Abstract
The vasculature of tumours is highly abnormal and dysfunctional. Consequently, immune effector cells have an impaired ability to penetrate into solid tumours and often exhibit compromised functions. Normalization of the tumour vasculature can enhance tissue perfusion and improve immune effector cell infiltration, leading to immunotherapy potentiation. However, recent studies, have demonstrated that stimulation of immune cell functions can also help to normalize tumour vessels. In this Opinion article, we propose that the reciprocal regulation between tumour vascular normalization and immune reprogramming forms a reinforcing loop that reconditions the tumour immune microenvironment to induce durable antitumour immunity. A deeper understanding of these pathways could pave the way for identifying new biomarkers and developing more effective combination treatment strategies for patients with cancer.
Introduction
The recent clinical successes of cancer immunotherapies such as immune-checkpoint blockade have reaffirmed the importance of the host immune system in preventing and eliminating cancer1–4. In contrast to conventional cytotoxic agents, which directly target cancer cells, a major goal of cancer immunotherapy is to alleviate tumour-associated suppression of anticancer immune responses. A significant portion of cancer immunotherapy research over the past decade has focused on heightening the functions of effector T cells, which play a direct role in recognizing tumour-associated antigens and in mediating tumouricidal responses5. In this context, therapeutic cancer vaccines that are based on tumour antigens, or engineered chimeric antigen receptor (CAR) T cell therapies both rely on the formation of antigen-specific T lymphocyte clones that can recognize and eradicate tumours harboring a particular antigen or a mutant protein. Both approaches have shown promise in preclinical studies and early-phase clinical trials; in particular, CAR T cell therapy has demonstrated potential as a treatment for hematologic malignancies6–12 On the other hand, immune checkpoint inhibitors target inhibitory ligand–receptor interactions between T cells and immune suppressor cells within the tumor microenvironment (TME), in particularly those mediated by tumour cells13. Ipilimumab, a monoclonal antibody that targets the cytotoxic T lymphocyte associated protein-4 (CTLA4) and antibodies blocking the programmed cell death-1 (PD1)/programmed cell death ligand-1 (PDL1, also known as B7-H1) axis, have led to remarkable clinical responses in several types of metastatic cancer, for example melanoma14, non-small cell lung cancer (NSCLC)15,16, Merkel cell carcinoma17, and renal cell carcinoma18.
In this Opinion article, we highlight emerging evidence that cancer immunotherapies, such as immune checkpoint blockade, can promote T lymphocyte activation beyond modulating the stimulatory–inhibitory axis and this activation may extend to normalize the immunosuppressive TME. We propose that a reciprocal regulation between immune cells and the tumour vasculature is critical in dictating the antitumour efficacy of cancer immunotherapy and forms the basis of a larger interacting network consisting of tumour vascular remodelling, metabolic homeostasis, and immune reprogramming. Together, these elements establish a positive feedback loop, in which changes in one will reinforce the effect of others. Understanding the mutual regulation among these processes and their collective effects on promoting antitumour immunity is crucial for elucidating the mechanisms of tumour immune evasion and for developing effective combinational cancer immunotherapies.
The aberrant tumour vasculature
The stimulation of local and systemic antitumour immune responses is essential for successful cancer immunotherapy19. For activated immune cells to eradicate cancer cells, they first need to penetrate deep into the tumour parenchyma and identify cancer cells as their intended targets. Once inside the tumour, immune cells also need to overcome many of the immune suppressive mechanisms within the TME20–22.
Effect on immune cell infiltration
Immune cells, like nutrients or oxygen, rely on a functional vascular network to enter tissues23. The hypoxic environment within solid tumours induces the continued production of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), transforming growth factor-β (TGFβ), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF)24–26. This results in an imbalance between the levels of pro-angiogenic and anti-angiogenic factors, which are tightly regulated in healthy tissues, and promotes rapid but aberrant tumour blood vessel formation24,27,28. Morphologically, tumour blood vessels are tortuous, dilated, and unevenly distributed, with adjacent endothelial cells being loosely attached to one another. Pericytes, which surround the blood vessels and regulate vascular permeability, are usually detached from the endothelial cells, resulting in leaky tumour blood vessels that are characterized by dysfunctional flow characteristics22,28. Tumour-associated endothelial cells also express lower levels of cell adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM1) and intercellular adhesion molecule-1 (ICAM1), which promotes endothelial anergy and reduces the trafficking of immune effector cells into tumours29–32. Together, the structural and functional abnormalities of tumour blood vessels decreases the recruitment of immune effector cells, thus limiting the effectiveness of cancer immunotherapies (Fig 1). Strategies to convert a “cold” tumour that is devoid of immune effector cells into a “hot” tumour by increasing tumour infiltration of T lymphocytes is an active area of research, which aims to enhance the effectiveness of cancer immunotherapies33–35. It is not surprising that poorly vascularized tumours, for example pancreatic adenocarcinoma, which is densely packed with fibrous stroma with a sparse immune cell presence, are often highly resistant to cancer immunotherapies36.
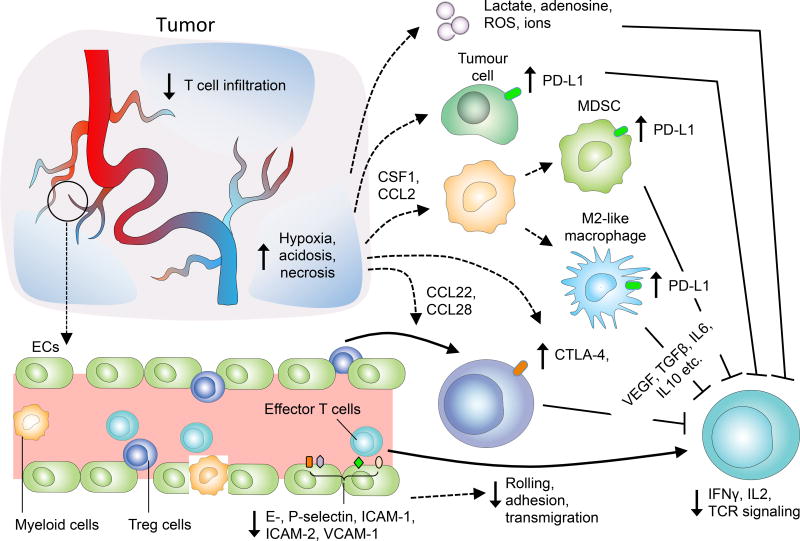
Figure 1. Abnormalities in the tumour vasculature contribute to immune suppression via multiple mechanisms.
Impaired vessel perfusion and increased vascular permeability promote tissue hypoxia, acidosis and necrosis, which activate immune suppressive processes to inhibit effector T cell functions. Hypoxia not only induces the secretion of cytokines and chemoattractants to increase the recruitment of immunosuppressor cells, but also upregulates the expression of CTLA4 or LAG3 on Treg, and PDL1 on MDSCs, TAMs131 and tumour cells.45 The endothelial cells of tumour vessels also express lower levels of cell adhesion molecules causing endothelial anergy, thereby reducing the ability of effector T cells to infiltrate into tumours. ECs: endothelial cells; ROS, reactive oxygen species; MDSC, myeloid-derived suppressor cell, Treg, regulatory T cells; TCR, T cell receptor; CTLA4, cytotoxic T -lymphocyte associated protein-4, PDL1, programmed cell death ligand-1; TAMs, tumour associated macrophages.
Effect on Tumour Immune Microenvironment
In addition to having direct effects on immune cell adhesion and extravasation, the abnormal tumour vasculature also indirectly antagonizes the effectiveness of cancer immunotherapy by promoting TME-mediated immune suppression. The impaired perfusion capacity of tumour blood vessels helps to create a highly hypoxic TME22,25. Hypoxia contributes to immunosuppression via several mechanisms (regulation of immunity by hypoxia is Reviewed in ref 37). First, hypoxia promotes the accumulation of myeloid-derived suppressor cells (MDSCs) and facilitates the differentiation and polarization of tumour-associated macrophages (TAMs) into an immunosuppressive M2-like phenotype38–40. Second, hypoxia indirectly increases the accumulation of regulatory T cells (Tregs) within the TME by upregulating expression of the chemoattractant chemokines (C-C motif) ligand 22 (CCL22) and CCL28 on tumour cells and TAMs41,42. Furthermore, hypoxia promotes resident immune suppressor cells within the TME to secrete immunosuppressive factors such as VEGF, TGFβ, and IL-1022,41–44. Hypoxia also induces the expression of immune checkpoint molecules such as PDL1 on tumour cells45 as well as PDL1, T cell immunoglobulin and mucin-domain containing-3 (TIM-3) and CTLA4 on TAMs, MDSCs, and Tregs. Moreover, through VEGF, hypoxia can indirectly upregulate PD1 expression on CD8+ T lymphocytes, thus further inhibiting immune effector cell activation and function (Fig 1)46–51. Last, hypoxia along with tumour cell necrosis, increase the extracellular concentrations of the immune-inhibitory metabolites adenosine and lactate.52–55 The accumulation of lactate further leads to metabolic lactic acidosis (a low pH in the blood due to build up of lactic acid) and results in impaired cytotoxic T cell functions by interfering with T cell receptor (TCR)–triggered production of interferon-γ (IFNγ)56. These processes act together to inhibit effector immune cell maturation and promote T cell anergy and exhaustion (Fig 1). Therefore, dysfunctional tumour vessels have a major role in contributing to the immunosuppressive nature of the TME and countering the effect of cancer immunotherapies.
Tumour vascular normalization
Given that the abnormal tumour vasculature promotes immune suppression within the TME, strategies that normalize these aberrant blood vessels may therefore restore immune cell functions and facilitate their antitumour activities. Although initially proposed as a way to improve the delivery of systemic drugs into tumours27,57,58, the process of vascular normalization was recently shown to potentiate cancer immunotherapy, by promoting immune cell infiltration into tumours and reducing the immune-suppressive elements within the TME.
Genetically induced vascular normalization improves immunotherapy
Genetic disruption of regulator of G-protein signaling 5 (Rgs5) expression in mice promoted immature PDGFRβ+ pericytes to become mature αSMA+NG2+ pericytes without affecting their overall coverage of the vasculature. This phenotypic change within tumour vessels consequently reduced tumour tissue hypoxia and vascular leakiness, leading to an influx of immune effector cells into the tumour parenchyma59. This study attributed the reduction in intratumoural pressure and hypoxia due to pericyte maturation as the main reasons for the increased T cell entry. However, vasculature normalization also results in an increased and more uniform distribution of adhesion molecules on the luminal surface of endothelial cells lining the tumour blood vessels, thus allowing more efficient immune cell docking and rolling, both of which facilitate immune cell infiltration into tumours60.
Therapeutically induced vascular normalization improves immunotherapy
Beyond genetic manipulations, the therapeutic blockade of proangiogenic factors can also normalize tumour vasculature and improve cancer immunotherapies. Traditional high-dose antiangiogenic therapy destroys tumour vessels, leading to further hypoxia and inhibition of immune cell recruitment. However, appropriate low-dose antiangiogenic therapy against VEGF/VEGFR was found to induce tumour vascular normalization, reduce hypoxia, facilitate tumour infiltration of CD8+ T lymphocytes, and potentiate cancer immunotherapy61–65. The importance of hypoxia in promoting immune suppression within solid tumours was further illustrated by a recent study demonstrating that respiratory hyperoxia induced by breathing high concentration (60%) of oxygen alone could convert the TME from an immunosuppressive to an immunosupportive phenotype, by decreasing intratumoural hypoxia and concentrations of extracellular adenosine66. As a result, respiratory hyperoxia resulted in the regression of spontaneously developed tumours in a T cell- and natural killer (NK) cell-dependent manner66.
Although earlier studies of tumour vascular normalization mostly focused on disruption of the VEGF–VEGFR axis, recent studies have revealed the angiopoietin (Ang) –Tie2 signalling pathway as a promising new target for normalizing tumour vessels67. Simultaneous blockade of angiopoietin-2 (Ang2) and activation of Tie2 signalling normalized tumour vessels and induced favorable immunological alterations within the TME57. Moreover, dual targeting of Ang2 and VEGF signaling pathways often results in improved vascular normalization relative to VEGF inhibition alone68,69 and in various tumour models enhanced the effects of PD1 blockade70. The vascular normalization effect of dual Ang2 and VEGF blockade was associated with more efficient lymphocyte priming by antigen-presenting cells, TAM polarization to an M1-like phenotype, and accumulation of activated, IFNγ-expressing CD8+ T cells within the perivascular space70,71. Although depletion of CD8+ T cells completely eliminated the antitumor effects of Ang2 and VEGF blockade, it remains to be seen whether the absence of CD8+ T cells will also abrogate the accompanied vascular normalization effect.69. Interestingly, the dual blockade of Ang2 and VEGF upregulated PDL1 expression in tumor endothelial cells via IFNγ70. This observation raises the possibility that resistance to antiangiogenic therapy may arise, at least in part, due to the development of adaptive immune suppressive processes within tumours70,72. Furthermore, IFNγ can induce both PDL1-dependent and PDL1-independent resistance within tumours in the setting of immune checkpoint blockade73. Together, these findings further highlight the intricate relationship between immune cells and tumour blood vessels, and provide new rationales for combining antiangiogenic treatments with immunotherapies.
Activated eosinophils promote tumour vascular normalization
In addition to blocking proangiogenic factors, a recent study demonstrated that activated CD11b+Gr1loF4/80+Siglec-F+ eosinophils can also promote the normalization of tumour vessels, which subsequently help to mediate tumour rejection by CD8+ T cells74. The exact mechanism by which the pro-angiogenic eosinophils induce vessel normalization is unclear, but it may be that they act through the polarization of TAMs into the M1-like phenotype via eosinophil-derived IFNγ and tumour necrosis factor (TNF) signalling, resulting in decreased VEGF production. The normalized blood vessels improve T cell infiltration, which results in a positive feedback loop that facilitate further M1-like macrophage polarization, vessel normalization, and VCAM1 expression on endothelial cells to promote more efficient T cell entry.74 Together, these studies confirm that vascular normalization has an immune supportive role in enhancing antitumour immunity through a multitude of mechanisms, which include: reducing tumour tissue hypoxia, improving the access of tumour-infiltrating T lymphocytes to tumour cells, and polarizing immune suppressive cells toward immune stimulatory phenotypes.
Immune-vascular crosstalk
The interplay between tumour blood vessel remodelling and tumour immune microenvironment reprogramming has led to studies that examined the effect of vascular normalization on immune checkpoint blockade and vice versa70,72,75. In CD4+ T cell-deficient mice, pericyte coverage of blood vessels was reduced and tumour tissue hypoxia was increased in breast tumour models, thus indicating that a lack of CD4+ T cells causes vascular abnormalities75. Treatment with dual anti-CTLA4 and anti-PD1 therapy, which has been thought to mainly affect T cells76–81, induced tumour vessel normalization75. Although it is well known that immune cell populations possess anti-angiogenic and/or pro-angiogenic activities82–84, the results of that study suggest that the antitumor effects of immune checkpoint blockade may also stem from its ability to remodel the tumour vasculature75. With this new understanding, it is now conceivable that cancer immunotherapies, such as immune checkpoint blockade, may exert effects beyond immune cells and act on non-immune cells within the stromal microenvironment to indirectly enhance their antitumour activities.
A feedback loop of vascular normalization and immune reprogramming
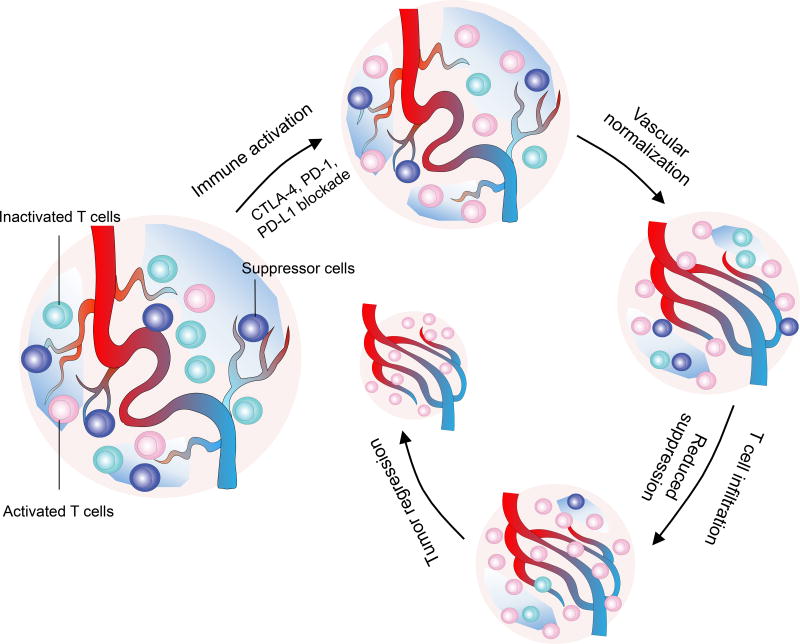
This unexpected action of immune checkpoint inhibitors thus completes a positive feedback loop, in which immune checkpoint inhibitors activate T cells to normalize tumour blood vessels, resulting in the polarization of the immunosuppressive TME into an immune-supportive environment. This in turn facilitates the expansion and improves the functions of intratumoural effector T cells, thus lead to more vascular and TME remodelling, which ultimately produces long-term tumour control (Fig 2). Thus, vascular normalization in the setting of immune stimulation represents a novel mechanism for the antitumor effects of immune checkpoint blockade and provides a new understanding of tumour vascular remodelling and immune reprogramming. Although these findings are exciting, the properties of immunotherapy-induced tumour vascular normalization require further characterization and validation in clinical settings. For example, the conditions required for immunotherapy-induced vascular normalization, the duration of the response, and the differences from antiangiogenic therapy-mediated vascular normalization remain unclear. In addition, the distinct effects of CD4+ and CD8+ T cells populations on tumour blood vessels in the setting of immune checkpoint blockade need further analysis. Thus far, the role of CD4+ T cells on tumour vessels was elucidated through genetic manipulation or antibody-mediated depletion, in which tumour implantation occurred in the absence of CD4+ T cells75. It is well known that tissue hypoxia increases as a tumour grows28, resulting in TME changes that increase Treg population within the tumour41,42,52. Several elegant studies have demonstrated that Tregs can promote tumor angiogenesis and that Treg depletion activates CD8+ T cells and induces vascular normalization42,74. Therefore, the tumour vascular normalization effect of immune checkpoint blockade is likely a dynamic process that involves different immune cell populations at different stages of tumour development.
Figure 2. A reinforcing feedback loop of immune reprogramming and tumour vascular normalization.
The highly immune suppressive tumour microenvironment is often dominated by the presence of immune suppressor cells and dysfunctional effector T cells. Immune checkpoint blockers activate effector T cells, which in turn promote the normalization of tumour blood vessels. The initial vascular normalization decreases immune suppressive processes within the tumour microenvironment, facilitates the infiltration and enhances the function of effector T cells, leading to further normalization of tumour blood vessels. This feedback loop between immune reprogramming and tumour vascular normalization reinforces each other, ultimately promotes immune-mediated tumour eradication. The disruption or the inability to establish such a positive reinforcement process may lead to transient therapeutic efficacy and decrease long-term tumour control of immune checkpoint inhibitors.
Molecular mechanisms of the feedback loop
At the molecular level, the interconnection between angiogenesis and immune activation relies on dual-functional signalling of many proangiogenic factors and immune regulator proteins. Previous studies have demonstrated that proangiogenic factors are usually immunosuppressive, whereas immune effector cells and antitumour cytokines possess angiostatic effects. For example, potent angiogenic factors (for example, VEGF, which is secreted by tumour and stromal cells) can interfere with both the maturation of dendritic cells from CD34+ precursors, and the development of T lymphocytes85–88. By contrast, major mediators of antitumour immune responses such as IFNγ, the CXC-chemokine ligands (CXCL) 9 and 10, and TNF also inhibit tumour angiogenesis89–93. Moreover, IFNγ is a major mediator of antitumor immunity with robust antiangiogenic activity3,83,84,93. A recent study induced IFNγ expression in murine fibrosarcoma and adenocarcinoma cells and selectively expressed IFNγ receptor in specific stromal cell types, including myeloid cells, fibroblasts, T cells, and endothelial cells; this study found that IFNγ directly promoted tumour vessel regression and blood flow arrest via its interactions with tumour endothelial cell IFNγ receptors, leading to intratumoral ischemia and subsequent collapse of the tumour92. The apparent discrepancy between this finding and the recent evidence that IFNγ promoted vessel normalization75 could be explained by potential differences in exposure of endothelial cells to IFNγ75,92. The induction of IFNγ expression in tumour cells produced high systemic IFNγ concentrations (~10 ng/mL), which were maintained for a more extended period than the transient IFNγ elevation that can be elicited by adoptively transferred antigen-specific T cells91. Therefore, it is likely that the inducible IFNγ system produced more intense and sustained antiangiogenic effects on tumour blood vessels in comparison with IFNγ stimulation from immune checkpoint blockade75. Excess antiangiogenic activities mediated by both higher dose and prolonged IFNγ exposure likely promote the transition from vascular normalization to vessel regression, a phenomenon that was also observed in previous studies using anti-VEGFR2 agents61,63.
In addition to IFNγ, T helper 1 (TH1) type chemokines (such as CXCL9 and CXCL10) can produce angiostatic effects, in addition to acting as chemoattractants for effector T cells33. Human microvascular endothelial cells that express high levels of CXCR3, the receptor for the angiostatic chemokines CXCL4, CXCL9, CXCL10 and CXCL11, were found to exhibit low rates of proliferation and high rates of apoptosis, as well as decreased capability to undergo tube-like vessel formation89. Several recent studies revealed that the elevation of histone and DNA methylation within tumours and activation of intrinsic oncogenic signalling suppressed the expression of TH1 type chemokines and resulted in ’cold’ tumour type that is resistant to immunotherapy33,94,95. Treatment with epigenetic modulators was found to restore the expression of CXCL9 and CXCL10, promoted CD8+ T cell tumour infiltration, and enhanced the efficacy of immune checkpoint therapy94. These studies further underscore the complexity of the interactions between immune cells and tumor blood vessels at the genetic and molecular levels.
Finally, immunosuppressive cell populations such as Tregs, MDSCs, and M2-like TAMs not only promote tumour immune evasion, but also foster tumour angiogenesis by secreting VEGF, placental growth factor (PIGF), IL-1β, IL-6, FGF2, stromal cell-derived factor 1 (SDF1), and PDGF, among others33,93,96,97. Both TAMs and MDSCs are often intimately associated with tumour blood vessels98 and secrete membrane-bound or soluble proteases (for example, matrix metalloproteinase (MMP)2, MMP9, and MMP12), which facilitates the growth of tumour blood vessels by degrading the extracellular matrix and improving the availability of proangiogenic growth factors99,100. Interestingly, MDSCs that reside within the perivascular space can adopt an endothelial cell-like morphology and express markers such as CD31 and VEGFR2, suggesting that these immature cells may possess the potential to differentiate into cells that become part of the tumour vasculature96,97. Therefore, strategies that deplete or polarize immune suppressive myeloid cells within tumours have been investigated to normalize tumour vasculatures. For example, histidine-rich glycoprotein was found to induce TAM polarization away from the immune-suppressive phenotype by downregulating macrophage-derived PIGF. This response resulted in sustained tumour vascular normalization that inhibited tumour growth and metastases101,102.
New biomarkers for immuno-oncology
The discovery that activated T cells can induce morphologic and functional tumour vessel normalization in the setting of immune checkpoint blockade provides a new opportunity to identify novel biomarkers for cancer immunotherapy103.
Serum-based biomarkers for immuno-oncology
Serum-based biomarkers reflecting the functional status of tumour blood vessels were previously used to monitor responses to angiogenic therapies104,105 and may now be explored as predictors of response to cancer immunotherapies. Serum levels of Ang2, a vessel-destabilizing ligand of Tie2 and a critical regulator of blood vessel maturation106, were found to inversely correlate with both clinical response rate and survival in melanoma patients treated with the anti-CTLA4 antibody, ipilimumab107. Similarly, humoral responses against proangiogenic cytokines including Ang2 and VEGFA were found to predict long-term remission and survival in patients with acute myeloid leukemia that had received tumour cell vaccines after allogeneic hematopoietic stem cell transplantation, and also in patients with NSCLC treated with tumour vaccines108,109. Together, these findings support the interconnecting relationship between tumour vascular remodeling and the generation of antitumor immune responses, and they further highlight the potential role of using vascular-related biomarkers as a surrogate to predict clinical responses to cancer immunotherapies.
Vessel-based biomarkers for immuno-oncology
Beyond circulating biomarkers, functional measurements of vascular changes with Doppler ultrasonography, perfusion scans, or dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI)110,111, may also provide important information regarding the changes that occur within the TME as a surrogate to tumour responses to immune checkpoint inhibitors (Fig 3). These noninvasive measurements also allow longitudinal monitoring during therapy, a limitation that faces tissue-based biomarkers because of the need for repeat biopsies. Measurements of tumour vascular remodelling more closely reflect changes within the TME in the setting of immune checkpoint blockade therapy, which may not be perfectly represented by systemic biomarkers such as circulatory cytokine levels, immune cell subset counts, or lactate dehydrogenase levels103. Finally, tumour vascularity112 and the degree of perfusion impairment may also serve as a predictor of response to cancer immunotherapy at baseline, before treatment is begun. These new imaging biomarkers can be incorporated into future clinical trial designs, and potentially be used to stratify patients for treatments, as has been done successfully for PDL1 expression in advanced-stage NSCLC103. Again, establishing robust vascular biomarkers for cancer immunotherapy requires carefully designed biomarker exploratory studies with independent validation and is still far from clinical translation at this time. Nevertheless, noninvasive measurement techniques provide a unique opportunity to explore TME-based biomarkers based on functional changes induced by immune checkpoint blockade therapy, which extends beyond traditional tumour cell and immune cell analyses.
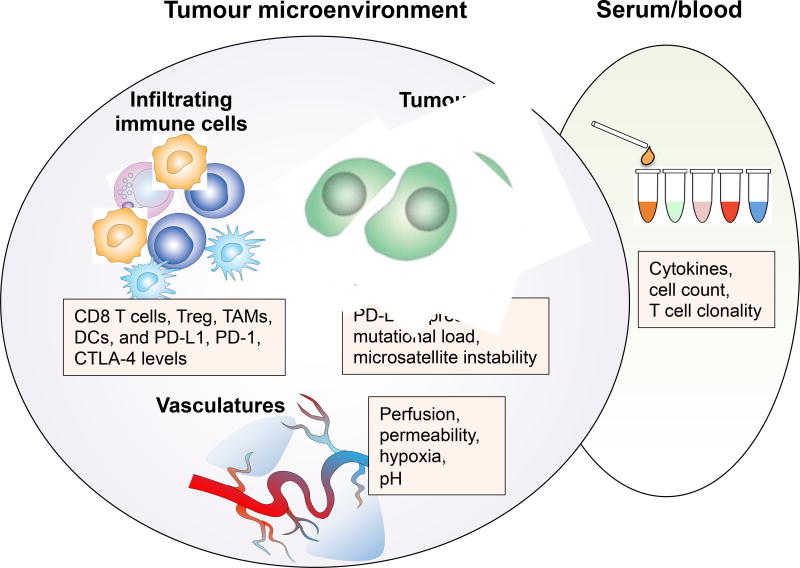
Figure 3. Biomarker discovery for immuno-oncology.
The current tissue-based biomarker analysis for immuno-oncology largely focuses on intrinsic tumour cell properties and immune cell profiles within the tumour microenvironment, including PDL1 expression levels, mutational load, as well as the number of infiltrating effector T cells, immune suppressor cells, and their ratios. The vascular remodeling effects of immune checkpoint therapy provide a new rationale for assessing tumour microenvironment-based biomarkers beyond tumour and immune cells. Examination of tumour vascular-related changes such as alterations to tissue perfusion, hypoxia, pH and vascular permeability, in combination with immune cell profiling and tumour cell characterization may provide a more sensitive and dynamic way of monitoring tumour responses to immune checkpoint blockade. Together with serum-based biomarkers, tumour microenvironment-based biomarkers incorporating tumour cell, immune cell, as well as blood vessel analyses will provide a complete picture of cancer immunotherapy induced immunological changes to accurately monitor clinical responses in patients.
Combination immunotherapies
The interdependence between vascular normalization and immune reprogramming provides a unique opportunity to identify new and more efficient combination therapy strategies to enhance antitumour immunity. Since the first clinical trial of ipilimumab monotherapy for patients with advanced melanoma nearly a decade ago, research has focused on combining immunotherapies with standard-of-care chemotherapy or radiotherapy to achieve the optimal therapeutic effects14,113–115. However, in some cases, it is unclear whether a mechanistic basis exists to support the specific combination regimen used. For example, several institutional and cooperative group trials are ongoing to study the usefulness of combining radiation with cancer immunotherapies. These studies are based largely on the premise that radiation can facilitate the release of tumour-associated antigens, which may help to broaden the antitumour responses in the setting of immune stimulation116. However, it is far from clear how the two regimens should be delivered in relation to one another (for example, concurrently versus sequentially) and what type of radiotherapy should be used (for example, conventionally fractionated, where a large number of treatments are delivery with small doses of radiation, versus hypofractionated or stereotactic radiotherapy, where a few large doses of radiation are used to ablate the tumour to yield the best response117,118. Ionizing radiation is known to induce changes within the tumour immune microenvironment119, but the nature of those changes has not always been consistent. For example, studies using a single fraction of low-dose radiation (~2 Gy) have been shown to normalize tumour vasculatures and improve T cell recruitment by inducing inducible nitric oxide synthase (iNOS) expression in TAMs in mouse and human pancreatic tumours120,121. However, other studies have found that a single-fraction ablative dose of radiation (as high as 30 Gy) promotes enhanced CD8+ T cell infiltration and decreases MDSCs in murine colorectal tumours122. It is unclear whether the differences in these findings mainly reflect variations in the tumour models used or are due to the involvement of distinct molecular processes. Nevertheless, the fact that stimulated T cells in the setting of immune checkpoint blockade also promote tumour vascular normalization, improve tissue perfusion, and alleviate tumour hypoxia provides a strong rationale that a mutual benefit may exist between cancer immunotherapy and radiotherapy. Beyond the traditional belief that radiotherapy serves as an immune adjuvant119, it is now conceivable that immunotherapy may also have radiosensitizing effects by increasing oxygenation within the tumour, thus paving the way for future studies to examine this reciprocal effect.
The relationship between cancer immunotherapy and conventional systemic therapy also warrants a more detailed examination in light of these new findings. By normalizing the tumour vasculature and improving tissue perfusion, cancer immunotherapy may, at least in theory, improve the delivery of systemic agents into tumours123. However, similar to vascular normalization strategies using antiangiogenic agents, the optimal conditions needed to produce notable therapeutic benefits may be technically difficult to accomplish and may vary among different tumours and different patients61. Nevertheless, new mechanistic insights into the interplay between vascular normalization and immune reprogramming provide us with a theoretical foundation to explore new combination approaches using cancer immunotherapy and systemic therapies.
Vasculature and beyond
Vascular normalization can also lead to changes in the acellular components of the TME that may facilitate the infiltration and activation of immune effector cells to complement cancer immunotherapies. For example, increased tumour blood vessel permeability and decreased lymphatic drainage lead to an elevated intratumoural interstitial fluid pressure (IFP)124. This pathophysiological state in the TME makes it difficult for immune effector cells to enter the tumour parenchyma and they accumulate primarily around the tumour edge124. By restoring the transluminal pressure gradient across tumour blood vessels, vascular normalization has been shown to decrease IFP within the TME125, therefore reducing the restrictions on immune effector cell mobilization and tumouricidal functions.
Another potential benefit of tumour vascular normalization is through its effect on intratumoural lymphatic vessels. Elevated IFP may lead to the compression of intratumoural lymphatic vessels, which may be important for antigen-presenting cells to home to regional lymph nodes and prime T cells126. Previous studies of tumour vascular normalization showed that despite restoration of blood flow within previously compressed blood vessels, lymphatic drainage remained sluggish, suggesting that different processes are involved in blood and lymphatic vessel impairment within tumours124. Investigations into whether immune cells possess a similar ability to remodel dysfunctional tumour lymphatics, in addition to blood vessels, may further illuminate the antitumour effects of cancer immunotherapies.
Finally, tumour vascular normalization may also help to restore aberrant concentrations of ions within the TME, which are largely caused by increased cell turnover and the inadequate removal of intracellular ions released from cancer cells127,128. The ionic imbalance can directly inhibit the functions of effector T cells. A recent study showed that elevations in the extracellular potassium concentration ([K+]e) in the TME impairs TCR–driven Akt–mTOR phosphorylation via the activities of the serine/threonine phosphatase PP2A128, thus inhibiting T cell activation. Similarly, Tregs that were induced to undergo apoptosis from oxidative stress were found to release and convert a large amount of ATP to adenosine, which had the downstream effect of amplifying immune suppressive signals within the TME129. This seminal finding demonstrates that local concentrations of cellular metabolites, such as reactive oxygen species, also have critical roles in regulating the immune TME. The identification of different ionic and metabolic checkpoints further highlights the complexities associated with tumour immune escape, which involves both cellular and acellular elements within the TME. Further investigations into the effect of tumour vascular normalization on the ionic profiles within the TME are therefore needed to fully understand the reciprocal interactions between tumour blood vessel remodeling and immune reprogramming.
Outlook
Although the evaluation of antitumour immune responses has traditionally been focused on assessing the interactions between cancer and immune effector cells, growing evidence now suggests that other components of the TME also have critical roles in determining the efficacy of cancer immunotherapies22,56,130,131. The constituents of the TME form a sophisticated interaction network that ultimately determines the immune landscape within the TME. The complexity and heterogeneity of this immune TME requires a systematic assessment of different molecular (for example, IFNγ, Granzyme B, and various cytokines), cellular (for example, CD8+ T cell activation and myeloid cell polarization), vascular (for example, vessel normalization and tissue perfusion), and acellular responses (for example, metabolic and ionic changes) to cancer immunotherapies. The finding of mutual regulation between tumour vascular normalization and immune reprogramming supports the notion that the antitumour effects of immune checkpoint inhibition may depend not only on the restoration of effector T cell functions within the TME, but also on the normalization of the TME itself. Therefore, the effects of immune checkpoint blockade on the TME, such as the vascular changes it induces, may provide novel variables with which to evaluate and predict cancer immunotherapy responses. The insights gained from studying this reciprocal regulation between immune stimulation and TME normalization has thus opened new avenues to understand how tumours undergo immune evasion and to identify strategies to further boost the antitumour effects of cancer immunotherapies.
Footnotes
Author contributions
Y.H. and W.J. conceived the study. Y.H. and W.J. performed the literature search. Y.H., B.Y.S.K. and W.J. designed and generated the figures. All authors helped to write the manuscript.
Competing interests
The authors declare no competing financial interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burnet FM. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 2.Burnet M. Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br. Med. J. 1957;1:841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016;13:273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eshhar Z, Gross G. Chimeric T cell receptor which incorporates the anti-tumour specificity of a monoclonal antibody with the cytolytic activity of T cells: a model system for immunotherapeutical approach. Br. J. Cancer. Suppl. 1990;10:27–29. [PMC free article] [PubMed] [Google Scholar]
- 9.Mayordomo JI, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J. Natl. Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst RS, et al. embrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 16.Reck M, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 17.Nghiem PT, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer MH, et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell. 2017;168:487–502. doi: 10.1016/j.cell.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganss R, Arnold B, Hammerling GJ. Mini-review: overcoming tumor-intrinsic resistance to immune effector function. Eur. J. Immunol. 2004;34:2635–2641. doi: 10.1002/eji.200425474. [DOI] [PubMed] [Google Scholar]
- 21.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr. Opin. Immunol. 2015;33:55–63. doi: 10.1016/j.coi.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 25.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 26.Kourembanas S, Hannan RL, Faller DV. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J. Clin. Invest. 1990;86:670–674. doi: 10.1172/JCI114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 28.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckanovich RJ, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 30.Wu NZ, Klitzman B, Dodge R, Dewhirst MW. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res. 1992;52:4265–4268. [PubMed] [Google Scholar]
- 31.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffioen AW, et al. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res. 1996;56:1111–1117. [PubMed] [Google Scholar]
- 33.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang H, et al. Facilitating T Cell Infiltration in Tumor Microenvironment Overcomes Resistance to PD-L1 Blockade. Cancer Cell. 2016;29:285–296. doi: 10.1016/j.ccell.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang W, Chan CK, Weissman IL, Kim BYS, Hahn SM. Immune Priming of the Tumor Microenvironment by Radiation. Trends Cancer. 2016;2:I638–645. doi: 10.1016/j.trecan.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Jiang H, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 2016;22:851–860. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017 doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corzo CA, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 42.Facciabene A, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 43.Doedens AL, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klages K, et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70:7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 45.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y, et al. PD-1 and PD-L1 expression in 132 recurrent nasopharyngeal carcinoma: the correlation with anemia and outcomes. Oncotarget. 2017;8:51210–51223. doi: 10.18632/oncotarget.17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruf M, Moch H, Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int. J. Cancer. 2016;139:396–403. doi: 10.1002/ijc.30077. [DOI] [PubMed] [Google Scholar]
- 48.Koh J, et al. EML4-ALK enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1alpha and STAT3. Oncoimmunology. 2016;5:e1108514. doi: 10.1080/2162402X.2015.1108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koh HS, et al. The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat. Commun. 2015;6:6340. doi: 10.1038/ncomms7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noman MZ, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voron T, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin. Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 53.Fischer K, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 54.Gottfried E, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 55.Huber V, et al. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 2017;43:74–89. doi: 10.1016/j.semcancer.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Mendler AN, et al. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int. J. Cancer. 2012;131:633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- 57.Park JS, et al. Normalization of Tumor Vessels by Tie2 Activation and Ang2 Inhibition Enhances Drug Delivery and Produces a Favorable Tumor Microenvironment. Cancer Cell. 2016;30:953–967. doi: 10.1016/j.ccell.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 58.Jiang W, Huang Y, An Y, Kim BYS. Remodeling Tumor Vasculature to Enhance Delivery of Intermediate-Sized Nanoparticles. ACS Nano. 2015;9:8689–8696. doi: 10.1021/acsnano.5b02028. [DOI] [PubMed] [Google Scholar]
- 59.Hamzah J, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, et al. Regulation of leukocyte transmigration: cell surface interactions and signaling events. J. Immunol. 2004;172:7–13. doi: 10.4049/jimmunol.172.1.7. [DOI] [PubMed] [Google Scholar]
- 61.Huang Y, Stylianopoulos T, Duda DG, Fukumura D, Jain RK. Benefits of vascular normalization are dose and time dependent. Cancer Res. 2013;73:7144–6. doi: 10.1158/0008-5472.CAN-13-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shrimali RK, et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang Y, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. U.S.A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rahbari NN, et al. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl. Med. 2016;8:360ra135. doi: 10.1126/scitranslmed.aaf5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung K, et al. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. J. Clin. Invest. 2017;127:3039–3051. doi: 10.1172/JCI93182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatfield SM, et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 2015;7:277ra230. doi: 10.1126/scitranslmed.aaa1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazzieri R. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Hashizume H, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70:2213–2223. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peterson TE, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl. Acad. Sci. U.S.A. 2016;113:4470–4475. doi: 10.1073/pnas.1525349113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmittnaegel M, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 2017;9:eaak9670. doi: 10.1126/scitranslmed.aak9670. [DOI] [PubMed] [Google Scholar]
- 71.Kloepper J, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc. Natl. Acad. Sci. U.S.A. 2016;113:4476–4481. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allen E, et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 2017;9:eaak9679. doi: 10.1126/scitranslmed.aak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benci JL, et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167:1540–1554. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carretero R, et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 75.Tian L, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 78.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 79.June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat. Med. 2017;23:540–547. doi: 10.1038/nm.4321. [DOI] [PubMed] [Google Scholar]
- 80.Kamphorst AO, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang AC, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruegg C, et al. Evidence for the involvement of endothelial cell integrin alphaVbeta3 in the disruption of the tumor vasculature induced by TNF and IFN-gamma. Nat. Med. 1998;4:408–414. doi: 10.1038/nm0498-408. [DOI] [PubMed] [Google Scholar]
- 83.Beatty GL, Paterson Y. IFN-γ-Dependent Inhibition of Tumor Angiogenesis by Tumor-Infiltrating CD4+ T Cells Requires Tumor Responsiveness to IFN-γ. J. Immunol. 2001;166:2276–2282. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- 84.Hayakawa Y, et al. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood. 2002;100:1728–33. [PubMed] [Google Scholar]
- 85.Ohm JE, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–86. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 86.Gabrilovich DI, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 87.Huang Y, et al. Resuscitating cancer immunosurveillance: selective stimulation of DLL1-Notch signaling in T cells rescues T-cell function and inhibits tumor growth. Cancer Res. 2011;71:6122–6131. doi: 10.1158/0008-5472.CAN-10-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang Y, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110:624–631. doi: 10.1182/blood-2007-01-065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Arenberg DA, et al. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J. Exp. Med. 1996;184:981–992. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fathallah-Shaykh HM, Zhao LJ, Kafrouni AI, Smith GM, Forman J. Gene transfer of IFN-gamma into established brain tumors represses growth by antiangiogenesis. J. Immunol. 2000;164:217–222. doi: 10.4049/jimmunol.164.1.217. [DOI] [PubMed] [Google Scholar]
- 92.Kammertoens T, et al. Tumour ischaemia by interferon-gamma resembles physiological blood vessel regression. Nature. 2017;545:98–102. doi: 10.1038/nature22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer. 2017;17:457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 94.Peng D, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 96.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 97.Yang L, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 98.Lewis CE, Harney AS, Pollard JW. The multifaceted role of perivascular macrophages in tumors. Cancer Cell. 2016;30:18–25. doi: 10.1016/j.ccell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J. Natl Cancer Inst. 2002;94:1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 101.Huang Y, Snuderl M, Jain RK. Polarization of tumor-associated macrophages: a novel strategy for vascular normalization and antitumor immunity. Cancer Cell. 2011;19:1–2. doi: 10.1016/j.ccr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rolny C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 103.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 2017 doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rigamonti N, et al. Role of Angiopoietin-2 in Adaptive Tumor Resistance to VEGF Signaling Blockade. Cell Rep. 2014;8:696–706. doi: 10.1016/j.celrep.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 105.Goede V, et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br. J. Can. 2010;103:1407–1414. doi: 10.1038/sj.bjc.6605925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Palma M, Naldini L. Angiopoietin-2 TIEs up macrophages in tumour angiogenesis. Clin. Cancer Res. 2011;17:5226–32. doi: 10.1158/1078-0432.CCR-10-0171. [DOI] [PubMed] [Google Scholar]
- 107.Wu W, et al. Angiopoietin-2 as a Biomarker and Target for Immune Checkpoint Therapy. Can. Immunol. Res. 2017;5:17–28. doi: 10.1158/2326-6066.CIR-16-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Piesche M, et al. Angiogenic cytokines are antibody targets during graft-versus-leukemia reactions. Clin. Cancer Res. 2015;21:1010–8. doi: 10.1158/1078-0432.CCR-14-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schoenfeld J, et al. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 2010;70:10150–10160. doi: 10.1158/0008-5472.CAN-10-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zahra MA, et al. Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol. 2007;8:63–74. doi: 10.1016/S1470-2045(06)71012-9. [DOI] [PubMed] [Google Scholar]
- 111.Padhani AR, Miles KA. Multiparametric Imaging of Tumor Response to Therapy. Radiology. 2010;256:348–364. doi: 10.1148/radiol.10091760. [DOI] [PubMed] [Google Scholar]
- 112.Martinet L, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–87. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- 113.Twyman-Saint Victor C, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gotwals P, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 116.Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J. Immunother. Cancer. 2016;4:51–70. doi: 10.1186/s40425-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat. Rev. Clin. Oncol. 2016;13:516–524. doi: 10.1038/nrclinonc.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Demaria S, Coleman CN, Formenti SC. Radiotherapy: Changing the Game in Immunotherapy. Trend Cancer. 2016;2:286–294. doi: 10.1016/j.trecan.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang W, Chan CK, Weissman IL, Kim BYS, Hahn SM. Immune Priming of the Tumor Microenvironment by Radiation. Trend Cancer. 2016;2:638–645. doi: 10.1016/j.trecan.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 120.Klug F, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 121.De Palma M, Coukos G, Hanahan D. A new twist on radiation oncology: low-dose irradiation elicits immunostimulatory macrophages that unlock barriers to tumor immunotherapy. Cancer Cell. 2013;24:559–61. doi: 10.1016/j.ccr.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 122.Filatenkov A, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer Res. 2015;21:3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stylianopoulosa T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc. Natl. Acad. Sci. U.S.A. 2013;110:18632–18637. doi: 10.1073/pnas.1318415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lund AW. Rethinking Lymphatic Vessels and Antitumor Immunity. Trend Cancer. 2016;2:548–551. doi: 10.1016/j.trecan.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 127.Rotin D, Robinson B, Tannock IF. Influence of hypoxia and an acidic environment on the metabolism and viability of cultured cells: potential implications for cell death in tumors. Cancer Res. 1986;46:2821–2826. [PubMed] [Google Scholar]
- 128.Eil R, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016;537:539–543. doi: 10.1038/nature19364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maj T, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumour. Nat. Immunol. 2017 doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tang H, Qiao J, Fu YX. Immunotherapy and tumor microenvironment. Cancer Lett. 2016;370:85–90. doi: 10.1016/j.canlet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Doedens AL, et al. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 2013;4:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]