Key Points
APRIL is a compact, self-protein that binds 2 MM antigens (BCMA and TACI) with high affinity; we present an APRIL-based CAR.
Dual-antigen targeting increases the availability of tumor-binding sites and reduces the risk of antigen-negative disease escape.
Abstract
B-cell maturation antigen (BCMA) is a promising therapeutic target for multiple myeloma (MM), but expression is variable, and early reports of BCMA targeting chimeric antigen receptors (CARs) suggest antigen downregulation at relapse. Dual-antigen targeting increases targetable tumor antigens and reduces the risk of antigen-negative disease escape. “A proliferation-inducing ligand” (APRIL) is a natural high-affinity ligand for BCMA and transmembrane activator and calcium-modulator and cyclophilin ligand (TACI). We quantified surface tumor expression of BCMA and TACI on primary MM cells (n = 50). All cases tested expressed BCMA, and 39 (78%) of them also expressed TACI. We engineered a third-generation APRIL-based CAR (ACAR), which killed targets expressing either BCMA or TACI (P < .01 and P < .05, respectively, cf. control, effector-to-target [E:T] ratio 16:1). We confirmed cytolysis at antigen levels similar to those on primary MM, at low E:T ratios (56.2% ± 3.9% killing of MM.1s at 48 h, E:T ratio 1:32; P < .01) and of primary MM cells (72.9% ± 12.2% killing at 3 days, E:T ratio 1:1; P < .05, n = 5). Demonstrating tumor control in the absence of BCMA, we maintained cytolysis of primary tumor expressing both BCMA and TACI in the presence of a BCMA-targeting antibody. Furthermore, using an intramedullary myeloma model, ACAR T cells caused regression of an established tumor within 2 days. Finally, in an in vivo model of tumor escape, there was complete ACAR-mediated tumor clearance of BCMA+TACI− and BCMA−TACI+ cells, and a single-chain variable fragment CAR targeting BCMA alone resulted in outgrowth of a BCMA-negative tumor. These results support the clinical potential of this approach.
Introduction
Multiple myeloma (MM) is a cancer of plasma cells (PC) that is responsible for 2% of cancer deaths.1 Myeloma remains largely incurable, despite significant progress seen with the inclusion of proteasome inhibitors and immunomodulatory drugs into the mainstay of treatment regimens.2 Furthermore, current therapeutic strategies fail to benefit approximately 15% of patients who have primary refractory disease, adverse genetics, or both.3 There remains a need for new myeloma therapies with different mechanisms of action, particularly those that can induce durable remissions.
Chimeric antigen receptors (CAR) typically graft the specificity of a monoclonal antibody (mAb) onto a T cell, redirecting T-cell cytotoxicity to tumor by a mechanism unimpeded by major histocompatibility complex class restriction.4 CAR T cells may have advantages over mAb-based approaches because CAR T cells can actively migrate to sites of disease and persist, thus engendering a sustained rejection of target cells. CD19-directed CAR T-cell therapy has been effective against refractory B-cell malignancies, and sustained responses are seen in the face of chemotherapy-resistant disease.5-9 Applying CAR T-cell therapy to MM, however, faces several challenges, not least of which is target antigen selection. CD19 is expressed in only a small proportion of tumor cells,10 and well-characterized antigens expressed by myeloma such as CD38,11,12 CD56,13,14 and CD13815 may not be suitable targets because of expression outside the lymphoid compartment.
B-cell maturation antigen (BCMA) is a member of the tumor necrosis factor (TNF) receptor superfamily, is upregulated at the terminal stages of B-cell maturation, and is selectively expressed on PC.16,17 BCMA is absent on haemopoietic stem cells16-18 and is expressed by nearly all cases of MM, albeit at variable, and often low, density.16 Consequently, BCMA has been targeted by several immunotherapeutic strategies in MM, including CAR approaches and bispecific T-cell engager therapies.17,19-23 In the first reported clinical trial investigating a BCMA targeting CAR, rapid and dose-dependent disease response was seen in 4 of 12 patients despite substantial tumor load and heavy pretreatment.24 However, relatively high T-cell doses were needed to achieve durable remissions, and possibly akin to CD19 downregulation in CD19 CAR T-cell studies,25 loss of BCMA expression at relapse was reported.24
Thus, although BCMA is a promising target, the challenges of low-target density and target escape may compromise clinical efficacy. To address this, we hypothesized that dual-antigen binding would increase the level of targetable antigen on tumor cells, while potentially reducing the incidence of antigen-negative escape, in this way enhancing therapeutic potential and capacity for long-term disease control. The transmembrane activator and calcium-modulator and cyclophilin ligand (TACI) is also a TNF receptor and is involved in maturation of B cells, including their maturation to PC.26,27 Importantly, TACI is also expressed on MM cells.18,28,29 A proliferation-inducing ligand (APRIL) is a natural ligand of both BCMA and TACI and is an attractive antigen binder because it is a compact, oligomerizing, single-domain self-protein that binds both MM antigens with high, nanomolar affinity.30,31
In this work, we describe a novel CAR construct using a truncated form of APRIL as the tumor-targeting domain (APRIL-based chimeric antigen receptors; ACAR), which recognizes both BCMA and TACI on MM cells. We establish ACAR potency at antigen levels seen in clinical samples, at low effector-to-target ratios (E:T), against primary cells, as well as in murine models of myeloma and tumor escape.
Method
BCMA/TACI quantification
Mononuclear cells (MNCs) were stained with CD138 allophycocyanin (APC) (clone MI15) to identify tumor and murine immunoglobulin G2a (IgG2a) phycoerythrin (PE) isotype control, rat IgG2a PE isotype control, anti-BCMA PE (clone 19F2), or anti-TACI PE (clone 1A1) (all antibodies from BioLegend). BD Fortessa was used for cell acquisition, and data were analyzed using FlowJo_V10 (Treestar). Antibodies bound per cell (ABC) was calculated using BD QuantiBRITE beads and subtracting ABC of isotype control (>100 ABC was considered positive).
Cloning
All plasmids were cloned in-house32 into the oncoretroviral vector SFG,33 and RD114-pseudotyped supernatant was produced as has been previously described.32 Sequence coding for residues 116 to 250 of the canonical sequence for APRIL (Uniprot 075888) was cloned between signal peptide from IgG κ chain V-III to CAR scaffolds comprising IgG1 hinge spacer, CD8 α spacer, or IgG1 Fc domain34 coexpressed with RQR8 using an in-frame foot-and-mouth–like 2A peptide, TaV.35 Epidermal growth factor receptor vIII (EGFRvIII) and BCMA targeting CARs were engineered using MR1-136 or 11-D-5-317 single-chain variable fragments (scFvs), respectively, a CD8 spacer, and a CD28-OX40-CD3ζ endodomain.
CAR T cells
Peripheral blood mononuclear cells (PBMC) obtained by density gradient centrifugation (Ficoll Paque, GE Lifesciences) were stimulated with CD3 and CD28 antibodies (0.5 µg/mL; Miltenyl) and interleukin-2 (100 IU/mL; Genescript) then transduced as before32 to obtain CAR T cells. Transduction efficiency was assessed by fluorescence-activated cell sorter (FACS) of cells stained for RQR8 (Qbend10 antibody; R&D) or APRIL (anti-APRIL biotin) and RQR8 for ACAR T cells.
Further methods are available in the supplemental Data, available on the Blood Web site.
Results
Primary myeloma cells express BCMA and TACI
We have previously reported the variable surface expression of BCMA on tumor.16 Here, we sought to quantify expression levels of both BCMA and TACI on the cell surface of primary bone marrow (BM)–derived MM cells.
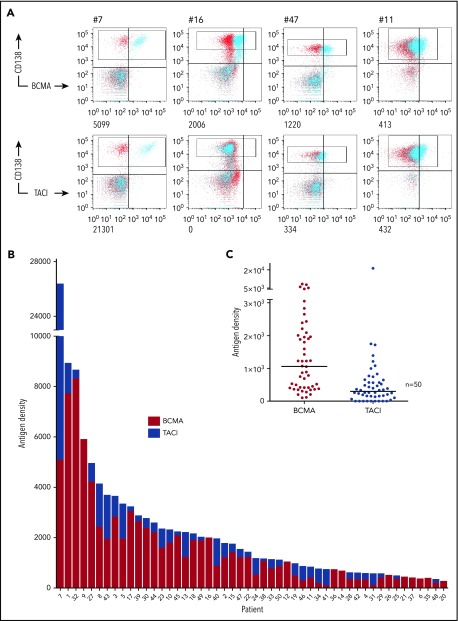
Ficolled BM MNCs from 50 patients were stained for CD138 to identify tumor and anti-BCMA or TACI (Figure 1A; supplemental Table 1, supplemental Figure 1A) by using QuantiBRITE beads for antigen quantification. We found expression of BCMA on CD138+ tumor cells from all patients tested (median: 1061; range: 105-8323 ABC) (Figure 1B). TACI was coexpressed on the tumor (supplemental Figure 1B) and detected on MM cells from 39 of these patients, at generally lower levels (median: 333; range: 0-21 301 ABC) (Figure 1C). Thus, we calculated that concurrent targeting of both antigens in comparison with BCMA alone would increase levels of target antigen in 78% of patients and result in an increased mean combined targetable antigen density on the tumor of 2458 ABC in comparison with 1623. TACI expression also exceeded BCMA in a subset of samples (16%) (supplemental Figure 1C). Notably, 7 of these 8 patients expressed less than the median level of BCMA, suggesting that concurrent TACI targeting may be particularly beneficial in a proportion of BCMAlo tumors.
Figure 1.
BCMA and TACI expression on primary myeloma cells. (A) Fresh BM MNCs were stained with CD138 APC and 1 of BCMA PE, TACI PE (blue) or isotype control (red). Antigen densities of BCMA and TACI on CD138+ tumor cells (gated) were then quantified using QuantiBRITE beads and subtracting ABC of isotype control. FACS plots from 4 representative patient samples with antigen densities (ABC) are shown. (B) Stacked plot of BCMA and TACI expression on CD138+ cells. Each bar represents a separate myeloma patient. (C) Distribution of BCMA and TACI expression on primary CD138+ myeloma cells (n = 50; medians shown. BCMA range: 105-8323, mean: 1623; TACI range: 0-21 301, mean: 853).
In keeping with our previous findings,16 patients with a new diagnosis of myeloma (54%) expressed lower levels of BCMA (P < .05) than did those with relapsed disease (46%), but there was no such correlation with TACI expression (P = .3; supplemental Figure 1D). Of the 42 (84%) patients for whom fluorescence in situ hybridization was available, the 25 (60%) patients with high-risk cytogenetic lesions had higher levels of BCMA (P < .05 by Mann-Whitney) but a trend to lower levels of TACI (P = .06) (supplemental Figure 1E).
Thus we confirm the surface tumor expression of BCMA on tumors from all patients tested and the coexpression of BCMA and TACI in the majority (78%) of patients, supporting a therapeutic strategy for myeloma that targets both of these antigens.
Similar expression pattern of BCMA and TACI in normal tissues
The selectivity of BCMA expression to lymphoid cells17 and more specifically PC has been previously described.16 TACI is also a known lymphoid antigen expressed mainly on B cells, but at an earlier stage of maturation and particularly in maturing subsets of splenic B cells.37 Because expression of TACI on normal tissues is less well known, we performed reverse transcription-polymerase chain reaction (qRT-PCR) of BCMA and TACI in a range of normal tissues.
Transcript analysis from 72 normal tissues, each from 3 donors, revealed the highest levels of BCMA and TACI expression in lymphoid tissues. Notable expression levels were also seen in gastrointestinal and bronchial tissues, which likely reflects the presence of lymphocytes at these anatomical sites. BCMA, but not TACI, expression was also noted in testes and gall bladder (supplemental Figure 2, supplemental Table 2). BCMA and TACI transcripts were equally high in the splenic parenchyma, but it is noteworthy that in other tissues, BCMA gene expression was up to 10-fold higher than was TACI.
These data are consistent with TACI expression being restricted to the lymphoid compartment, with distribution broadly similar to that of BCMA.
Optimization of APRIL-based CAR constructs
APRIL is a soluble ligand that binds BCMA and TACI. Additionally, the amino terminus of APRIL binds proteoglycans 38,39 but is not involved in the interaction with BCMA or TACI. To confirm that a truncated form of APRIL could bind BCMA and TACI when expressed on a cell surface, we fused truncated APRIL to the CD8 transmembrane domain and expressed this on SUPT1 cells. Staining with recombinant soluble BCMA and TACI confirmed that truncated APRIL is both stably expressed and maintains BCMA and TACI binding when membrane bound (supplemental Figure 3A). Furthermore, surface plasmon resonance analysis of soluble, truncated APRIL binding to TACI and BCMA confirmed previously described binding kinetics (supplemental Figure 3B).40
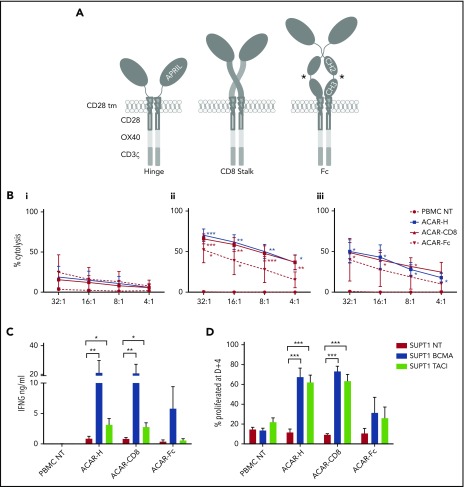
Next, 3 ACARs were constructed, consisting of truncated APRIL fused to a spacer domain, a CD28 transmembrane, and tripartite endodomain (CD28-OX40-CD3ζ).41 Spacers were either the hinge of human IgG1 (ACAR-H), the stalk of human CD8α (ACAR-CD8), or the hinge, CH2, and CH3 domains of human IgG1 modified to reduce Fc receptor binding42 (ACAR-Fc) (Figure 2A).
Figure 2.
Optimization of APRIL-based chimeric antigen receptors. (A) Three third-generation ACARs were constructed, consisting of a truncated APRIL molecule, fused to a tripartite endodomain (CD28-OX40-CD3ζ) via 1 of 3 spacers: the hinge of IgG1 (ACAR-H), the stalk of human CD8α (ACAR-CD8), or modified Fc (ACAR-Fc). *FcR mutations as per Hombach.42 PBMCs were activated by CD3/CD28/interleukin-2, transduced with ACAR constructs using RD114-pseodotyped retrovirus, and CD56 depleted before testing against SUPT1 cells expressing high levels of BCMA (8 × 104 ABC) or TACI (16.2 × 105 ABC). (B) Target cell death as determined by 4-hour 51Cr release assay with SUPT1NT (i), SUPT1BCMA (ii), or SUPT1TACI (iii) on coculture with PBMCs transduced with ACAR-H (n = 5), ACAR-CD8 (n = 6), and ACAR-Fc (n = 3). Significance values indicated are compared with cytolysis with PBMC NT by paired t test. (C) ACAR-transduced T cells were also cocultured (1:1) with SUPT1 targets and IFNG release at D+1 measured by enzyme-linked immunosorbent assay (ELISA) (same number of experiments as before). *P < .05, **P < .01. (D) ACAR- transduced PBMC from further donors were labeled with Cell Trace Violet prior to coculture with SUPT1 targets (1:1) and FACS at D+4. Percentage of ACAR-positive cells proliferated with antigen-expressing targets was then defined in relation to coculture with SUPT1NT control (n = 6 for ACAR-H and ACAR-CD8, n = 4 for ACAR-Fc). Mean ± SEM indicated by paired t test. ***P < .001.
PBMCs from normal donors were activated with IL-2, anti-CD28, and CD3 antibodies, retrovirally transduced with ACAR constructs, CD56 depleted, and tested against SUPT1 cells modified to express high levels of BCMA, TACI, or nontransduced (NT) targets. Using 4-hour 51Cr release assay, T cells transduced with ACAR-H (n = 5), and ACAR-CD8 (n = 6) spacer variants caused cytolysis of SUPT1BCMA (P < .01 for both ACAR constructs in comparison with PBMC NT at an E:T ratio of 16:1, paired t test) and SUPT1TACI (P < .05 for both ACAR constructs) targets. In comparison, ACAR-Fc–transduced T cells killed SUPT1BCMA targets (n = 3, P < .05) but not TACI-expressing targets (Figure 2B).
After coculture with antigen-expressing target cells (1:1 with irradiated, SUPT1 cells) for 24 hours, interferon γ (IFNG) release from ACAR-H (n = 5) and ACAR-CD8 (n = 6) T cells was detected. There was significant cytokine release observed on coculture of both of these ACAR constructs with SUPT1BCMA (P < .01 for both) and SUPT1TACI (P < .05 for both) in comparison with control targets. In comparison, ACAR-Fc did not result in cytokine release against TACI- or BCMA-expressing SUPT1 cells (n = 3) (Figure 2C).
To assess proliferation of ACAR T cells, we stained effector T cells with Cell Trace Violet prior to 1:1 coculture with SUPT1 targets and analyzed them by FACS at 4 days. In comparison with control cocultures with SUPT1NT targets, there was a significant increase in the percentage of proliferated ACAR-H–transduced and ACAR-CD8–transduced T cells with SUPT1BCMA and SUPT1TACI (P < .001 for both effectors with BCMA- and TACI-expressing targets) (Figure 2D; supplemental Figure 4).
Taken together, these data indicate that both ACAR-CD8 and ACAR-H demonstrated greater in vitro activity, compared with ACAR-Fc–transduced T cells. Both spacer variants resulted in target cytolysis, cytokine release, and effector proliferation in response to SUPT1BCMA or SUPT1TACI.
APRIL CAR causes target cytolysis at low antigen densities, at a low E:T ratio, and in the presence of soluble APRIL, BCMA, and TACI
Clinical responses will likely require ACAR activity against the low levels of BCMA and TACI found on some primary MM cells, and at low E:T ratios. We thus explored the in vitro cytolytic potential of the 2 most promising ACAR constructs (ACAR-H and ACAR-CD8) under these conditions.
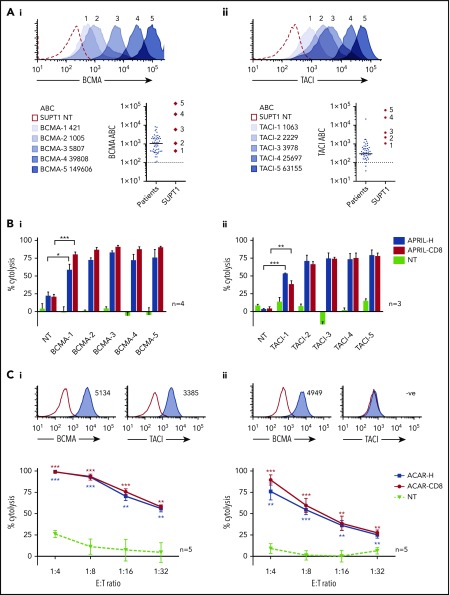
ACAR-transduced PBMCs were tested against SUPT1 targets expressing a wide range of surface BCMA (421 to 1.5 × 105 ABC) and TACI (1063 to 6.3 × 104 ABC) (Figure 3A). By 51Cr release, T cells transduced with either ACAR construct caused significant cytolysis of all BCMA- and TACI-expressing targets in comparison with control at all E:T ratios tested (32:1 to 4:1, 16:1, shown in supplemental Figure 5A). In an attempt to more closely replicate physiological conditions, cocultures were then extended to 48 hours and the E:T ratio lowered to 1:10 and target kill assessed by FACS. In these conditions, T cells transduced with ACAR-H and ACAR-CD8 both caused significant target cytolysis of unirradiated targets expressing even the lowest levels of BCMA and TACI (Figure 3B).
Figure 3.
ACAR-mediated cytolysis seen at low target density and low E:T ratios. (A) SUPT1 targets were engineered to express a wide range of BCMA (i) and TACI (ii). Antigen densities of targets are indicated. Dot plots depict receptor levels found on primary MM tumor cells from 50 patients (population median is indicated, and dashed line represents threshold for positive expression) in comparison with engineered SUPT1 targets. (B) These SUPT1BCMA (i) and SUPT1TACI (ii) targets were then cocultured with ACAR-CD8 and H-spacer variants at a low E:T ratio (1:10), and target death determined at 48 hours by FACS and expressed as percentage cytolysis in comparison with media control. (C) Specific cytolysis at 48 hours of human myeloma cell lines MM.1s (i) and U266 (ii) when cocultured with ACAR-transduced T cells at reducing E:T ratios. Inset histograms show BCMA and TACI expression by FACS (blue shaded curves) in comparison with staining with isotype control (open curves). Mean ± SEM of number of experiments are indicated. *P < .05, **P < .01, ***P < .001 by t test, in comparison with PBMC NT.
ACAR-mediated cytolysis of MM cells was confirmed in a number of human myeloma cell lines (HMCLs; supplemental Figure 5B). ACAR activity was also demonstrated at lower E:T ratios against MM.1s and U266 HMCL with significant target cytolysis down to an E:T ratio of 1:32 on coculture with T cells transduced with both ACAR constructs (Figure 3C). T cells transduced with a BCMA-targeting CAR (BCMA CAR) based on the 11-D-5-317,24 scFv were also compared with the ACAR, and despite low E:T ratios, there was no statistically significant difference in kill of MM.1s or U266 by the BCMA CAR and ACAR (supplemental Figure 5C).
Members of the TNF receptor superfamily found in the sera of MM patients may interfere with an APRIL-based therapeutic strategy by blockade or inadvertent T-cell activation. We therefore quantified APRIL, BCMA, and TACI in MM BM (supplemental Figure 6A), repeated cytotoxicity assays with ACAR-H against MM.1s at low E:T ratios (supplemental Figure 6B), and measured IFNG release (supplemental Figure 6C) in the presence of physiological levels of these proteins. There was no significant cytokine release, and ACAR-mediated target cytolysis was unaffected by soluble APRIL and soluble TACI but was reduced at the highest levels of soluble BCMA tested (P < .001 at 1000 ng/mL in comparison with media control).
Therefore in vitro, T cells transduced with both ACAR-H and ACAR-CD8 demonstrate equivalent cytolytic activity, and we consistently observed significant cytolysis of the lowest BCMA and TACI expressers even at low E:T ratios. Furthermore, ACAR killing was equivalent to that demonstrated by a scFv BCMA targeting CAR when used against BCMA-expressing targets. We also observed that ACAR T cells are not activated by soluble ligand, and though tumor kill was also unaffected by physiological levels of soluble APRIL or TACI, attenuation of target kill was seen at the highest levels of sBCMA.
APRIL CAR causes cytolysis of primary myeloma cells
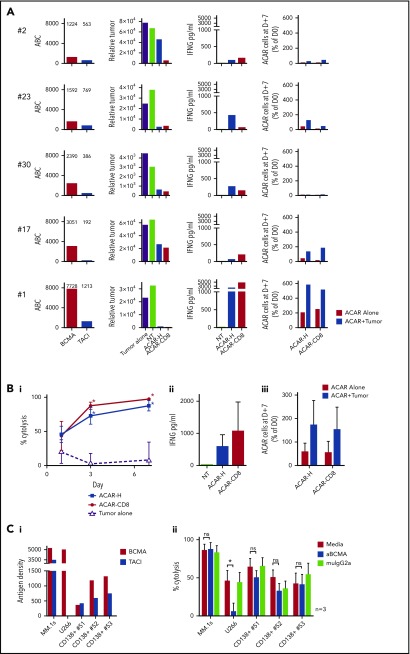
To test ACAR activity on primary tumor cells, allogeneic PBMCs transduced with ACAR-H and ACAR-CD8 variants were CD56 depleted, then cocultured 1:1 with CD138-selected BM derived MM cells from 5 patients. Although BCMA and TACI expression varied between patient samples (BCMA 1224-7728 and TACI 563-1213 ABC; Figure 4A), tumor cytolysis and IFNG release were seen with both ACAR constructs in all samples. Survival and proliferation of ACAR T cells was seen with 3 patient samples (patients 23, 17, and 1 in Figure 4A).
Figure 4.
ACAR causes cytolysis of primary myeloma cells in vitro. (A) CD138-selected BM-derived primary myeloma cells from 5 patients were cultured in media alone (labeled “Tumor alone”), with allogeneic NT T cells or T cells transduced to express ACAR-H.2A.RQR8 or ACAR-CD8.2A.RQR8. Patients are identified by the numbers allocated in Figure 1B and supplemental Table 1. Tumor antigen densities of BCMA and TACI are indicated (ABC). Relative number of viable tumor cells at D+3 are shown. Cytokine release was determined at D+1 by ELISA, and T-cell numbers after 7 days of coculture with or without tumor cells were determined by staining for RQR8 transgene by using FACS. (B) Summarized tumor kill (percentage cytolysis determined in relation to viable tumor cells on coculture with NT T cells) (i), cytokine release (ii), and T-cell expansion (iii). (C) ACAR-H– transduced PBMCs from 3 donors were cocultured 1:4 with MM.1s, U266, or CD138-selected primary BM-derived tumor cells from 3 further patients. Effectors and targets were cultured in media alone, 150 µg/mL of anti-BCMA antibody (S307118G03), or the equivalent concentration of IgG2a control: BCMA and TACI expression on targets (i) and target kill at 48 hours by FACS (ii). Mean ± SEM, *P < .05 in comparison with control by paired t test. ns, not significant.
Combining the results from the 5 patient samples, at D+3, ACAR-H and ACAR-CD8 resulted in 72.9% ± 12.2% and 87.7% ± 5.4% tumor deaths, respectively (mean ± SEM cytolysis in relation to control). In comparison, baseline tumor cell death was 2.8% ± 15.3% (P < .05 for both ACAR constructs by paired t test). There was no significant difference in target kill, cytokine release, or T-cell expansion between the 2 ACAR spacer variants (Figure 4B).
We tested the ability of ACAR constructs to induce cytolysis by TACI alone by conducting cytotoxicity assays in the presence of high concentrations of anti-BCMA monoclonal antibody (S307118G03). We observed that the antibody blocked ACAR-mediated cytolysis of U266 (BCMA+TACI−) but not MM.1s cells (BCMA+TACI+). Encouragingly, anti-BCMA antibody did not attenuate killing of primary MM cells from 3 patients who expressed both BCMA and TACI (Figure 4C).
Taken together, these experiments confirm ACAR-mediated cell death of primary MM cells and support the assumption that in the event of BCMA downregulation, tumor control could be maintained by TACI expression on primary cells.
Efficacy of APRIL CAR against myeloma in vivo
Because these in vitro assays did not show a significant difference in efficacy of ACAR-H and ACAR-CD8, we proceeded to test the smaller and thus simpler of the 2 constructs, ACAR-H in an in vivo model.
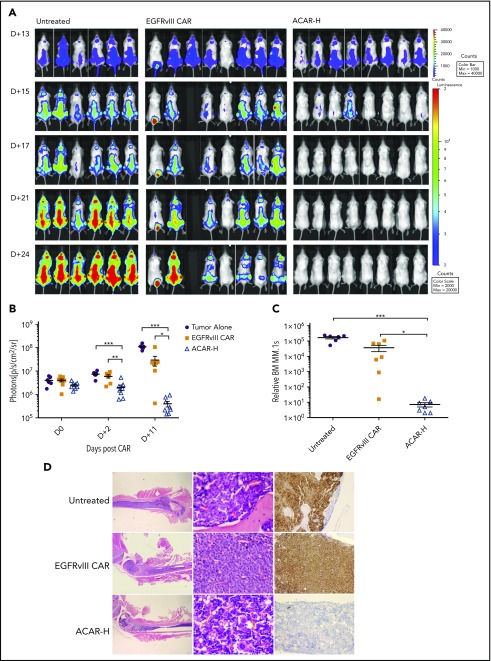
To establish an intramedullary myeloma model, we injected 22 NOD scid gamma mice intravenously with 10 × 106 HA+Fluc+MM.1s cells. Thirteen days later, there was intramedullary disease by bioluminescent imaging (BLI) in all mice (Figure 5A), at which point 5 × 106 EGFRvIII CAR or ACAR-H T cells (Figure 5B) were administered by tail vein injection into 8 animals. A single animal in the EGFRvIII CAR group did not recover following T cells, and a further mouse (with the lowest disease burden pre-CAR) had disease clearance. Nonetheless, after 2 days, there was less disease in ACAR- than in EGFRvIII CAR-treated animals by BLI (P < .01 by t test) and continued disease suppression in ACAR-treated mice (Figure 5A-B). At termination of the experiment (D+12 post-ACAR T-cells, D+25 posttumor cells), FACS of the BM confirmed significant tumor clearance in ACAR-treated animals in comparison with both control cohorts (P < .05 and P < .001 in comparison with EGFRvIII CAR and untreated cohorts; Figure 5C). Tumor clearance in the ACAR-H–treated cohort was confirmed by immunohistochemistry (Figure 5D).
Figure 5.
ACAR-H mediated tumor clearance in vivo. (A) Twenty-two NSG mice were injected IV with 10 × 106 HA+Fluc+MM.1s cells at D0 and monitored by BLI for tumor burden at different time points (dorsal views shown). On D+13 there was clear evidence of intramedullary tumor in all animals, at which point 6 animals were left untreated, and 8 animals were intravenously injected with T cells transduced with a control EGFRvIII targeting CAR (EGFRvIII CAR) or ACAR-H (5 × 106 CAR cells/animal). (B) Average radiance (p/s/cm2/sr) of whole mice in the 3 groups at different timepoints. (C) At termination of experiment (D+25 posttumor, D+12 post-CAR) by FACS, there was significant reduction of tumor in the bone marrow of ACAR-treated mice in comparison with EGFRvIII-treated and untreated animals. Tumor cells were identified as live/single/muCD11b−/HA+ with numbers normalized to Flow-Check beads to calculate relative engraftment. (D) Eradication of CD138+ tumor cells by ACAR was confirmed in bone marrow by IHC of femur. Hematoxylin and eosin staining shown at ×12.5 and ×400 (left and central panels) and immunostaining for CD138 (right panels) at ×200 original magnification. Mean ± SEM shown. *P < .05, **P < .01, ***P < .001, by t test.
Human APRIL binds murine BCMA and TACI at affinities similar to those of their human isoforms,43 and ACAR causes equivalent cytolysis of SUPT1 targets expressing human or murine BCMA and TACI (supplemental Figure 7A). This provided the unique opportunity to investigate possible off-target toxicity in our mouse xenograft model without modification to the ACAR construct. Numerous tissues were harvested from test mice (full list in the supplemental data), and on examination of formalin-fixed and paraffin-embedded, hematoxylin and eosin–stained tissue sections, we found there to be no treatment-related histopathological findings in ACAR-treated animals (supplemental Figure 7B).
Persistent disease control in an in vivo escape model
We propose dual antigen targeting as a means of reducing the risk of antigen-negative disease escape. To model the capacity for ACAR-mediated tumor control despite BCMA downregulation, we engrafted NSG mice with a mix of SUPT1BCMA and SUPT1TACI (4:1 ratio) tumor cells by tail vein before administration of ACAR or BCMA CAR.
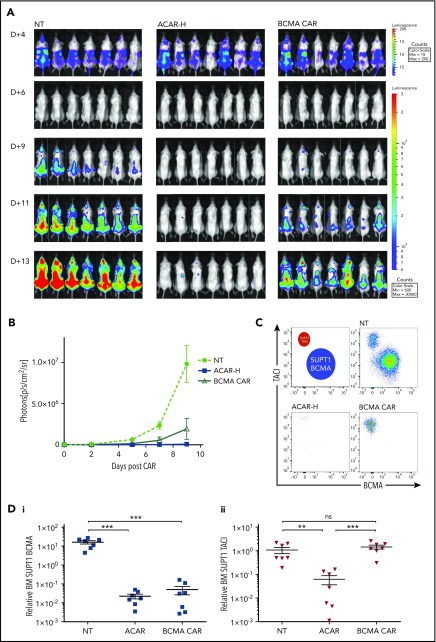
Twenty-one NSG mice were injected with 3.5 × 106 Fluc-expressing SUPT1 cells comprising SUPT1BCMA (5807 ABC) and SUPT1TACI (2229 ABC) (80%:20%). At 4 days, mice received 5 × 106 NT T cells, ACAR, or BCMA CAR T cells (n = 7 per group) by tail vein injection. By BLI, there was continued tumor growth with NT T cells, partial disease suppression with BCMA CAR, and greatest tumor clearance in ACAR-treated animals (Figure 6A-B). On termination of the experiment (D+13 and D+9 posttumor and CAR, respectively), FACS of BM from animals receiving ACAR T cells showed clearance of both SUPT1BCMA and SUPT1TACI (P < .001 and P < .01 in comparison with NT, respectively), and BM from animals receiving BCMA CAR showed persistence of SUPT1TACI (P values nonsignificant in comparison with NT). There was continued engraftment of both tumor populations in animals receiving NT T cells (Figure 6C-D) and evident T-cell persistence in all mice (supplemental Figure 8).
Figure 6.
ACAR-H mediated clearance of BCMA negative tumor. (A) BCMA-3 (5807 ABC) and TACI-2 (2229 ABC) SUPT1 targets were transduced with RQR8.2A.Fluc and HA.2A.Fluc, respectively, and used in an in vivo tumor escape model. Twenty-one NSG mice were intravenously injected with a total of 3.5 × 106 BCMA- and TACI-expressing SUPT1 cells at a ratio of 4:1. At D+4, mice were intravenously injected with NT PBMCs, and T cells transduced with ACAR-H or a CAR construct targeting BCMA alone (BCMA CAR) at a dose of 5 × 106 CAR cells/animal (n = 7 per cohort). Tumor burden was monitored by BLI at different time points (dorsal views shown). (B) Average radiance (p/s/cm2/sr) of whole mice in the 3 groups at different time points. (C) Nine days post-CAR T cells, the experiment was terminated, and FACS of BM MNCs showed persistent engraftment of BCMA and TACI SUPT1 cells following NT T cells, clearance of both cell populations by ACAR-H T cells, and eradication of BCMA expressing tumor only by BCMA CAR (single example from 3 cohorts shown). (D) SUPT1 cells were identified as live/single/muCD11b−/CD2−/CD4+/CD8+ and BCMA (i) and TACI (ii) expression determined by RQR8 and HA staining, respectively, with numbers normalized to Flow-Check beads to calculate relative engraftment. Mean ± SEM shown. **P < .01, ***P < .001 by t test.
These data support the assumption that in comparison with targeting BCMA alone, dual-antigen targeting of BCMA and TACI facilitates continued disease suppression in the event of BCMA downregulation or loss in patients who have tumor coexpression of both antigens.
Discussion
BCMA is emerging as a lead therapeutic target in MM, as is indicated by several on-going clinical studies. The NCI group have reported 12 patients treated with their BCMA targeting CD28-CD3ζ CAR44 observing sustained responses at the highest-dose level of 9 × 106 T cells/kg24 and a further 21 patients treated with a separate (bb2121) 4-1BB-CD3ζ CAR, with consistent responses in patients administered at least 150 × 106 CAR T cells.45 Cohen et al have described their preliminary results of the first cohort treated with a 4-1BB-CD3ζ BCMA CAR.46 In this study, 3 of 9 patients developed grade 3-4 cytokine release syndrome, but notably, there were deep responses and evidence of CAR T-cell expansion without prior lymphodepleting chemotherapy. Alternative T-cell redirecting therapies are CD3-BCMA bispecific molecules on a common IgG arm,47,48 or bispecific T-cell engagers, in which scFvs to CD3 and BCMA are joined by a small peptide linker.23,49 Additionally, a phase I study of an antibody-drug conjugate utilizing the antitubulin agent, monomethyl auristatin F, reported an overall response rate of 67% in their high-dose groups in multiply relapsed patients.50
Although these BCMA-targeted therapies show promise, this receptor is present on tumor cells at variable and often low levels.16,17 We found the median surface BCMA expression on MM cells to be over a log less than CD19 on acute lymphoblastic leukemia (ALL).6 Moreover, antigen-negative tumor escape is well described in B-cell malignancies, with an incidence exceeding 10% in patients with ALL treated with a CD19 CAR.6,25 In the BCMA CAR study described by the University of Pennsylvania group,46 disease progression in 2 patients was associated with reduction in BCMA expression, reminiscent of the report from the NCI group using their first BCMA CAR.24 These observations prompt a re-evaluation of such therapies targeting a single antigen.
We found BCMA and TACI to be coexpressed on the tumor for the majority (78%) of patients, and we hypothesized that targeting 2 tumor antigens could overcome the challenges of low target levels and antigen escape when targeting BCMA alone. To date, there have been several approaches to creating dual-targeting CARs. These strategies have included the admixing of 2 populations of CAR-transduced T cells,51 engineering a single CAR construct containing 2 separate scFvs in tandem (TanCAR)52,53 or the coexpression of 2 CARs on T cells using a bicistronic vector or double transduction (OR gate).54,55 In the context of low levels of target antigen, the first approach may not ensure maximal T-cell activation, because only BCMA or TACI would be recognized by individual T cells. A bivalent TanCAR may result in lower numbers of ligated receptors per target cell in target-limited conditions; finally, an OR gate requires a large, complex bicistronic vector or a complex double transduction.
In comparison, APRIL is compact (135aa), nonimmunogenic, and natively bispecific, binding either MM antigen31 with high affinity. Using APRIL as the CAR binder, we report target cytolysis at low E:T ratios that enforce an assessment of serial kill and at low levels of target antigen, such as are present on primary tumor cells. ACAR-mediated cytolysis was also achieved at low levels of TACI, when BCMA targeting was blocked, thus indicating the possibility of ACAR-mediated disease control, even with BCMA downregulation or loss. Data exist that demonstrate resistance of CARs to blocking by avidity effects,56,57 and we observed reduction in ACAR killing at the highest levels of sBCMA found in MM BM but not with physiological concentrations of APRIL or TACI. In confirmation of our in vitro findings, we observed tumor regression of established disease after only 48 hours of ACAR T-cell infusion in an intramedullary murine myeloma model. Notably, using an in vivo model of tumor escape, we observed improved disease control in comparison with a CAR targeting BCMA alone.
BCMA and TACI are both lymphoid antigens. BCMA is vital for the survival of long-lived PC,58 is upregulated in late-memory B cells on committing to the PC lineage,59,60 and is thus present on normal and malignant PC.16,17 In comparison, TACI expression is found primarily on maturing B cells, particularly marginal-zone B cells, CD27+ memory B-cell subsets and PC.37,59 Our qRT-PCR analysis of TACI transcripts indicates expression restricted to the lymphoid compartments. Furthermore, ACAR does not appear to result in tissue toxicity in an animal model. We expect that ACAR therapy would result in loss of the entire plasma cell compartment and a subset of the B-cell compartment. The subsequent hypogammaglobinaemia may be more profound than that of CD19 targeting61 but should not be more severe than that of BCMA targeting alone.
TACI has been implicated as both a positive and a negative immune regulator,62-65 and gene disruptions are found in 8% of patients with common variable immunodeficiency.27,63 TACI also drives PC differentiation,66 suggesting that TACI is expressed early in PC development. We describe tumor TACI expression in the majority of patients and, given the ontogeny of TACI expression, speculate that in these patients at least, expression of this antigen on putative myeloma stem cells that have a role in disease relapse and drug resistance67,68 would add a further advantage to this approach.
In summary, using a novel ligand-based approach, we have demonstrated that the ACAR can concurrently target BCMA and TACI to increase the number of targetable tumor antigens in the majority of MM patients. ACAR T cells were able to kill targets expressing either receptor, and significant killing was seen at physiological receptor levels, at low E:T ratios, or with BCMA blockade. ACAR T cells also killed primary myeloma cells in vitro, and we observed rapid and complete tumor clearance in vivo in comparison with an irrelevant CAR, as well as in our tumor escape model in comparison with a CAR targeting BCMA alone. These observations suggest that dual-antigen targeting of BCMA and TACI by ACAR T cells may improve on the initial clinical responses seen with BCMA targeting CARs, both by extending clinical applicability to those patients with low levels of tumor BCMA and by reducing the risk of antigen-negative escape.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from Bloodwise (12062) (L.L.) and the Kay Kendall Leukaemia Fund (KKL726) (N.C., B.D.). The work was undertaken at University College London/University College London Hospitals, which is an National Institute for Health Research Biomedical Research Centre, a Cancer Research UK Cancer Centre, and a Bloodwise Research Centre of Excellence.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.P. conceived the project; L.L., K.Y., and M.P. designed the study; S.O., M.R.-J., K.Y., and M.P. supervised the work; L.L., B.D., N.C., B.P., M.C., D.G.-F., S.O., S.T., V.B., R.B., P.M., E.K., M.P.N., and D.P. performed the work; L.L., S.O., and J.F. analyzed the data; L.L. wrote the paper; and J.F., K.Y., and M.P. reviewed the paper.
Conflict-of-interest disclosure: L.L. received funding from Bloodwise Research. K.Y. received funding from Janssen Research. L.L., B.D., N.C., P.M., K.Y., and M.P. own equity in Autolus. L.L., B.D., N.C., K.Y., and M.P. own the patent for APRIL CAR. S.O., S.T., V.B., R.B., E.K., J.F., and M.P. are employees of Autolus. M.P. has received honoraria from Amgen and Roche. The remaining authors declare no competing financial interests.
Correspondence: Lydia Lee, University College London Cancer Institute, Department of Haematology, 72 Huntley St, London, WC1E 6DD, United Kingdom; e-mail: l.lee@ucl.ac.uk.
References
- 1.National Center for Health Statistics, Centers for Disease Control and Prevention. Mortality data. https://www.cdc.gov/nchs/nvss/deaths.htm. Accessed 5 September 2016.
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Lee JH, Lahuerta JJ, et al. ; International Myeloma Working Group. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125(9):3392-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garfall AL, Maus MV, Hwang WT, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373(11):1040-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horenstein AL, Sizzano F, Lusso R, et al. CD38 and CD157 ectoenzymes mark cell subsets in the human corneal limbus. Mol Med. 2009;15(3-4):76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quarona V, Zaccarello G, Chillemi A, et al. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom. 2013;84(4):207-217. [DOI] [PubMed] [Google Scholar]
- 13.Grumet M, Rutishauser U, Edelman GM. Neural cell adhesion molecule is on embryonic muscle cells and mediates adhesion to nerve cells in vitro. Nature. 1982;295(5851):693-695. [DOI] [PubMed] [Google Scholar]
- 14.Edelman GM. Cell adhesion molecules in neural histogenesis. Annu Rev Physiol. 1986;48(1):417-430. [DOI] [PubMed] [Google Scholar]
- 15.Elenius K, Jalkanen M. Function of the syndecans—a family of cell surface proteoglycans. J Cell Sci. 1994;107(Pt 11):2975-2982. [DOI] [PubMed] [Google Scholar]
- 16.Lee L, Bounds D, Paterson J, et al. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br J Haematol. 2016;174(6):911-922. [DOI] [PubMed] [Google Scholar]
- 17.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novak AJ, Darce JR, Arendt BK, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103(2):689-694. [DOI] [PubMed] [Google Scholar]
- 19.Ryan MC, Hering M, Peckham D, et al. Antibody targeting of B-cell maturation antigen on malignant plasma cells. Mol Cancer Ther. 2007;6(11):3009-3018. [DOI] [PubMed] [Google Scholar]
- 20.Tai YT, Mayes PA, Acharya C, et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123(20):3128-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramadoss NS, Schulman AD, Choi SH, et al. An anti-B cell maturation antigen bispecific antibody for multiple myeloma. J Am Chem Soc. 2015;137(16):5288-5291. [DOI] [PubMed] [Google Scholar]
- 22.Chekmasova AA, Horton HM, Garrett TE, et al. A novel and highly potent CAR T cell drug product for treatment of BCMA-expressing hematological malignances. Blood. 2015;126(23):3094. [Google Scholar]
- 23.Hipp S, Tai YT, Blanset D, et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia. 2017;31(10):2278. [DOI] [PubMed] [Google Scholar]
- 24.Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008;19(3-4):263-276. [DOI] [PubMed] [Google Scholar]
- 27.Castigli E, Wilson SA, Garibyan L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37(8):829-834. [DOI] [PubMed] [Google Scholar]
- 28.Tai YT, Li XF, Breitkreutz I, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66(13):6675-6682. [DOI] [PubMed] [Google Scholar]
- 29.Quinn J, Glassford J, Percy L, et al. APRIL promotes cell-cycle progression in primary multiple myeloma cells: influence of D-type cyclin group and translocation status. Blood. 2011;117:890-901. [DOI] [PubMed] [Google Scholar]
- 30.Patel DR, Wallweber HJ, Yin J, et al. Engineering an APRIL-specific B cell maturation antigen. J Biol Chem. 2004;279(16):16727-16735. [DOI] [PubMed] [Google Scholar]
- 31.Hymowitz SG, Patel DR, Wallweber HJ, et al. Structures of APRIL-receptor complexes: like BCMA, TACI employs only a single cysteine-rich domain for high affinity ligand binding. J Biol Chem. 2005;280(8):7218-7227. [DOI] [PubMed] [Google Scholar]
- 32.Philip B, Kokalaki E, Mekkaoui L, et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood. 2014;124(8):1277-1287. [DOI] [PubMed] [Google Scholar]
- 33.Rivière I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA. 1995;92(15):6733-6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas S, Straathof K, Himoudi N, Anderson J, Pule M. An optimized GD2-targeting retroviral cassette for more potent and safer cellular therapy of neuroblastoma and other cancers. PLoS One. 2016;11(3):e0152196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnelly ML, Hughes LE, Luke G, et al. The ‘cleavage’ activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’ sequences. J Gen Virol. 2001;82(Pt 5):1027-1041. [DOI] [PubMed] [Google Scholar]
- 36.Beers R, Chowdhury P, Bigner D, Pastan I. Immunotoxins with increased activity against epidermal growth factor receptor vIII-expressing cells produced by antibody phage display. Clin Cancer Res. 2000;6(7):2835-2843. [PubMed] [Google Scholar]
- 37.Ng LG, Sutherland AP, Newton R, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;173(2):807-817. [DOI] [PubMed] [Google Scholar]
- 38.Ingold K, Zumsteg A, Tardivel A, et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201(9):1375-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendriks J, Planelles L, de Jong-Odding J, et al. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005;12(6):637-648. [DOI] [PubMed] [Google Scholar]
- 40.Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18(5):263-275. [DOI] [PubMed] [Google Scholar]
- 41.Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12(5):933-941. [DOI] [PubMed] [Google Scholar]
- 42.Hombach A, Hombach AA, Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther. 2010;17(10):1206-1213. [DOI] [PubMed] [Google Scholar]
- 43.Kimberley FC, van der Sloot AM, Guadagnoli M, et al. The design and characterization of receptor-selective APRIL variants. J Biol Chem. 2012;287(44):37434-37446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kochenderfer JN. Chimeric antigen receptors/genetically modified T-cells. Blood. 2016;128(22):SCI-37. [Google Scholar]
- 45.Berdeja JG, Lin Y, Raje NS, et al. First-in-human multicenter study of bb2121 anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: updated results. J Clin Oncol. 2017;35(suppl 15):3010.28715249 [Google Scholar]
- 46.Cohen AD, Garfall AL, Stadtmauer EA, et al. B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (MM): initial safety and efficacy from a phase I study. Blood. 2016;128(22):1147. [Google Scholar]
- 47.Panowski SH, Kuo T, Chen A, et al. Preclinical evaluation of a potent anti-Bcma CD3 bispecific molecule for the treatment of multiple myeloma [abstract]. Blood. 2016;128(22). Abstract 383. [Google Scholar]
- 48.Seckinger A, Delgado JA, Moser S, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31(3):396-410. [DOI] [PubMed] [Google Scholar]
- 49.Topp MS, Attal M, Langer C, et al. Phase 1 dose-escalation study of BI 836909, an anti-BCMA bi-specific T-cell engager, in relapsed and/or refractory multiple myeloma (RRMM). J Clin Oncol. 2016;34(suppl 15). Abstract TPS8067. [Google Scholar]
- 50.Cohen AD, Popat R, Trudel S, et al. First in human study with GSK2857916, an antibody drug conjugated to microtubule-disrupting agent directed against B-cell maturation antigen (BCMA) in patients with relapsed/refractory multiple myeloma (MM): results from study BMA117159: part 1. Dose escalation [abstract]. Blood. 2016;128(22). Abstract 1148. [Google Scholar]
- 51.Hegde M, Corder A, Chow KK, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21(11):2087-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4(6):498-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grada Z, Hegde M, Byrd T, et al. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruella M, Barrett DM, Kenderian SS, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126(10):3814-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen KH, Wada M, Pinz KG, et al. A compound chimeric antigen receptor strategy for targeting multiple myeloma [published online ahead of print 27 September 2017]. Leukemia. doi:10.1038/leu.2017.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rufener GA, Press OW, Olsen P, et al. Preserved activity of CD20-specific chimeric antigen receptor-expressing T cells in the presence of rituximab. Cancer Immunol Res. 2016;4(6):509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramos CA, Savoldo B, Torrano V, et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest. 2016;126(7):2588-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Connor BP, Raman VS, Erickson LD, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199(1):91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avery DT, Kalled SL, Ellyard JI, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112(2):286-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179(11):7276-7286. [DOI] [PubMed] [Google Scholar]
- 61.Bhoj VG, Arhontoulis D, Wertheim G, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 2016;128(3):360-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan M, Wang H, Chan B, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol. 2001;2(7):638-643. [DOI] [PubMed] [Google Scholar]
- 63.Salzer U, Bacchelli C, Buckridge S, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009;113(9):1967-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castigli E, Wilson SA, Scott S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201(1):35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18(2):279-288. [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Carmona Y, Cols M, Ting AT, et al. Differential induction of plasma cells by isoforms of human TACI. Blood. 2015;125(11):1749-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsui W, Huff CA, Wang Q, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103(6):2332-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsui W, Wang Q, Barber JP, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68(1):190-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.