Abstract
Inflammation is an important component of the tumor microenvironment. IL-1 is an inflammatory cytokine which plays a key role in carcinogenesis and tumor progression. IL-1 is subject to regulation by components of the IL-1 and IL-1 receptor (ILR) families. Negative regulators include a decoy receptor (IL-1R2), receptor antagonists (IL-1Ra), IL-1R8, and anti-inflammatory IL-37. IL-1 acts at different levels in tumor initiation and progression, including driving chronic non-resolving inflammation, tumor angiogenesis, activation of the IL-17 pathway, induction of myeloid-derived suppressor cells (MDSC) and macrophage recruitment, invasion and metastasis. Based on initial clinical results, the translation potential of IL-1 targeting deserves extensive analysis.
Keywords: interleukin-1, inflammation, inflammation-associated cancer, therapy
Introduction
Selected chronic inflammatory conditions increase the risk of developing cancer (the extrinsic pathway) (1). These include infection, autoimmunity and autoinflammation. Colitis-associated cancer has served as a typical example of this connection. In the intrinsic pathway, genetic events, which drive carcinogenesis, orchestrate the construction of an inflammatory microenvironment by direct induction of inflammatory genes or through activation of downstream transcription factors (nuclear factor-κB (NF-κB), signal transducer and activator of transcription 3 (STAT3) and hypoxia-inducible factor 1α (HIF-1α)).
Among leukocytes, tumor-associated macrophages (TAM) have served as a paradigm for the connection between inflammation and cancer (2). TAM influence all aspects of tumor growth and progression, including providing a nurturing niche for cancer stem cells, promoting angiogenesis, paving the way to invasion and metastasis, taming adaptive immune responses (3–5). Cytokines play a key role in the tumor promoting functions of TAM. The macrophage cytokine repertoire is vast and includes primary inflammatory mediators (e.g. IL-1 and TNFα), anti-inflammatory cytokines (TGF-β and IL-10) and chemokines, which are differentially expressed depending of the state of activation (2, 6). IL-1 is a prototypic inflammatory cytokine upstream in the cytokine cascade (7, 8). Several lines of evidence indicate that IL-1 and its regulation play a pivotal role in cancer-related inflammation. Here we will review how IL-1 affects various aspects of tumor progression and clinical results highlighting its role as a therapeutic target.
1. An overview of the IL-1 and IL-1 receptor family and of their regulation
IL-1 is the first member of a family of structurally related cytokines. Ligands include two isoforms of IL-1 (α and β), three receptor antagonists (IL-1Ra in three isoforms; IL-36Ra; IL-38), IL-33, IL-18, IL-36 (α and γ) and IL-37 (9). The IL-1 receptor (IL-1R) family is composed of 10 members which assemble as heterodimers and signal via the MyD88-IRAK-NFκB pathway (9).
A key feature of IL-1 is the existence of multiple levels of negative regulation within the system. IL-1R2 is a decoy receptor which in membrane bound or released forms acts as a molecular trap for IL-1β (10). In addition it also traps extracellularly IL-1α in concert with a released isoform of the accessory protein (IL-1R3) and blocks intracellular processing (9). IL-18 binding protein (IL-18BP) is a secreted non-signalling inhibitor of IL-18. Receptor antagonists in the system include IL-1Ra, IL-36γ, IL-38. IL-37 is a member of the family which interacts with IL-1R8 and IL-1R5/IL-1R8α and has anti-inflammatory activity (11, 12). Finally, IL-1R8 is a fringe member of the IL-1R family with distinct structural features and functions as negative regulator. In addition to being part of the receptor complex which recognizes IL-37, IL-1R8 is recruited at signalling receptor complexes of the IL-1 and TLR family and dampens the response by interfering with MyD88 multimer formation (13, 14).
In recent years, IL-1 has emerged as a key cytokine involved in innate and adaptive immunity with new, unexpected vistas. IL-1 is downstream of inflammasome activation and its role in sensing microbial invasion, tissue and cellular damage (9). Moreover, IL-1 is a key driver of innate (ILC3) and adaptive (Th17) lymphocyte differentiation thus playing a role in polarized lymphoid cell-orchestrated responses. These general features of IL-1 and its complex regulation are relevant for its role in carcinogenesis and tumor progression.
2. Promotion of carcinogenesis
Schematically, inflammation and cancer are linked by an intrinsic pathway, whereby genetic events which cause cancer orchestrate the construction of an inflammatory microenvironment, and an extrinsic pathway whereby non-resolving inflammation, drives carcinogenesis (1). IL-1 has been associated to both pathways by genetic studies in mice and man. In a model of epithelial carcinogenesis, IL-1α has been shown to be downstream of Ras activation and to be an essential driver for the activation of NFκB–regulated genes, including cytokines and chemokines required for the establishment of a protumoral microenvironment. In addition, IL-1α is involved in the suppression of keratinocyte differentiation markers, leading to neoplastic transformation in a cell-autonomous manner (15). In a series of seminal studies Ron Apte and colleagues found that mice genetically deficient in IL-1β or IL-1R1 are protected against methylcholanthrene carcinogenesis (16–18). The role of IL-1α is less evident in this model of carcinogenesis in terms of inflammation and tumorigenicity. However, studies with 3-MCA-induced fibrosarcoma cell lines from IL-1α-deficient mice showed that host-derived IL-1α is involved in cancer immunoediting, by affecting innate and adaptive immunosurveillance mechanisms (19).
Intriguingly, IL-1β deficiency had a more profound impact, in terms of increased susceptibility also to skin carcinogenesis, than genetic inactivation of IL-1α (18). Indeed, IL-1α overexpression in transgenic mice was associated with reduced incidence of DMBA/TPA-induced skin tumors (20). It is tempting to speculate that this difference is related to the “alarmin” function of IL-1α triggering protective immunity.
Chronic non-resolving inflammatory conditions such as inflammatory bowel disease, non alcoholic liver steatohepatitis and obesity increase the propensity of developing tumors (1, 4) and gastrointestinal neoplasias have been invaluable to define the clinical significance and mechanisms of cancer-related inflammation. Genetic polymorphisms at the IL-1 locus in humans have been shown to be strongly associated with susceptibility to gastritis which drives gastric carcinoma (21). Analysis of mice deficient in IL-1 family molecules and MyD88 have phenotypes which are at the interception of tumor promoting inflammation, promoting mucosal integrity and microbial sensing (for review (22)).
3. Promotion of metastasis
In early studies it was observed that IL-1β augments lung metastasis after i.v. injection of tumor cells (23). These early observations were confirmed and extended in models of spontaneous lung metastasis and carcinogenesis (16, 24–26). Increased secondary localization after treatment with IL-1 was also observed in models of liver metastasis. IL-1- mediated promotion of metastasis was inhibited by IL-1Ra (27, 28). The mechanisms underlying IL-1 mediated stimulation of metastasis are complex and include promotion of angiogenesis and induction of endothelial cell adhesion molecules recognized by tumor cells. For instance, VCAM-1 induced by IL-1 is recognized by VLA4 which is expressed on some melanoma cells, whereas the E-selectin ligand is present on colon cancer cells (17).
4. Mechanisms of IL-1-driven tumor promotion
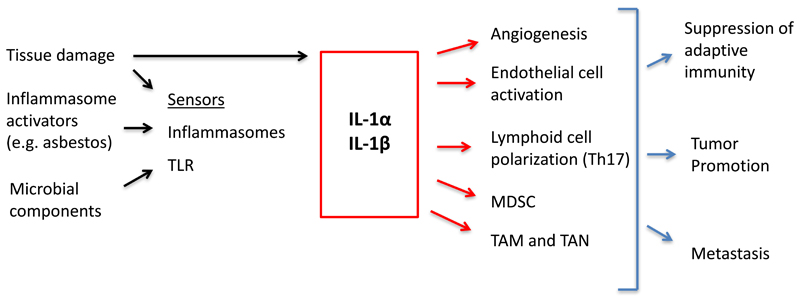
IL-1 affects multiple aspects of the tumor microenvironment which underlie tumor promotion discussed under point 2 and presumably mediate clinical observations to be discussed below (Fig. 1). IL-1-driven tumor promotion has been associated with promotion of angiogenesis (17, 29, 30). IL-1 has complex effects on the vascular endothelium (9, 31). In general, IL-1 activates endothelial cells in a prothrombotic/proinflammatory direction by inducing procoagulant activity and inducing expression of adhesion molecules and inflammatory cytokines. Moreover, IL-1 induces in endothelial cells and surrounding stromal cells the production of proangiogenic cytokines such as IL-8. In this general perspective, it is interesting that IL-1 is downstream of metabolic rewiring of macrophages with succinate being a key inducing product activating the HIF-1α pathway (32).
Figure 1. IL-1 in cancer related inflammation.
Key mechanisms by which IL-1 promotes carcinogenesis, progression and metastasis are summarized in a schematic form.
Colitis-associated intestinal cancer has served as a paradigm for the connection between inflammation and cancer (1). Signals originating from microbes and dysbiosis are key drivers of gastrointestinal tumorigenesis (33, 34). IL-1 is downstream of microbial sensing by epithelial cells or innate immunity cells. In agreement, mice deficient of IL-1R8/SIGIRR, a negative regulator of the signaling of IL-1R family members and TLR, exhibited a dramatic intestinal inflammation in response to dextran sulfate sodium salt (DSS) administration, with more severe weight loss, intestinal bleeding, and mortality, and showed increased susceptibility to carcinogenesis in response to azoxymethane and DSS (35, 36).
The differentiation of innate and adaptive lymphoid cells polarized in an ILC3 and Th17 direction, respectively, is sustained by IL-1. The IL-17 pathway has been shown to support carcinogenesis in the gastrointestinal tract (37). There is evidence that neutrophils are an important component of tumor promoting inflammation (38). It remains to be established whether neutrophils are a component of the IL-1-IL-17 pathway of tumor promotion.
IL-1 promotes recruitment of myeloid cells in tumors and their tumor promoting function. Chemokines such as CCL2 and endothelial cell expression of adhesion molecules mediate leukocyte recruitment at sites of inflammation. In a recent study, the IL-1R-MyD88 pathway was shown to upregulate expression of ten-eleven-2 (tet2) in TAM in mouse models of melanoma and in melanoma patients. Tet2 is a DNA methylcytosine dioxigenase which was found responsible for the immunosuppressive program of TAM (39). Genetic inactivation of tet2 shifted macrophage polarization, with recruitment of effector T cells and antitumor activity. In early studies IL-1 was found to stimulate haematopoiesis (hemopoietin-1). Expansion of myeloid cells in cancer results in the appearance in peripheral blood and lymphoid tissues of a heterogeneous population of immature elements endowed with immunosuppressive activity, operationally defined myeloid derived suppressor cells (MDSC) (40). IL-1 sustains MDSCs generation and immunosuppressive function (41–43). Thus, IL-1 by promoting MDSCs and sustaining the immunosuppressive activity of TAM contributes to suppression of effective adaptive antitumor immune responses.
Clinical perspective
Human polymorphisms at the IL-1 locus have shown that an imbalance between proinflammatory IL-1 and anti-inflammatory IL-1Ra is a major risk factor for gastritis, ulcer, and hence gastric carcinoma (21). In addition polymorphisms in the IL-1Ra and IL-1 gene are associated with lung cancer risk (44–47), a consistent finding possibly relevant to anti-IL-1β mediated protection against this tumor, as discussed below.
At present clinically available anti-IL-1strategies include IL-1Ra (Anakinra), anti-IL-1β mAb and anti-IL-1α mAb. Anakinra has been studied in patients with smouldering myeloma with evidence suggesting inhibition of progression to frank neoplasia (48), although this observation has not been followed up.
In a phase I study in patients with end-stage cancers of various origins, a naturally occurring human neutralizing anti-IL-1α mAb has shown activity in reducing cancer cachexia (i.e. in increasing lean body mass and decreasing fatigue, pain, and appetite loss, as well as circulating IL-6 levels) (49). Since IL-1α is barely detectable in plasma, the mAb was supposed to target IL-1α expressed on the surface of platelets, CD14+ and CD16+ monocytes, and in particular malignant cells, which contain IL-1 precursor. Neutralization of tumor-associated IL-1α as well as platelet or monocyte-associated IL-1α would reduce local and systemic inflammatory processes, leading to correction of a metabolic defect and increased lean body mass, as well as potentially reducing IL-1-dependent angiogenesis and immune suppression.
Atherosclerosis is a manifestation of vessel wall inflammation and IL-1 has long been known to drive atherosclerosis and its complications. On this basis a large prospective study was conducted using anti-IL-1β (Canakinumab) in high risk atherosclerosis patients (50). In the same study involving 10,061 patients, a dramatic (>50%) reduction of the incidence and mortality from lung cancer was observed (51). These impressive results have broad implications and are thought provoking. Given the relatively short follow up (3.7 years) and natural timeframe of carcinogenesis in humans, it is likely that mechanisms other than blocking of IL-1-driven cancer progression are involved. As discussed above, IL-1 drives pathways of myeloid cell-mediated suppression of specific antitumor immunity. It is therefore tempting to speculate that IL-1β blockade interferes with MDSCs and TAM-mediated immune suppression and that the tumor protection observed is a reflection of unleashed T cell mediated responses.
Concluding remarks
IL-1 is a key mediator of innate and adaptive immunity at the crossroad of diverse pathways of microbial recognition and activation and orientation of lymphoid cell function. IL-1 has emerged as a key component of tumor promoting inflammation by shaping different constituents of the tumor microenvironment including tumor infiltrating myeloid cell recruitment, angiogenesis, and skewing and suppression of anti-tumor immunity. Available information suggests that IL-1 targeting in the clinic may affect cancer-associated cachexia (49) and incidence and mortality from cancer, based on a large cohort (10,061) of patients at risk of atherosclerosis related complications (51). These impressive results need confirmation and extension. As they stand, they raise the issue of anti-IL-1 strategies as a form of immunotherapy. Preclinical results summarized here and available clinical observations raise the issue of combining IL-1 inhibition with checkpoint blockade.
Fundings
The financial support of Fondazione Cariplo (Contract n. 2015-0564), Ministero della Salute (RF-2011-02348358 and RF-2013-02355470), Ministry of Education, University and Research (PRIN 2015YYKPNN), the European Research Council (ERC – N° 669415 to AM), and the Italian Association for Cancer Research (AIRC IG-19014 and AIRC 5x1000 -9962) is gratefully acknowledged.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Netea MG, Balkwill F, Chonchol M, et al. A guiding map for inflammation. Nat Immunol. 2017;18:826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colotta F, Re F, Muzio M, et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Neff CP, Barber K, et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci U S A. 2015;112:2497–2502. doi: 10.1073/pnas.1424626112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nold-Petry CA, Lo CY, Rudloff I, et al. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol. 2015;16:354–365. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- 13.Molgora M, Barajon I, Mantovani A, Garlanda C. Regulatory Role of IL-1R8 in Immunity and Disease. Front Immunol. 2016;7:149. doi: 10.3389/fimmu.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molgora M, Bonavita E, Ponzetta A, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature. 2017;551:110–114. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cataisson C, Salcedo R, Hakim S, et al. IL-1R-MyD88 signaling in keratinocyte transformation and carcinogenesis. J Exp Med. 2012;209:1689–1702. doi: 10.1084/jem.20101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apte RN, Dotan S, Elkabets M, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 17.Voronov E, Shouval DS, Krelin Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krelin Y, Voronov E, Dotan S, et al. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007;67:1062–1071. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- 19.Elkabets M, Krelin Y, Dotan S, et al. Host-derived interleukin-1alpha is important in determining the immunogenicity of 3-methylcholantrene tumor cells. J Immunol. 2009;182:4874–4881. doi: 10.4049/jimmunol.0803916. [DOI] [PubMed] [Google Scholar]
- 20.Murphy JE, Morales RE, Scott J, Kupper TS. IL-1 alpha, innate immunity, and skin carcinogenesis: the effect of constitutive expression of IL-1 alpha in epidermis on chemical carcinogenesis. J Immunol. 2003;170:5697–5703. doi: 10.4049/jimmunol.170.11.5697. [DOI] [PubMed] [Google Scholar]
- 21.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 22.Salcedo R, Cataisson C, Hasan U, Yuspa SH, Trinchieri G. MyD88 and its divergent toll in carcinogenesis. Trends Immunol. 2013;34:379–389. doi: 10.1016/j.it.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giavazzi R, Garofalo A, Bani MR, et al. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res. 1990;50:4771–4775. [PubMed] [Google Scholar]
- 24.Vidal-Vanaclocha F, Amezaga C, Asumendi A, Kaplanski G, Dinarello CA. Interleukin-1 receptor blockade reduces the number and size of murine B16 melanoma hepatic metastases. Cancer Res. 1994;54:2667–2672. [PubMed] [Google Scholar]
- 25.Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, Dinarello CA. Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro. J Natl Cancer Inst. 1996;88:198–205. doi: 10.1093/jnci/88.3-4.198. [DOI] [PubMed] [Google Scholar]
- 26.Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, et al. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci U S A. 2000;97:734–739. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenzie RC, Oran A, Dinarello CA, Sauder DN. Interleukin-1 receptor antagonist inhibits subcutaneous B16 melanoma growth in vivo. Anticancer Res. 1996;16:437–441. [PubMed] [Google Scholar]
- 28.Chirivi RG, Garofalo A, Padura IM, Mantovani A, Giavazzi R. Interleukin 1 receptor antagonist inhibits the augmentation of metastasis induced by interleukin 1 or lipopolysaccharide in a human melanoma/nude mouse system. Cancer Res. 1993;53:5051–5054. [PubMed] [Google Scholar]
- 29.Carmi Y, Voronov E, Dotan S, et al. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol. 2009;183:4705–4714. doi: 10.4049/jimmunol.0901511. [DOI] [PubMed] [Google Scholar]
- 30.Voronov E, Carmi Y, Apte RN. The role IL-1 in tumor-mediated angiogenesis. Front Physiol. 2014;5:114. doi: 10.3389/fphys.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6:2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- 32.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 34.Thomas S, Izard J, Walsh E, et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017;77:1783–1812. doi: 10.1158/0008-5472.CAN-16-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garlanda C, Riva F, Veliz T, et al. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007;67:6017–6021. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- 36.Xiao H, Gulen MF, Qin J, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Wang K, Kim MK, Di Caro G, et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41:1052–1063. doi: 10.1016/j.immuni.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galdiero MR, Bianchi P, Grizzi F, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016;139:446–456. doi: 10.1002/ijc.30076. [DOI] [PubMed] [Google Scholar]
- 39.Pan W, Zhu S, Qu K, et al. The DNA Methylcytosine Dioxygenase Tet2 Sustains Immunosuppressive Function of Tumor-Infiltrating Myeloid Cells to Promote Melanoma Progression. Immunity. 2017;47:284–297 e285. doi: 10.1016/j.immuni.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7 doi: 10.1038/ncomms12150. 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elkabets M, Ribeiro VS, Dinarello CA, et al. IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010;40:3347–3357. doi: 10.1002/eji.201041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A. The growing diversity and spectrum of action of myeloid-derived suppressor cells. Eur J Immunol. 2010;40:3317–3320. doi: 10.1002/eji.201041170. [DOI] [PubMed] [Google Scholar]
- 44.Bhat IA, Naykoo NA, Qasim I, et al. Association of interleukin 1 beta (IL-1beta) polymorphism with mRNA expression and risk of non small cell lung cancer. Meta Gene. 2014;2:123–133. doi: 10.1016/j.mgene.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lind H, Zienolddiny S, Ryberg D, Skaug V, Phillips DH, Haugen A. Interleukin 1 receptor antagonist gene polymorphism and risk of lung cancer: a possible interaction with polymorphisms in the interleukin 1 beta gene. Lung Cancer. 2005;50:285–290. doi: 10.1016/j.lungcan.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Zienolddiny S, Ryberg D, Maggini V, Skaug V, Canzian F, Haugen A. Polymorphisms of the interleukin-1 beta gene are associated with increased risk of non-small cell lung cancer. Int J Cancer. 2004;109:353–356. doi: 10.1002/ijc.11695. [DOI] [PubMed] [Google Scholar]
- 47.Hu Z, Shao M, Chen Y, et al. Allele 2 of the interleukin-1 receptor antagonist gene (IL1RN*2) is associated with a decreased risk of primary lung cancer. Cancer Lett. 2006;236:269–275. doi: 10.1016/j.canlet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong DS, Hui D, Bruera E, et al. MABp1, a first-in-class true human antibody targeting interleukin-1alpha in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15:656–666. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 51.Ridker PM, MacFadyen JG, Thuren T, et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]