impairment must be considered (eg, vascular brain injury, HS and CARTS, argyrophilic grain disease, and PART). This point is illustrated below in Example 1.
Another reason for applying an age restriction to the use of CARTS terminology is to ensure that FTLD-TDP with HS is not misdiagnosed. Frontotemporal lobar degeneration may also have pathogenic mutations (ie, GRN mutation and c9orf72 expansion among others). At first glance, this concern may seem unwarranted, given that (1) autopsy studies have shown late-onset FTLD to be less common than younger-onset FTLD (<60 years of age)3; (2) the clinical features of CARTS and FTLD are distinct9; and (3) FTLD-TDP is far less common than CARTS.13,17,31,32 Nonetheless, FTLD can occur in older patients, for instance, in older brains with morphologic HS and without significant AD neuropathology (up to 4 of 18 patients with HS in one study).17 Late-onset FTLD has been reported between 65 and 86 years of age, accounting for 25% of all patients with FTLD in one autopsy series33 (see below). Furthermore, neuropathologically, FTLD with HS may overlap with CARTS (Example 4 illustrates one such example). Histopathologic features, not yet widely adopted, have been proposed to distinguish between older-age FTLD with HS and HS-aging.18
The second question relates to the necessity of including TDP-43 pathology as a core diagnostic feature of CARTS. TDP-43 pathology is likely the most specific biomarker of degenerative disease–associated HS with a detected prevalence in HS-aging of 85% to 100%.17,11,13,34 TDP-43 pathology is visualized as abundant immunostained short neurites and intracellular inclusions in hippocampal formation, amygdala, and adjacent limbic cortices. TDP-43 pathology may also extend outside of the mesial temporal lobe.17,25 One recent study identified that only HS was associated with basal forebrain TDP-43 pathology,26 whereas no significant association was seen with TDP-43 pathology in this region and either early AD or LBD pathologies.
The third question refers to why morphologic HS is not required for the application of the term CARTS, particularly as “sclerosis” is included in the acronym; for example, can TDP-43–positive and HS-negative CARTS occur? Studies indicate that TDP-43 pathology in mesial temporal lobe may precede generalized HS34,35 as defined on hematoxylin-eosin staining. TDP-43 pathology in CARTS may be bilateral even when morphologic HS (loss of neurons, astrogliosis) is present in only 1 hippocampus.13 In such cases, the associated cognitive impact of unilateral HS is still approximately the same as that seen with bilateral HS.36 Example 1 refers to a patient with dementia who was older than 85 years at death. Hippocampal sclerosis was absent on bilateral sampling of the hippocampus; however, limbic TDP-43 pathology was present. For this and similar cases, we recommend the term CARTS be applied because this TDP-43 pathology of aging is associated with cognitive impairment, but is not associated with either FTLD or AD pathologies.
The fourth and last question considers how TDP-43 pathology with or without concomitant HS should be reported in the setting of “high” AD neuropathologic change. Unlike in CARTS, TDP-43 pathology is currently held to be a secondary neuropathologic process to AD neuropathology in advanced pathologic stages,37,38 analogous to the α-synucleinopathy that often accompanies advanced AD. An observation supporting the link between AD neuropathology and TDP-43 pathology is that early-onset, genetically driven AD can be accompanied by TDP-43 pathology.39 Furthermore, TDP-43 pathology may contribute to a more aggressive course of AD even in the absence of morphologic HS,17,36,40 and a staging system has been put forth for TDP-43 pathology in this setting.37 Example 5 highlights a patient with clinical AD, “high” AD neuropathologic change at autopsy, HS, and TDP-43 pathology, including extrahippocampal TDP-43 pathology. The relationships between TDP-43, advanced AD pathology, hippocampal sclerosis, and clinical findings currently are areas of intense investigation.
EXAMPLE 1: CARTS WITH COMORBID PATHOLOGIES BUT WITHOUT HS
The patient, an 89-year-old man at the time of his death, had a medical history of atrial fibrillation, remote cerebrovascular accident, coronary artery disease, and carotid stenosis with carotid endarterectomy. The patient resided in a skilled nursing facility and was known to have dementia. No family history of dementia was provided and APOE status was unknown.
Autopsy identified severe atherosclerosis, coronary artery disease, and remote myocardial infarction. Brain autopsy revealed a fresh brain weight of 1450 g, and macroscopic and microscopic examination showed vascular brain injury in the form of bilateral, multiple acute and remote infarcts. Histologic examination showed preservation of pyramidal neurons in bilateral hippocampi. Sections of the amygdala demonstrate gliosis, scattered neurofibrillary tangles, and scattered ballooned neurons (Figure 1, A through F). Tau immunostaining demonstrated characteristic pathology of argyrophilic grains in the amygdala, entorhinal cortex, and hippocampus proper as well as scattered oligodendroglial “coiled” bodies in white matter. TDP-43 pathology (neuronal inclusions and dystrophic neurites) was observed within the amygdala but not in the hippocampi or elsewhere. No Aβ plaque deposition was identified and α-synuclein immunostaining revealed no pathologic inclusions.
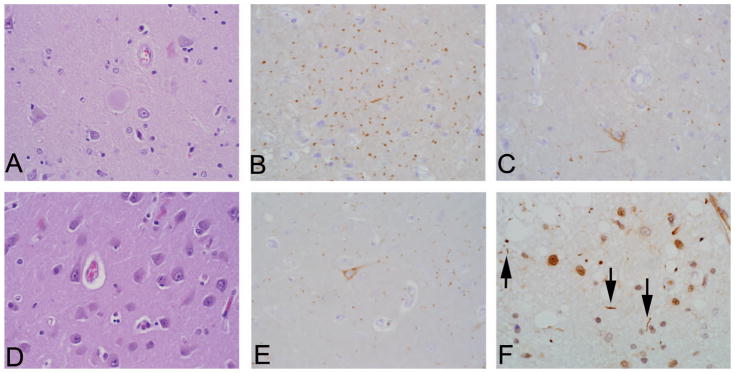
Figure 1.
Dementia with cerebral age-related TDP-43 with sclerosis (CARTS) without hippocampal sclerosis (HS) and with comorbid age-related pathologies (Example 1). Patient older than 85 years, with dementia, primary age-related tauopathy, arygyrophilic grain disease, and TAR DNA-binding protein 43 kDa (TDP-43) pathology without HS. Hematoxylin-eosin (H&E) staining of amygdala shows a balloon cell (center of photomicrograph) (A), and tau immunostaining shows innumerable “grains” (B) and superficial entorhinal cortex neurofibrillary tangle (NFT) pathology (C). An H&E-stained section through sector CA1 shows a normal complement of pyramidal neurons with rare Hirano bodies (eg, top left of image) (D), although grains and very mild NFT pathology are also evident in tau immunostaining (E). TDP-43 immunostaining of the corticomedial amygdala (F) shows a limited focus of pathology, shown here in the form of short, irregular neurites (indicated by the black arrows). Bilateral sampling of the hippocampi did not reveal any morphologic HS (H&E, original magnification ×400 [A and D]; tau, original magnification ×400 [B, C, and E]; TDP-43, original magnification ×600 [F]).
As illustrated in Figure 1, A through F, neuropathologic examination revealed 4 common pathologies of aging rather than AD neuropathology in this patient with dementia, namely: (1) a TDP-43 proteinopathy involving limbic structures without HS, that is, CARTS; (2) multifocal large-and small-vessel vascular brain injury; (3) Braak stage II–III without amyloid deposition, consistent with PART; and (4) the non-AD tauopathy of argyrophilic grains. Figure 2 provides a sample autopsy signout for Example 1.
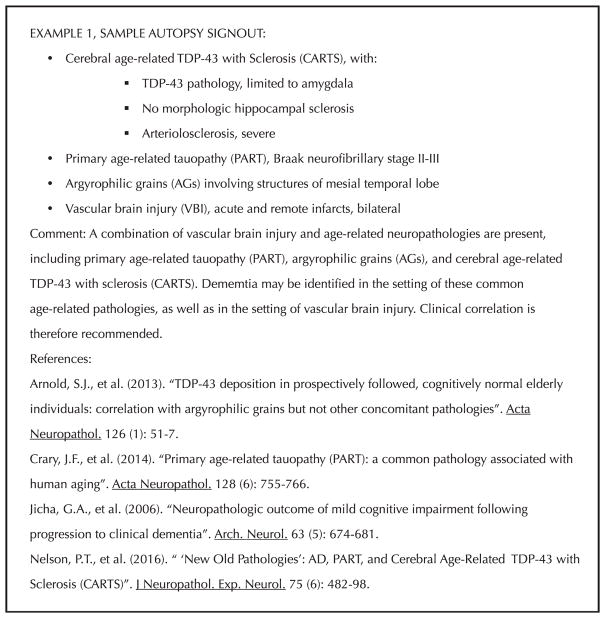
Figure 2.
Sample autopsy signout for Example 1, dementia with cerebral age-related TDP-43 with sclerosis (CARTS) without hip-pocampal sclerosis and with comorbid age-related pathologies. Abbreviation: TDP-43, TAR DNA-binding protein 43 kDa.
EXAMPLE 2: CARTS (HS + TDP-43) WITH COMORBID PATHOLOGY
The patient, 86 years old at the time of her death, had a medical history of hypertension and hyperthyroidism, and mild AD was diagnosed 7 years earlier. Initial symptoms included impairments in memory and spatial navigation. Two years before her death she developed parkinsonism and her condition progressed to clinically “moderate” AD with further disorientation, word-finding and speech difficulties. She was treated with donepezil and memantine. She had no hallucinations or fluctuations in alertness. Family history was significant for dementia in the patient’s father in the eighth decade of life and an older sibling (age of onset unknown).
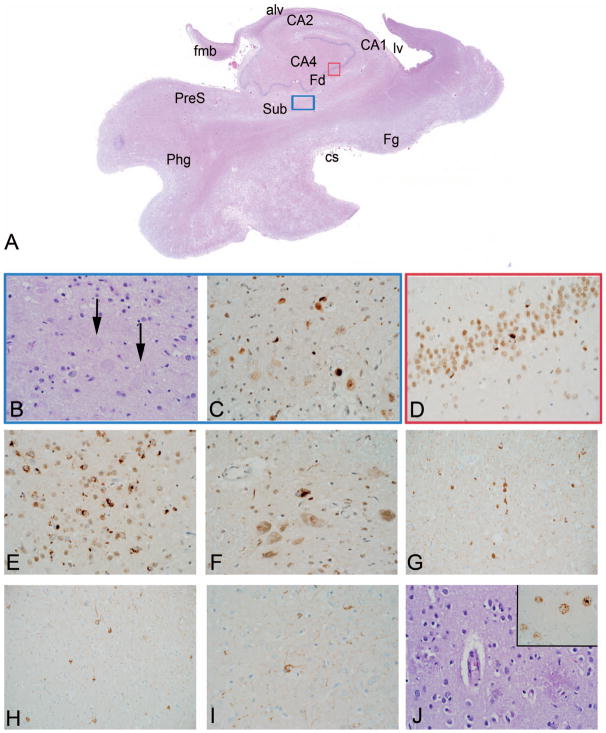
At autopsy, the fresh brain weight was 1120 g although cortical atrophy was not prominent. On gross examination, severe atherosclerosis was identified in large caliber vessels at the base of the brain and mild pallor was seen within substantia nigra and loci cerulei. Hippocampal atrophy was present bilaterally, and histologic examination revealed severe bilateral hippocampal sclerosis with loss of neurons in subiculum and hippocampal sector CA1 and associated reactive astrogliosis (Figure 3, A through J). Severe arteriosclerosis with thickening of vessel walls was identified in subcortical nuclei, including thalamus and basal ganglia. Scattered microinfarcts were present in cerebellum, lateral thalamus, and frontal cortex. Pallor was present in cerebral subcortical white matter.
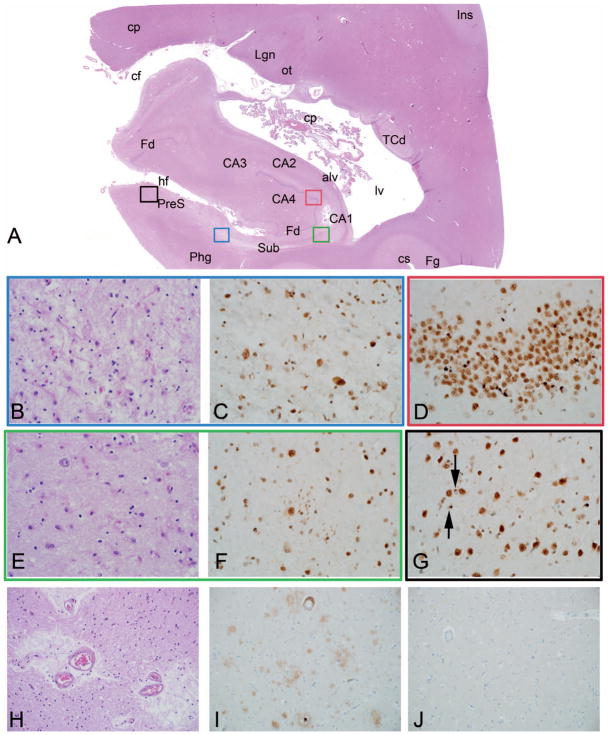
Figure 3.
Cerebral age-related TDP-43 with sclerosis (CARTS) with TAR DNA-binding protein 43 kDa (TDP-43) and hippocampal sclerosis (HS) and comorbid pathologies (Example 2). Patient older than 85 years with a clinical diagnosis of Alzheimer disease (see text for details). Scanned hematoxylin-eosin (H&E) slide of the mesial temporal lobe shows profound atrophy with neuronal loss and collapse in hippocampal sector CA1 (CA1) and subiculum (Sub) (A). Colored region-of-interest boxes in the scanned image correspond to areas shown in (B through G) below (subiculum = blue, presubiculum = black, CA1 = green, and dentate gyrus = red). (B, C) Within subiculum, there is nearly complete neuronal loss with rarefaction of neuropil and reactive astrogliosis in H&E staining, and TDP-43 shows fine neurites and neuronal cytoplasmic inclusions. (D) Small granule cells of dentate gyrus (Fd) show round cytoplasmic inclusions by TDP-43 immunostain. (E, F) Within sector CA1, the H&E image is similar to that of subiculum, and TDP-43 shows small, fine neurites in the area of neuronal loss. (G) TDP-43 immunostain shows neurites as well as very small cytoplasmic inclusions, possibly of small neurons or glial cells, in presubiculum (black arrows). Severe small vessel disease, which is characteristic of CARTS, is present in the thalamus with associated gliosis and perivascular rarefaction (H). Alzheimer disease neuropathologies are not significant; they include predominantly diffuse parenchymal plaques composed of amyloid β, or Aβ (I), and no tau pathology is seen in neocortex (J). Lewy body pathologies were also present (see text, not shown). Anatomic labels in this figure, as well as Figures 7 and 9, represent findings on microscopy of the section in tandem with terminology used in several references.42–47 Abbreviations: alv, alveus; CA1–CA4, hippocampal sectors CA1 to 4; cf, choroidal fissure; cp, cerebral peduncle and choroid plexus; cs, collateral sulcus; Fd, fascia dentata (dentate gyrus); Fg, fusiform gyrus; hf, hippocampal fissure; Ins, insula; Lgn, lateral geniculate nucleus; lv, lateral ventricle; ot, optic tract; Phg, parahippocampal gyrus; PreS, presubiculum; Sub, subiculum; TCd, tail of the caudate nucleus (H&E, original magnifications ×400 [B and E] and ×200 [H]); TDP-43, original magnification ×400 [C, D, F, and G]; β amyloid, original magnification ×200 [I]; tau, original magnification ×200 [J]).
Immunohistochemistry for TDP-43 demonstrated a severe TDP-43 proteinopathy in limbic regions, including neuronal inclusions in hippocampal dentate granule cells and within CA1 in the form of slender neurites. TDP-43 proteinopathy was also identified in superficial laminae of temporal and frontal isocortices and focally within brainstem (substantia nigra). α-Synuclein immunostaining indicated LBD within the brainstem, limbic structures of mesial temporal lobe, and basal forebrain (not shown), and the presence of only sparse Lewy bodies in the temporal neocortex. Diffuse Aβ immunoreactive plaques were present, including within the cerebellum (Thal phase 5), whereas neuritic plaque pathology was predominantly sparse. Cortical and leptomeningeal arterioles had mild cerebral amyloid angiopathy. Tau immunostaining demonstrated neurofibrillary pathology, largely restricted to limbic regions with mild hippocampal NFT pathology. Rare, superficial NFTs were present in the parastriate cortex of the occipital region (Braak NFT stage IV).
In summary, this example illustrates the co-occurrence of CARTS pathology with 2 other common dementia-related neurodegenerative pathologies—limbic LBD and “intermediate”-level AD pathologies—in the setting of the clinical diagnosis of AD. A key point of this example (illustrated in Figure 3, A through J), is that a fundamental substrate of the patient’s clinical “AD” was CARTS pathology, and not “high” AD neuropathologic change. Figure 4 provides a sample autopsy signout for Example 2.
Figure 4.
Sample autopsy signout for Example 2, cerebral age-related TDP-43 with sclerosis (CARTS) with hippocampal sclerosis and comorbid pathologies. Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; TDP-43, TAR DNA-binding protein 43 kDa.
EXAMPLE 3: DIFFUSE LEWY BODY DISEASE AND TDP-43-POSITIVE HS
The patient, 76 years old at the time of her death, initially had Parkinson disease without cognitive impairment 17 years before succumbing to her dementia. She had a very complex medical history significant for pernicious anemia, celiac disease with “gluten encephalopathy,” normal pressure hydrocephalus, and partial situs inversus abdominalis. Clinical features of AD developed 8 years before her death. During the course of her illness the patient developed a REM (rapid eye movement) sleep behavior disorder, severe cognitive impairment, peripheral neuropathies, visual hallucinations, and her condition progressed to a virtually nonverbal state. She was mute, bedridden, and unable to take food by mouth. Gluten encephalopathy was felt to be the driving force behind her clinical deterioration. Once a feeding gastrostomy tube was placed and strict gluten-free diet was used, she gained strength, began to verbalize, ambulate, and even returned to painting. However, within 4 years she again experienced cognitive decline. Enlarging ventricles were seen on brain MRI and she underwent ventriculoperitoneal shunting and a brain biopsy, which showed parenchymal amyloid deposition and no tau pathology. The patient was treated with rivastigmine transdermal patch, memantine, and levodopa and carbidopa, among other medications. APOE status was unknown and no family history of dementia was identified.
At autopsy, the fresh brain weight was 1030 g. Diffuse, mild cortical atrophy was present and internal examination was most notable for pallor of substantia nigra and loci cerulei. Bilaterally, hippocampi and adjacent limbic structures were atrophic. Microscopic examination, including α-synuclein immunostaining, demonstrated severe and diffuse LBD pathology, with characteristic involvement of brainstem nuclei, limbic structures, and basal forebrain, as well as temporal neocortex (Figure 5, A through F). In addition, profound hippocampal atrophy with focal, unilateral microscopic HS was identified. Characteristic, frequent TDP-43 immunoreactive lesions of HS were present in the subiculum, CA1, and dentate gyrus, as well as within adjacent limbic structures (amygdalae, entorhinal cortex) and in basal forebrain. No neocortical TDP-43 pathology was present. Aβ and tau immunostaining demonstrated only sparse neuritic plaques, a Braak NFT stage of II, and a Thal phase of 3, despite the 8-year clinical history of AD superimposed on the patient’s known LBD. This was consistent with the absence of tau pathology in neocortex with parenchymal amyloid deposition on antemortem brain biopsy (see above).
Figure 5.
Hippocampal sclerosis and TAR DNA-binding protein 43 kDa (TDP-43) with diffuse Lewy body disease (Parkinson disease dementia) (Example 3). Patient in the eighth decade of life with symptoms of Lewy body disease and a concomitant clinical diagnosis of Alzheimer disease. As in examples 1, 2, and 5, there is marked neuronal loss in sector CA1 on hematoxylin-eosin (H&E) staining (A), and as in example 2, TDP-43 immunostaining shows innumerable small neurites (B). TDP-43 pathology is also present within small neurons of ventromedial basal forebrain (black arrows) in the area of ventral striatum (C). In addition, conspicuous Lewy body pathology is present in H&E sections within dorsal nucleus of X in the medulla (D) and in cholinergic, magnocellular neurons of basal forebrain (E) (black arrows in [D and E] indicate Lewy bodies). Lewy bodies were conspicuous in H&E-stained sections of temporal cortex, and a single ×400 field stained with α-synuclein identified multiple cortical Lewy bodies and neurites (F) (H&E, original magnification ×400 [A, D, and E]; TDP-43, original magnification ×400 [B and C]; α-synuclein, original magnification × 400 [F]).
This example demonstrates the diagnostic challenge of HS and TDP-43 immunoreactive lesions, in the setting of patients with a well-characterized non-CARTS degenerative disease (Parkinson disease dementia in this example) and a complex medical history. In addition to the fact that the patient had a specific degenerative disease, the patient was younger than 85 years, currently considered as the threshold for the use of CARTS terminology. Another important feature about this example is the diagnosis of AD, approximately 12 years after diagnosis of Parkinson disease. Given the very limited AD neuropathology present at autopsy, it is likely that TDP-43–positive HS was a significant contributor to the additional AD-like amnestic symptoms. Figure 6 provides a sample autopsy signout that incorporates the multifaceted pathology and corresponds to the spectrum of clinical findings in Example 3.
Figure 6.
Sample autopsy signout for Example 3, hippocampal sclerosis and TAR DNA-binding protein 43 kDa (TDP-43) with diffuse Lewy body disease (Parkinson disease dementia). Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; NIA/AA, National Institute on Aging/Alzheimer’s Association.
EXAMPLE 4: HS AND TDP-43 IN RELATIVELY LATE-ONSET FTLD
The patient, 83 years old at the time of her death, developed dementia 8 years earlier with memory loss and word-finding difficulties at symptom onset. The clinical diagnosis at that time was probable AD. Memory and speech output difficulties intensified over time and during the last 2 to 3 years of life, her language skills deteriorated and the patient became mute. The patient did not carry an APOE ε4 allele but did have a strong family history of dementia with similar symptoms in multiple siblings.
At autopsy the fresh brain weight was 1020 g and the most notable finding on external examination was severe, circumscribed, and knife-edge atrophy of the anteromedial temporal lobes bilaterally, extending caudally to involve parahippocampal gyri, amygdalae, and hippocampi (Figure 7, A through J). Frontal atrophy was mild and anterior cingulate cortex was very thin. Subcortical nuclei appeared within normal limits and mild ventricular enlargement was present. Histologic examination demonstrated characteristic findings of bilateral HS. In addition, parahippocampal and temporal cortices, including cortex of the superior temporal gyrus, were very profoundly atrophic with “status spongiosus”—a characteristic finding of FTLD with nearly complete neuronal loss, cortical thinning, a reactive vascular pattern, and reactive astrogliosis. Moderate to severe arteriosclerotic changes were present in subcortical nuclei.
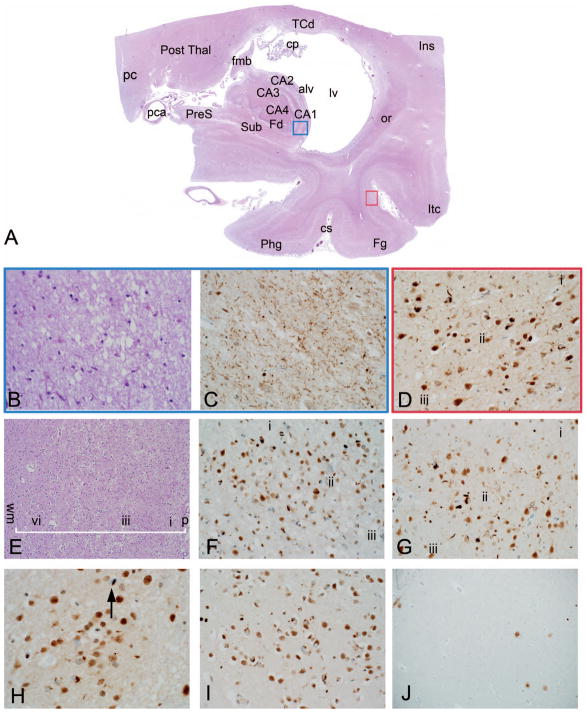
Figure 7.
Hippocampal sclerosis and TAR DNA-binding protein 43 kDa (TDP-43) in late-onset frontotemporal lobar degeneration (Example 4). A patient with clinical features of Alzheimer disease and semantic dementia progressing to mutism, as well as a strong family history of dementia (see text for details). A scanned hematoxylin-eosin (H&E) slide of the mesial temporal lobe shows profound atrophy in hippocampus, but also in parahippocampal, fusiform, and inferior temporal gyri, with marked dilatation of the lateral ventricle (A). Colored region-of-interest boxes on the scanned image correspond to areas shown in (B through D) (hippocampal sector CA1 =blue, and occipitotemporal (OT) cortex of fusiform gyrus = red). (B, C) Within CA1, on H&E staining (B) there is complete neuronal loss as in patient 1, and TDP-43 immunostain (C) shows innumerable fine neurites. (D) Within OT cortex, by TDP-43 immunostain, neuritic and neuronal inclusion pathology is present centered on cortical lamina ii (laminae i and iii also shown for orientation). (E) Low-power H&E section through temporal neocortex shows massive neuronal loss (“status spongiosus”). For orientation, a white batter indicates the full thickness of the cortex and labels indicate cortical layers i, iii, vi, as well as the pial surface (p) superficially and the subcortical white matter (wm) deep to cortex. (F, G) TDP-43 immunostaining shows dense neurite and neuronal pathology centered on cortical lamina ii in temporal (F) and anterior cingulate cortex (G). Within caudate nucleus (H), TDP-43 pathology is present including an intranuclear lentiform inclusion (black arrow); basal forebrain TDP-43 pathology was conspicuous (I). Amyloid β (Aβ pathology was very mild in comparison [see text for detail]) (J). Abbreviations: alv, alveus; CA1–CA4, hippocampal sectors CA1 to 4; cp, choroid plexus; cs, collateral sulcus; Fd, fascia dentata (dentate gyrus); Fg, fusiform gyrus; fmb, fimbria; Ins, insula; Itc, inferior temporal cortex; lv, lateral ventricle; or, optic radiations; pc, posterior commissure; pca, posterior cerebral artery; Phg, parahippocampal gyrus; Post Thal, posterior thalamus (pulvinar); PreS, presubiculum; Sub, subiculum; TCd, tail of the caudate nucleus (H&E, original magnifications ×400 [B] and ×100 [E]; TDP-43, original magnifications ×400 [C, D, F, G, I] and ×600 [H]; β amyloid, original magnification ×100 [J]).
TDP-43 immunostaining showed diffuse TDP-43 pathology in hippocampi, amygdalae, and adjacent allocortices. Temporal neocortex, as well as anterior cingultate cortex and subcortical nuclei, were severely affected by TDP-43 pathology. The pathologic pattern was most consistent with FTLD-TDP type A, characterized by innumerable, superficial isocortical neuronal inclusions and neurites in affected areas and intranuclear “lentiform” inclusions in both cortex and affected subcortical nuclei. The frontal cortex was mildly affected by TDP-43 pathology. “Intermediate” AD neuropathologies were identified by Aβ and tau immunostaining (Thal phase 3, Braak stage V, and CERAD “sparse” neuritic plaque pathology). No LBD pathology was present.
Although the histopathology of HS was evident in this patient, and it was indistinguishable in many respects from CARTS, several findings suggest a different diagnosis. These include the following: (1) the patient was 83 years old at the time of her death; (2) the patient had a clinical history of aphasia at the onset of symptoms, progressing to mutism; (3) the patient had unusually extensive TDP-43 pathology, as well as profound cortical atrophy in the temporal lobe; and (4) the patient had a strong family history of neurodegenerative disease. Further, arguing against an atypical presentation of AD, the extensive and severe amyloid pathologies typical of advanced AD neuropathology were not seen (Thal phase was 3, neuritic plaque pathology was sparse, and no cerebral amyloid angiopathy was present). Postmortem clinicopathologic correlation and further discussions between physicians and family members led to the consensus opinion that the course and symptoms were most indicative of a late-onset FTLD. Figure 8 provides a sample autopsy signout for Example 4.
Figure 8.
Sample autopsy signout for Example 4, hippocampal sclerosis and TAR DNA-binding protein 43 kDa (TDP-43) in late-onset frontotemporal lobar degeneration (FTLD). Abbreviations: AD, Alzheimer disease; CE-RAD, Consortium to Establish a Registry for Alzheimer’s Disease.
EXAMPLE 5: ADVANCED AD PATHOLOGY PLUS HS AND TDP-43
The patient, 83 years old at the time of her death, had a 20-year history of dementia with a clinical diagnosis of AD and carried the APOE ε4/ε4 genotype. Among other symptoms of AD, the patient developed difficulty in finding words 5 years before death.
At autopsy the fresh brain weight was 940 g, and diffuse cerebral atrophy was identified with the greatest extent of atrophy being in the temporal lobe. Internal examination revealed marked, bilateral atrophy of hippocampi, amygdalae, and surrounding cortices with dilatation of the temporal horn of the lateral ventricle and the entire ventricular system (Figure 9, A through J). Pallor of pigmented brainstem nuclei was present, atherosclerotic changes were severe, and a subacute infarct was identified in the distribution of the left middle cerebral artery. Histologic examination showed extensive neuronal loss in CA1 and subiculum with frequent extracellular “ghost” tangles (a finding absent in the examples previously described here), as well as frequent and obvious AD-type neuropathologies elsewhere in the hippocampus (senile plaques, NFTs in sector CA4) and neocortex. Status spongiosus and severe volume loss were present in the entorhinal and transentorhinal cortex, and some regions of entorhinal cortex were nearly devoid of residual, viable neurons.
Figure 9.
Hippocampal sclerosis and TAR DNA-binding protein 43 kDa (TDP-43) in advanced Alzheimer disease (AD) (Example 5). A patient with a very longstanding clinical diagnosis of AD before death and high-risk apolipoprotein E genotype (see text for details). Scanned hematoxylin-eosin (H&E) slide of the mesial temporal lobe shows hippocampal atrophy with marked neuronal loss in hippocampal sector CA1, subiculum, and also within parahippocampal and fusiform gyri (A). Colored region-of-interest boxes on the scanned image correspond to areas shown in (B through D) below (subiculum = blue, and dentate gyrus = red). (B) Hematoxylin-eosin staining shows massive neuronal loss within the subiculum, but extracellular “ghost” tangles are still evident (B, arrows); (C) TDP-43 immunostaining shows fine neurites and dense neuronal cytoplasmic inclusions. Round, intraneuronal inclusions of TDP-43 are also present in granule cells of dentate gyrus (D) and TDP-43 pathology is also present in basal forebrain (E) and substantia nigra (F), among other sites (see text). In contrast to Examples 2 and 3, tau pathology confirms abundant neurofibrillary tangle pathology, including within anterior cingulate cortex (G), temporal neocortex of middle temporal gyrus (H), and primary visual area (I). Amyloid plaque pathologies are obvious by H&E staining (J) and were confirmed by β amyloid immunostaining (J, inset). Abbreviations: alv, alveus; CA1–CA4, hippocampal sectors CA1 to 4; cs, collateral sulcus; Fd, fascia dentata (dentate gyrus); Fg, fusiform gyrus; fmb, fimbria; lv, lateral ventricle; Phg, parahippocampal gyrus; PreS, presubiculum; Sub, subiculum (H&E, original magnification ×400 [B and J]; TDP-43, original magnification ×400 [C through F]; tau, original magnifications ×200 [G and H] and ×400 [I]; β-amyloid, original magnification ×400 [inset J]).
Immunostaining revealed extensive and severe tau pathology, which was characteristic of Braak stage VI (eg, with additional involvement of primary visual cortex and dentate gyrus in addition to hippocampus proper and subiculum, cingulate and association cortices, periventricular thalamus, and basal forebrain). Cerebral amyloid angiopathy was extensive and seen within leptomeningeal and cortical arterioles of cortex and cerebellum, as well as within intraparenchymal vessels of the basal forebrain and thalamus. Within deep laminae of occipital cortex, capillaries had conspicuous amyloid deposition as well. Thal phase was 5 and cored/neuritic plaque pathology was moderate. No LBD pathology was present.
TDP-43 demonstrated innumerable neurites in hippocampus proper and subiculum and neuronal cytoplasmic inclusions in the dentate gyrus. Also involved were the amygdala, parahippocampal cortex, striatum, basal fore-brain, and focally, the temporal neocortex. Neuronal inclusions were also identified in the thalamus, subthalamic nucleus, and substantia nigra. In the example of this patient where the genetic findings, clinical course, and pathologic observations were indicative of AD, the TDP-43 pathology was deemed likely to be secondary to AD pathogenesis. Figure 10 provides a sample autopsy signout for this example of advanced AD with TDP-43 pathology and HS.
Figure 10.
Sample autopsy signout for Example 5, hippocampal sclerosis and TAR DNA-binding protein 43 kDa (TDP-43) in advanced Alzheimer disease (AD). Abbreviations: CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; NIA/AA, National Institute on Aging/Alzheimer’s Association.
CONCLUSIONS
Here we discussed and illustrated common examples related to HS pathology in the elderly, with a focus on the recently revised entity of CARTS. We emphasize that CARTS describes a common neuropathology that is a strong contributor to dementia in the older US population. Demographic trends, which show growth in the “oldest old” segment of the population, indicate that age-related neuropathologies such as CARTS will continue to increase in prevalence and importance as a public health problem.41
Several questions require further clarification in future clinicopathologic studies. A critical question is whether CARTS can be teased out clinically, during life, in patients with overlapping neuropathologies (as in Example 2), particularly with the use of biomarkers and/or neuropsychological testing. An additional question is how often CARTS occurs in patients 85 years old and younger. Clinical experience of one of the authors (P.E.S., written communication, August 29, 2016) suggests there are patients younger than 85 years with MRI-confirmed HS and without clinical progression. Neuropathologically, the extent to which these cases can be distinguished from FTLD with HS remains another important question.
In advanced old age, the substrates of cognitive impairment are often multifactorial and include the entities illustrated in this article, including CARTS, AD, PART, LBD, and vascular brain injury. Many of these examples are clinically complex. Correspondingly, multiple neuropathologies are revealed at autopsy and “single” pathologic diagnoses are relatively rare. As such, it is incumbent upon physicians practicing in this area to be familiar with the common neuropathologic substrates of cognitive impairment in the older population.
Acknowledgments
We appreciate the collaboration with Lisa Lipscomb, RN, and Rachelle Doody, MD, PhD, of the Alzheimer’s Disease and Memory Disorders Center at Baylor College of Medicine, Houston, Texas. We are grateful to Kathryn Stockbauer, PhD, and Helen Chifotides, PhD, both of the Department of Pathology and Genomic Medicine at Houston Methodist Hospital, for their careful reading of the manuscript and edits. This work was supported in part by grants to Dr Cykowski from the Houston Methodist Specialty Physician Group and a Clinician-Scientist Research Award from the Houston Methodist Research Institute.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States. Washington, DC: US Department of Commerce, Economic and Statistics Administration, US Census Bureau; 2014. [Accessed November 27, 2016]. https://www.census.gov/prod/2014pubs/p25-1140.pdf. [Google Scholar]
- 2.Nelson PT, Trojanowski JQ, Abner EL, et al. “New old pathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS) J Neuropathol Exp Neurol. 2016;75(6):482–498. doi: 10.1093/jnen/nlw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16(4):203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136(pt 9):2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jellinger KA, Alafuzoff I, Attems J, et al. PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol. 2015;129(5):757–762. doi: 10.1007/s00401-015-1407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson PT, Smith CD, Abner EL, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol. 2013;126(2):161–177. doi: 10.1007/s00401-013-1154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson DW, Davies P, Bevona C, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88(3):212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 11.Pao WC, Dickson DW, Crook JE, Finch NA, Rademakers R, Graff-Radford NR. Hippocampal sclerosis in the elderly: genetic and pathologic findings, some mimicking Alzheimer disease clinically. Alzheimer Dis Assoc Disord. 2011;25(4):364–368. doi: 10.1097/WAD.0b013e31820f8f50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray ME, Cannon A, Graff-Radford NR, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014;128(3):411–421. doi: 10.1007/s00401-014-1302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson PT, Schmitt FA, Lin YS, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134:1506–1518. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson PT, Estus S, Abner EL, et al. ABCC9 gene polymorphism is associated with hippocampal sclerosis of aging pathology. Acta Neuropathol. 2014;127(6):825–843. doi: 10.1007/s00401-014-1282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One. 2011;6(10):e26286. doi: 10.1371/journal.pone.0026286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenowitz WD, Monsell SE, Schmitt FA, Kukull WA, Nelson PT. Hippocampal sclerosis of aging is a key Alzheimer’s disease mimic: clinical-pathologic correlations and comparisons with both Alzheimer’s disease and non-tauopathic frontotemporal lobar degeneration. J Alzheimers Dis. 2014;39(3):691–702. doi: 10.3233/JAD-131880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015;77(6):942–952. doi: 10.1002/ana.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol. 2007;113(3):245–252. doi: 10.1007/s00401-006-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickson DW, Baker M, Rademakers R. Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neurodegener Dis. 2010;7(1–3):170–174. doi: 10.1159/000289231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beecham GW, Hamilton K, Naj AC, et al. Genome-wide association meta-analysis of neuropathologic features of Alzheimer’s disease and related dementias. PLoS Genet. 2014;10(9):e1004606. doi: 10.1371/journal.pgen.1004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YJ, Xu YF, Cook C, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A. 2009;106(18):7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters OM, Ghasemi M, Brown RH., Jr Emerging mechanisms of molecular pathology in ALS. J Clin Invest. 2015;125(6):2548. doi: 10.1172/JCI82693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 24.Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9(10):995–1007. doi: 10.1016/S1474-4422(10)70195-2. [DOI] [PubMed] [Google Scholar]
- 25.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61(5):435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cykowski MD, Takei H, Van Eldik LJ, et al. Hippocampal sclerosis but not normal aging or Alzheimer disease is associated with TDP-43 pathology in the basal forebrain of aged persons. J Neuropathol Exp Neurol. 2016;75(5):397–407. doi: 10.1093/jnen/nlw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neltner JH, Abner EL, Baker S, et al. Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain. 2014;137:255–267. doi: 10.1093/brain/awt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neltner JH, Abner EL, Jicha GA, et al. Brain pathologies in extreme old age. Neurobiol Aging. 2016;37:1–11. doi: 10.1016/j.neurobiolaging.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee EB, Lee VM, Trojanowski JQ, Neumann M. TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system. Acta Neuropathol. 2008;115(3):305–311. doi: 10.1007/s00401-007-0331-5. [DOI] [PubMed] [Google Scholar]
- 30.Nelson PT, Head E, Schmitt FA, et al. Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;121(5):571–587. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coyle-Gilchrist IT, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knopman DS, Roberts RO. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci. 2011;45(3):330–335. doi: 10.1007/s12031-011-9538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baborie A, Griffiths TD, Jaros E, et al. Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol. 2011;121(3):365–371. doi: 10.1007/s00401-010-0765-z. [DOI] [PubMed] [Google Scholar]
- 34.Aoki N, Murray ME, Ogaki K, et al. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP type A. Acta Neuropathol. 2015;129(1):53–64. doi: 10.1007/s00401-014-1358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ighodaro ET, Jicha GA, Schmitt FA, et al. Hippocampal sclerosis of aging can be segmental: two cases and review of the literature. J Neuropathol Exp Neurol. 2015;74(7):642–652. doi: 10.1097/NEN.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20(1):66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Josephs KA, Murray ME, Whitwell JL, et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol. 2014;127(3):441–450. doi: 10.1007/s00401-013-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014;127(6):811–824. doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson YS, Raby S, Foulds PG, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011;122(6):703–713. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 40.Josephs KA, Whitwell JL, Tosakulwong N, et al. TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol. 2015;78(5):697–709. doi: 10.1002/ana.24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Werner CA. The Older Population: 2010. Washington, DC: United States Census Bureau; Nov, 2011. [Accessed November 27, 2016]. http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. [Google Scholar]
- 42.Braak H. Architectonics of the Human Telencephalic Cortex. Berlin, New York: Springer-Verlag; 1980. [Google Scholar]
- 43.Mai JK, Paxinos G. The Human Nervous System. 3. Amsterdam, Boston: Elsevier Academic Press; 2012. [Google Scholar]
- 44.Carpenter MB. Core Text of Neuroanatomy. 3. Baltimore: Williams & Wilkins; 1985. [Google Scholar]
- 45.DeArmond SJ, Fusco MM, Dewey MM. Structure of the Human Brain: A Photographic Atlas. 3. New York: Oxford University Press; 1989. [Google Scholar]
- 46.Economo C, Triarhou LC. Cellular Structure of the Human Cerebral Cortex. Basel, New York: Karger; 2009. [Google Scholar]
- 47.Crosby EC, Humphrey T, Lauer EW. Correlative Anatomy of the Nervous System. New York: MacMillan; 1962. [Google Scholar]