Abstract
Background
Vulvar lichen sclerosus et atrophicus (VLSA) is a chronic inflammatory skin disease of unknown etiology that mainly affects postmenopausal and perimenopausal women. The primary clinical symptoms of VLSA are itching, burning pain, and dyspareunia that can results in decreased quality of life. Existing therapies including topical corticosteroid ointment, topical calcineurin inhibitors, estrogens, are not very effective for treatment of VLSA.
Objective
To evaluate the effectiveness and safety of 5-aminolevulinic acid mediated photodynamic therapy (ALA-PDT) in the treatment of VLSA.
Materials and methods
Ten patients with VLSA who had failed conventional treatment received ALA-PDT. 10% 5-ALA in an oil-in-water emulsion was applied to the lesions and occluded with plastic film for 3 h, when the lesions were irradiated with 100 mW/cm2, 635 ± 15 nm red light for 20 min. Treatments were repeated three times at 2-week intervals. Objective and subjective symptoms and signs of the vulvar lesions based on horizontal visual analogue scales were recorded at each treatment and 1, 3, and 6 months after the last session. The quality of life was assessed using dermatology life quality index (DLQI) questionnaire.
Results
All patients completed three ALA-PDT treatments and the follow-up visits. Clinical symptoms of itching disappeared completely in nine patients, one patient had itching decreased from severe to mild. All subjects showed objective improvement in lesions. The DLQI of all cases improved after treatment. The main side-effects of ALA-PDT were pain, erythema, and swelling. Side-effects were transient and tolerable. All patients reported being “satisfied” or “very satisfied” with their outcomes.
Conclusions
ALA-PDT is an effective and safe approach for the treatment of VLSA
Keywords: Vulvar lichen sclerosus et atrophicus, 5-aminolevulinic acid, Photodynamic therapy, Refractory patients, Case series
1. Introduction
Vulvar lichen sclerosus et atrophicus (VLSA) is a chronic inflammatory skin disease that primarily affects postmenopausal and perimenopausal women, but younger women or even men may be affected. The etiology and pathogenesis of lichen sclerosus et atrophicus is still unknown; it has been suggested to be associated with genetic, environmental [1] or hormonal factors [2]. However, there are also suggestions that it may be related to infectious agents, including human papillomavirus, Borrelia burgdorferi (Lyme disease), or Epstein-Barr virus [3]. In addition, it has also been reported that autoimmune mechanisms may play a role in VLSA causation, because it often co-occurs with other autoimmune diseases such as autoimmune thyroiditis, alopecia areata, vitiligo and pernicious anemia. Moreover most VLSA patients have been found to have circulating autoantibodies [4,5]. VLSA is characterized by white well-defined papules and plaques, irreversible white wax-like, atrophic lesions, and is associated with an increased risk for vulvar squamous cell carcinoma [6]. Symptoms include itching, burning pain, and dyspareunia all of which can have a negative effect on patient quality of life.
The treatment of VLSA remains a clinical challenge, and treatment failures are common. It is important to treat the disease as early as possible, and to use an approach that can reverse the disease process. There is still no definitive medical cure for VLSA, so the purpose of treatment is largely to relieve or remove the symptoms (especially the vulvar itching) rather than definitive resolution of the lesions. Conventional medical measures include potent topical corticosteroids, topical calcineurin inhibitors, hormones (testosterone, estrogens, progesterone), 5-fluorouracil, and retinoids. Other therapeutic approaches have been attempted, including surgery, cryosurgery and carbon dioxide laser therapy [7–9]. Despite all these therapeutic approaches, the overall outcomes cannot be regarded as satisfactory. Among the above-mentioned therapies, potent topical corticosteroids are the first line of treatment. However, long-term use of topical corticosteroids may be associated with an increased risk of skin atrophy and alterations in pigmentation [10]. Surgical intervention is usually reserved for patients who have dyspareunia due to fissuring of the vulvar epithelium or narrowing of the vaginal entrance, or who fail medical treatment, or develop secondary scarring [11]. Nevertheless, surgery is often followed by infection, a high rate of recurrence of the disease, and unacceptable pain [11]. Therefore, more effective therapies for VLSA are required, that have lower incidence of side-effects and fewer relapses and recurrences.
5-Aminolevulinic acid photodynamic therapy (ALA-PDT) is a relatively novel technology for the treatment of epithelial, superficial, non-melanoma skin tumors, as well as for infectious agent-induced lesions and inflammatory diseases of the skin [12]. To assess the effectiveness and safety of ALA-PDT in the treatment of refractory VLSA, 10 patients were enrolled and received ALA-PDT treatments with a 6 month follow-up in our department.
2. Materials and methods
2.1. Patients
Ten postmenopausal or perimenopausal women were enrolled with clinical and histological confirmation of VLSA who complained of chronic vulvar itching and associated pain. The clinical manifestation of vulvar lesions were white, wax-like atrophic, lichenoid hyperkeratotic or sclerotic lesions. All patients had been treated with various conventional treatments before enrollment, including potent topical corticosteroids, topical calcineurin inhibitors, cryosurgery, and so on. However, these therapies had only led to temporary improvement or in some patients, no remission of symptoms. All patients wrote an informed consent to receive the treatment and attend follow-up sessions. There were no general exclusion criteria.
2.2. Methods
Prior to each treatment and follow-up visit photographs of the VLSA lesions were captured using the same digital camera. Freshly prepared 10% 5-aminolevulinic acid in an oil-in-water emulsion (Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co. Ltd, Shanghai, China) was applied to the lesions with a 1-cm margin and occluded with plastic film for 3 h. The lesions were irradiated with 100 mW/cm2, 100J/cm2, 635 ± 15 nm red light (Omnilux Revive, Photo Therapeutics, Inc, London, UK) over 20 min. All patients received the same ALA-PDT treatment regimen performed a total of three times at 2-weekly intervals and were followed-up at 1, 3, 6-months. No patients received any other medical treatment or any other conventional therapy during the whole treatment period.
2.3. Clinical evaluation
Before every treatment and follow-up visit, all patients completed the dermatology life quality index (DLQI) questionnaire to evaluate their quality of life (Annex 1) [13]. Patients were also asked to evaluate the degree of subjective symptoms include itching, burning pain and dyspareunia caused by the VLSA, based on a horizontal visual analogue scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe). Two independent dermatologists evaluated the objective clinical and morphological appearance of the lesions and the lesion sizes before and after treatment at 1, 3, and 6 month follow-up visits. Four objective parameters (atrophy, hyperkeratosis, depigmentation and sclerosis) were graded on the following scale: 3 = severe, 2 = moderate, 1 = mild, 0 = absent [14]. A grade scale was determined to evaluate the anatomical extent of the disease (0.5 = affected less than 30% of the vulvar surface, 1 = 30–60% of the vulvar surface and 1.5 = more than 60% of the vulvar surface) [14]. Total scores were obtained by summing the score of each parameter [14].
During each PDT session, the patients were asked to provide the level of treatment-related pain on a visual analogue scale from 0 to 10 (0 = no pain and 10 = extremely painful), and the duration of pain. In addition, patients were asked to assess their overall satisfaction using a grading scale (0 = dissatisfied, 1 = somewhat satisfied, 2 = satisfied, 3 = very satisfied) at every treatment and follow-up visit.
3. Results
All ten patients completed the entire series of treatment sessions and were followed up for 6 months. There were ten patients enrolled with an average age of 51years. The history of disease ranged from three to five years and the average disease duration was 40.54 months. Clinical characteristics of the patients are shown in Table 1.
Table 1.
Demographics and baseline characteristics of patients.
| Characteristic | Value |
|---|---|
| Age (years), mean ± SD | 51 ± 5.66 |
| Gender | |
| Postmenopausal women | 3 |
| Perimenopausal women | 7 |
| Duration (months), mean ± SD | 40.54 ± 10.20 |
| Macroscopic characteristics of lesions Atrophy | 10 |
| Hyperkeratosis | 7 |
| Depigmentation | 10 |
| Sclerosis | 5 |
| Previous treatment | 4 |
| Topical corticosteroids | 6 |
| Topical calcineurin inhibitors | 6 |
| Estrogens | 2 |
| Local injection | 3 |
| UVA1 | 4 |
| Others | 1 |
3.1. Clinical evaluation
Patients demonstrated a remarkable remission of itching, burning pain, and dyspareunia. Before ALA-PDT all patients complained of moderate and severe itching of the vulva. The mean scores of itching were 2.6 at the first ALA-PDT session. With the increasing number of sessions, the itching scores declined gradually. After the third ALA-PDT session, the itching disappeared completely in 9 patients and one patient had a decrease in itching symptoms from severe to mild. All patients initially complained of mild to severe burning pain, and 4 patients complained of moderate to severe dyspareunia. Pain and dyspareunia ceased completely after three ALA-PDT sessions. During the follow-up visits up to 6 months after the last session, there was no recurrence of these symptoms in nine of the patients. The subjective symptoms of patients at the first treatment and 6 months after the last session are shown in Table 2.
Table 2.
The subjective symptoms of patients at the first treatment and 6 months after the last session (3 = severe, 2 = moderate, 1 = mild, 0 = absent).
| No. | age | VAS of before the treatment
|
VAS of the last follow-up
|
||||
|---|---|---|---|---|---|---|---|
| itching | pain | dyspareunia | itching | pain | dyspareunia | ||
| 1 | 48 | 3 | 3 | 3 | 1 | 0 | 0 |
| 2 | 52 | 2 | 2 | 0 | 0 | 0 | 0 |
| 3 | 55 | 2 | 2 | 2 | 0 | 0 | 0 |
| 4 | 49 | 3 | 3 | 3 | 0 | 0 | 0 |
| 5 | 57 | 3 | 2 | 0 | 0 | 0 | 0 |
| 6 | 54 | 3 | 1 | 2 | 0 | 0 | 0 |
| 7 | 48 | 2 | 2 | 0 | 0 | 0 | 0 |
| 8 | 41 | 2 | 2 | 0 | 0 | 0 | 0 |
| 9 | 61 | 3 | 1 | 0 | 0 | 0 | 0 |
| 10 | 45 | 3 | 2 | 0 | 0 | 0 | 0 |
| Mean | 51 | 2.6 | 2.0 | 1.0 | 0.1 | 0 | 0 |
All patients showed a remarkable improvement in clinical signs at the six-month follow-up visit. The average score of clinical signs at baseline was significantly higher than the score recorded at last follow-up visit. Before the first PDT, all patients manifested depigmentation and atrophy, seven patients showed hyperkeratosis and five patients showed sclerosis in the vulval area. All patients showed a significant decrease in the size of the vulval lesions after treatment. The mean score at 6 months after the last session was lower than before treatment. Before treatment there were seven patients whose lesions were more than 60% of the vulvar surface and three patients involving 30–60%. After three treatments and six months follow-up visits, the area of all lesions in the vulval region reduced gradually in different degrees. No relapses were seen at the six-month follow-up visit. The therapeutic results and scores are presented in Fig. 1 and Table 3 respectively.
Fig. 1.
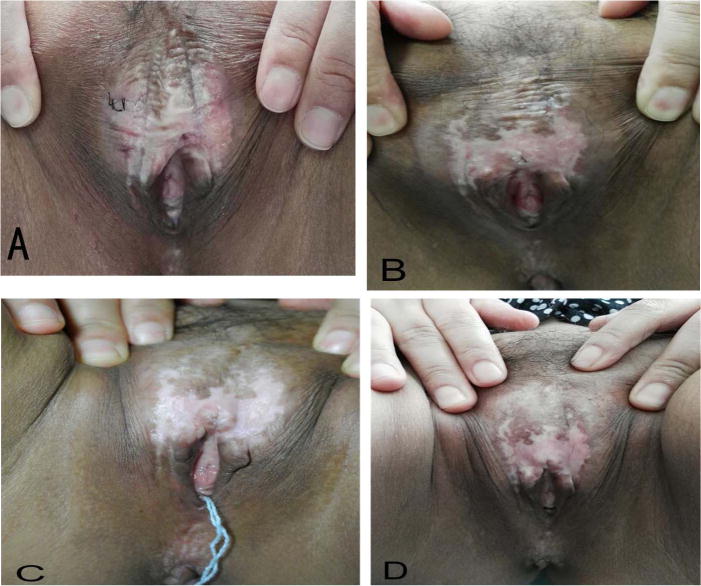
The macroscopy characteristics of vulvar lesions of one patient was received three times of ALA-PDT and followed-up in 6 months. (A) Pre-treatment. (B) One-month follow-up. (C) Three-month follow-up. (D) Six-month follow-up. These pictures show a significant clinical improvement with reduction of hyperkeratosis and decrease of the area of vulval lesions, even hypopigmentation was improved.
Table 3.
The clinical manifestation and lesions size and the scores before treatment and 6-months follow-up after the last session.
| No. | Baseline
|
Lesions size at baseline
|
Total scores | Last follow-up
|
Lesions size at last follow-up
|
Total scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| atrophy | depigmentation | hyperkeratosis | sclerosis | < 30% | 30–60% | > 60% | atrophy | depigmentation | hyperkeratosis | sclerosis | < 30% | 30–60% | > 60% | |||
| 1 | 2 | 3 | 1 | 1 | 0 | 0 | 1.5 | 8.5 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 4 |
| 2 | 2 | 3 | 2 | 2 | 0 | 0 | 1.5 | 10.5 | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 5 |
| 3 | 2 | 3 | 0 | 0 | 0 | 0 | 1.5 | 6.5 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| 4 | 2 | 3 | 1 | 1 | 0 | 0 | 1.5 | 8.5 | 1 | 2 | 0 | 1 | 0.5 | 0 | 0 | 4.5 |
| 5 | 3 | 3 | 2 | 0 | 0 | 0 | 1.5 | 9.5 | 1 | 2 | 0 | 0 | 0.5 | 0 | 0 | 3.5 |
| 6 | 2 | 3 | 2 | 2 | 0 | 0 | 1.5 | 10.5 | 0 | 1 | 0 | 1 | 0.5 | 0 | 0 | 2.5 |
| 7 | 2 | 3 | 0 | 0 | 0 | 1 | 0 | 6 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0.5 |
| 8 | 2 | 3 | 1 | 0 | 0 | 1 | 0 | 7 | 1 | 1 | 0 | 0 | 0.5 | 0 | 0 | 2.5 |
| 9 | 2 | 3 | 2 | 2 | 0 | 0 | 1.5 | 10.5 | 1 | 1 | 0 | 1 | 0.5 | 0 | 0 | 3.5 |
| 10 | 2 | 3 | 0 | 0 | 0 | 1 | 0 | 6 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0.5 |
| Mean | 2.1 | 3 | 1.1 | 0.8 | 0 | 0.3 | 1.05 | 8.35 | 0.6 | 1.1 | 0 | 0.5 | 0.35 | 0.3 | 0 | 2.85 |
3.2. Patients’ self-Evaluation
The mean scores of the dermatology life quality index (DLQI) and the scores of subjective symptoms reduced gradually over the course of the study. The mean scores of DLQI before treatment and one month, 3-month, 6-months follow-up are shown in Fig. 2. After three treatments the satisfaction scores showed that nine patients were “very satisfied” and one patient was “satisfied” because of residual mild itching. No patients were dissatisfied at the clinical response to the treatments. The average score of satisfaction with the therapeutic results was 2.9 at the last follow-up.
Fig. 2.
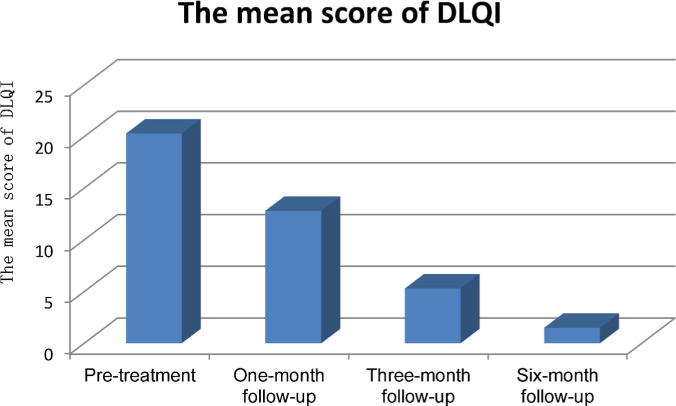
The mean scores of DLQI reported by patients at pretreatment, one, three and six-month follow-ups.
3.3. Adverse reactions
No severe adverse reactions appeared during the treatments or after the treatments. When treated with ALA-PDT, the main side effects were pain and burning sensation during the illumination period, which disappeared gradually within 3 h. All patients could tolerate the pain and no patients discontinued treatment or required analgesics. The treatment related pain scores reported by the patients ranged from 3 to 6 on a visual analogue scale of 10 and the average pain score was 3.93. Slight erythema and swelling were visible in all patients immediately after light irradiation in each session. These symptoms continued for 2–4 days, and mostly faded away by 3 days. Infections, hyperpigmentation, blistering or erosion were not observed during and after the treatments (Table 4).
Table 4.
Adverse reactions after treatment with ALA-PDT in patients.
| Adverse reactions | Value |
|---|---|
| Pain(incidence) | 10 |
| Pain (VAS/mean ± SD) | 3.93 ± 0.87 |
| Burning sensation(incidence) | 10 |
| Erythema(incidence) | 10 |
| Swelling(incidence) | 10 |
| Hyperpigmentation(incidence) | None |
| Infections(incidence) | None |
| Blister(incidence) | None |
| Erosion(incidence) | None |
4. Discussion
Lichen sclerosus et atrophicus (LSA) is a chronic inflammatory disease of the skin which appears mostly in the genital, perineal and perianal areas, and can cause intense discomfort include severe itching. Moreover it can cause narrowing of the vaginal and urethral entrances resulting in burning pain on urination and dyspareunia [15]. The disease can severely affect the quality of life and sexual well-being in patients, and is related to an increased risk of malignant transformation such as vulvar squamous cell carcinoma (SCC). Therefore, treatment is essential to relieve the symptoms and to prevent complications occurring. Various treatment methods have been tried with varying results. The current first-line therapy for VLSA is topical highly-potent corticosteroids, and 0.05% clobetasol propionate cream is considered the gold standard [16]. However, topical corticosteroids treatment can, not only cause relapse of symptoms, but can also cause side-effects include irritation, dryness, burning, dermal atrophy and hypopigmentation [10,15,16]. Therefore, more effective therapies with lower rates of side-effects and less incidence of relapse are necessary.
Recently, PDT has been widely applied to treat a diverse range of skin diseases and for non-melanoma skin tumors [12]. Light, oxygen, and a photosensitizer are the three basic components of PDT. The mechanism of PDT is based on the interaction of photosensitizer, visible light and oxygen, resulting in production of cytotoxic reactive oxygen species (such as singlet oxygen), which can damage cellular organelles, cause cell and tissue destruction, promote microvessel occlusion, and stimulate immune responses [12]. When 5-aminolevulinic acid (ALA) is applied to the surface of skin lesions, it penetrates through the skin barrier and accumulates selectively within the target cells, where is metabolized to the photoactive free porphyrin called protoporphyrin IX (PpIX, which is a heme precursor) [12]. When the optimum light is delivered to excite PpIX, it can produce singlet oxygen and destroy the target tissue. We applied 10% ALA to the lesions and occluded them with plastic film for 3 h to encourage ALA penetration, and followed this with irradiation using 100 mW/cm2 of 635 nm red light for 20 min, the results of the treatments were highly satisfactory. Pain, burning sensation and slight erythema and swelling were the main adverse effects in our study and all patients could tolerate them without any intervention.
Although there remain uncertainties about the exact mechanism to explain why ALA-PDT improves LSA lesions, a possibility is that PDT may affect the immunologic status of patients with LSA. This is particularly relevant as LSA is often associated with autoimmune diseases [5]. Moreover, Olejek et al. [17] performed immunohistochemical staining of biopsies and observed a decrease in inflammation and an increase in the number of microvessels in VLSA lesions after ALA-PDT.
Hillemanns et al. [18] first used PDT to treat 12 patients with VLSA and reported remission of symptoms, sustained over 6 months in 1999. Biniszkiewicz et al. [19] treated 24 patients with VLSA with three to six cycles of therapy at two-week intervals using PDT. All patients could tolerate the treatment procedure. The itching disappeared completely in 70.83% of the patients. Romero et al. [20] used PDT with two treatments at a 1-month interval to treat a single patient with recalcitrant erosive vulvar lichen sclerosus. The symptoms of the patient and the erosions and lesions had almost disappeared after treatment. The only discomfort was moderate pain during the treatment and it disappeared within several days. Sotiriou et al. [14] reported they applied PDT with two sessions at a 2-week interval in ten patients with vulvar lichen sclerosus. Treatment was well tolerated. All patients showed significant relief of symptoms and improved quality of life, but the objective clinical signs showed only a minor improvement in nine patients and one patient even showed no improvement. Sotiriouet al. [21] treated five patients with recalcitrant vulvar lichen sclerosis with ALA-PDT with a single session and reported complete alleviation of symptoms sustained over 3 months, but again with only minor improvement in the appearance of the vulval lesions. Imbernón-Moya et al. [22] used PDT to treat eight patients with vulvar lichen sclerosus. All patients could tolerate the treatments. After treatment, all patients showed significant improvement in symptoms and quality of life, but no improvement in the clinical signs. In our study, we treated 10 patients with VLSA by using PDT and achieved clear improvements of both subjective symptoms and also improvement in the clinical signs. Some lesions with depigmentation showed repigmentation at 6 months. The results were similar to those reported previously [14,18–23]. The mean VAS scores of subjective symptoms were clearly lower after treatment than before. There was no recurrence of the symptoms within the six-month follow-up. Among the ten patients, nine patients were “very satisfied” and only one patient was just “satisfied” with the treatment results. The quality of life of all patients improved and the mean score of the dermatology life quality index (DLQI) along with the reduction or disappearance of subjective symptoms reduced gradually over the treatment course. This confirms that subjective symptoms are closely related to quality of life.
In conclusion, our study provides evidence that ALA-PDT is a highly effective and safe method to treat VLSA, especially in patients who have failed conventional therapy, and who could therefore be considered “recalcitrant”. No severe adverse reactions appeared during the course of the treatment or post-treatment and all patients tolerated the therapy well. Although our study produced a highly satisfactory treatment outcome, the small sample size and short follow-up time were the main limitations. Therefore we suggest much larger trials of the effectiveness and safety of ALA-PDT in VLSA, should be conducted with longer follow-up periods, larger number of patients, and good objective evaluation of clinical lesions. Moreover, up to now there no consensus about parameters, such as concentration of ALA, incubation time, light source (power and wavelength), exposure time (energy density and power density), and number and frequency of treatment repetitions to treat VLSA. Therefore, considering the large potential benefit to patients, a multi-center clinical trial is needed to arrive at optimal parameters of PDT in a future study.
Supplementary Material
Acknowledgments
Dr. Rui Yin received research grant (No. 81571902) from National Natural Science Foundation of China and clinical research grant (SWH2014LC17) from Southwest Hospital, Third Military Medical University for this work. Michael R Hamblin was supported by US NIH grants R01AI050875 and R21AI121700.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.pdpdt.2017.12.003.
Footnotes
Conflict of interest disclosures
No authors hold stock, or receive royalties from any companies.
References
- 1.Doulaveri G, Armira K, Kouris A, et al. Genital vulvar lichen sclerosus in monozygotic twin women: a case report and review of the literature. Case Rep Dermatol. 2013;5(3):321–325. doi: 10.1159/000356775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedrich EG, Jr, Kalra PS. Serum levels of sex hormones in vulvar lichen sclerosus and the effect of topical testosterone. N Engl J Med. 1984;310(8):488–491. doi: 10.1056/NEJM198402233100803. [DOI] [PubMed] [Google Scholar]
- 3.Aidé S, Lattario FR, Almeida G, et al. Epstein-barr virus and human papillomavirus infection in vulvar lichen sclerosus. J Low Genit Tract Dis. 2010;14(4):319–322. doi: 10.1097/LGT.0b013e3181d734f1. [DOI] [PubMed] [Google Scholar]
- 4.Cooper SM, Ali I, Baldo M, Wojnarowska F. The association of lichen sclerosus and erosive lichen planus of the vulva with autoimmune disease: a case–control study. Arch Dermatol. 2008;144(11):1432–1435. doi: 10.1001/archderm.144.11.1432. [DOI] [PubMed] [Google Scholar]
- 5.Olejek A, Gabriel I, Bilska-Janosik A, et al. ALA – photodynamic treatment in Lichen sclerosus – clinical and immunological outcome focusing on the assessment of antinuclear antibodies. Photodiagn Photodyn Ther. 2017;18(6):128–132. doi: 10.1016/j.pdpdt.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Halonen P, Jakobsson M, Heikinheimo O, et al. Lichen sclerosus and risk of cancer. Int J Cancer. 2017;140(9):1998–2002. doi: 10.1002/ijc.30621. [DOI] [PubMed] [Google Scholar]
- 7.Smith YR, Haefner HK. Vulvar lichen sclerosus: pathophysiology and treatment. Am J Clin Dermatol. 2004;5(2):105–125. doi: 10.2165/00128071-200405020-00005. [DOI] [PubMed] [Google Scholar]
- 8.Neill SM, Tatnall FM, Cox NH. British Association of Dermatologists: guidelines for the management of lichen sclerosus. Br J Dermatol. 2002;147(4):640–649. doi: 10.1046/j.1365-2133.2002.05012.x. [DOI] [PubMed] [Google Scholar]
- 9.Peterson CM, Lane JE, Ratz JL. Successful carbon dioxide laser therapy for refractory anogenital lichen sclerosus. Dermatol Surg. 2004;30(8):1148–1151. doi: 10.1111/j.1524-4725.2004.30343.x. [DOI] [PubMed] [Google Scholar]
- 10.Hengge UR, Ruzicka T, Schwartz RA, et al. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1–15. doi: 10.1016/j.jaad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Burger MP, Obdeijn MC. Complications after surgery for the relief of dyspareunia in women with lichen sclerosus: a case series. Acta Obstet Gynecol Scand. 2016;95(4):467–472. doi: 10.1111/aogs.12852. [DOI] [PubMed] [Google Scholar]
- 12.Wen X, Li Y, Hamblin MR. Photodynamic therapy in dermatology beyond non-melanoma cancer: an update. Photodiagn Photodyn Ther. 2017;19(9):140–152. doi: 10.1016/j.pdpdt.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 14.Sotiriou E, Panagiotidou D, Ioannidis D. An open trial of 5-aminolevulinic acid photodynamic therapyfor vulvar lichen sclerosus. Eur J Obstet Gynecol Reprod Biol. 2008;141(2):187–188. doi: 10.1016/j.ejogrb.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-López FR, Ceausu I, Depypere H, et al. EMAS clinical guide: vulvar lichen sclerosus in peri and postmenopausal women. Maturitas. 2013;74(3):279–282. doi: 10.1016/j.maturitas.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Terras S, Gambichler T, Moritz RK, et al. UV-A1 phototherapy vs clobetasol propionate, 0.05%, in the treatment of vulvar lichen sclerosus: a randomized clinical trial. JAMA Dermatol. 2014;150(6):621–627. doi: 10.1001/jamadermatol.2013.7733. [DOI] [PubMed] [Google Scholar]
- 17.Olejek A, Kozak-Darmas I, Kellas-Sleczka S, et al. Effectiveness of photodynamic therapy in the treatment of lichen sclerosus: cell changes in immunohistochemistry. Neuro Endocrinol Lett. 2009;30(4):547–551. [PubMed] [Google Scholar]
- 18.Hillemanns P, Untch M, Pröve F, et al. Photodynamic therapy of vulvar lichen sclerosus with 5-aminolevulinic acid. Obstet Gynecol. 1999;93(1):71–74. doi: 10.1016/s0029-7844(98)00321-4. [DOI] [PubMed] [Google Scholar]
- 19.Biniszkiewicz T, Olejek A, Kozak-Darmas I, et al. Therapeutic effects of 5-Ala-induced photodynamic therapy in vulvar lichen sclerosus. Photodiagn Photodyn Ther. 2005;2(2):157–160. doi: 10.1016/S1572-1000(05)00062-1. [DOI] [PubMed] [Google Scholar]
- 20.Romero A, Hernández-Núñez A, Córdoba-Guijarro S, et al. Treatment of recalcitrant erosive vulvar lichensclerosus with photodynamic therapy. J Am Acad Dermatol. 2007;57(2):46–47. doi: 10.1016/j.jaad.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Sotiriou E, Apalla Z, Patsatsi A, et al. Recalcitrant vulvar lichen sclerosis treated with aminolevulinic acid-photodynamic therapy: a report of five cases. J Eur Acad Dermatol Venereol. 2008;22(11):1398–1399. doi: 10.1111/j.1468-3083.2008.02661.x. [DOI] [PubMed] [Google Scholar]
- 22.Imbernón-Moya A, Martínez-Pérez M, Churruca-Grijelmo M, et al. Photodynamic therapy as a therapeutic alternative in vulvar lichen sclerosus: series of 8 cases. Photodermatol Photoimmunol Photomed. 2016;32(5–6):307–310. doi: 10.1111/phpp.12242. [DOI] [PubMed] [Google Scholar]
- 23.Osiecka BJ, Nockowski P, Jurczyszyn K, et al. Photodynamic therapy of vulvar lichen sclerosus et atrophicus in a woman with hypothyreosis? case report. Photodiagnosis Photodyn Ther. 2012;9(2):186–188. doi: 10.1016/j.pdpdt.2012.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


