Abstract
A strong positive association has been observed between circulating anti-Müllerian hormone (AMH), a biomarker of ovarian reserve, and breast cancer risk in three prospective studies. Confirming this association is important because of the paucity of biomarkers of breast cancer risk in premenopausal women. We conducted a consortium study including ten prospective cohorts that had collected blood from premenopausal women. A nested case-control design was implemented within each cohort. A total of 2,835 invasive (80%) and in situ (20%) breast cancer cases were individually matched to controls (n = 3,122) on age at blood donation. AMH was measured using a high sensitivity enzyme-linked immunoabsorbent assay. Conditional logistic regression was applied to the aggregated dataset. There was a statistically significant trend of increasing breast cancer risk with increasing AMH concentration (ptrend across quartiles < 0.0001) after adjusting for breast cancer risk factors. The odds ratio (OR) for breast cancer in the top versus bottom quartile of AMH was 1.60 (95% CI = 1.31-1.94). Though the test for interaction was not statistically significant (pinteraction = 0.15), the trend was statistically significant only for tumors positive for both estrogen receptor (ER) and progesterone receptor (PR): ER+/PR+: ORQ4-Q1 = 1.96, 95% CI = 1.46-2.64, ptrend <0.0001; ER+/PR-: ORQ4-Q1 = 0.82, 95% CI = 0.40-1.68, ptrend = 0.51; ER-/PR+: ORQ4-Q1 = 3.23, 95% CI =0.48-21.9, ptrend = 0.26; ER-/PR-: ORQ4-Q1 = 1.15, 95% CI = 0.63-2.09, ptrend = 0.60. The association was observed for both pre- (ORQ4-Q1= 1.35, 95% CI= 1.05-1.73) and post-menopausal (ORQ4-Q1 =1.61, 95% CI = 1.03 - 2.53) breast cancer (pinteraction = 0.34). In this large consortium study, we confirmed that AMH is associated with breast cancer risk, with a 60% increase in risk for women in the top vs. bottom quartile of AMH.
Keywords: Breast cancer, anti-mullerian hormone, AMH, nested case-control study
Introduction
Anti-Müllerian hormone (AMH) is produced in the ovaries by the granulosa cells of pre-antral and early antral follicles 1. Circulating AMH is present in females at birth, peaks around age 20-25, and becomes undetectable after menopause, when the ovarian follicle reserve is depleted 2. AMH concentration has been shown to reflect the size of the follicular pool 3 and is a strong predictor of age at menopause 4-6.
The hypothesis that AMH plays a role in breast cancer development came from laboratory experiments that showed AMH stimulates apoptosis and reduces breast tumor growth 7-9, suggesting a protective role. On the other hand, the strong positive correlation of AMH with age at menopause suggests that women who have higher AMH could be at higher risk of breast cancer than women of the same age with lower AMH, because they are expected to reach menopause at a later age and thus have longer remaining duration of exposure to high concentrations of steroid sex hormones 10, 11.
A small cross-sectional study reported an inverse association of AMH concentration with breast cancer 12 and a case-control study found no association 13. However, AMH was measured at or after diagnosis, and might not reflect the AMH concentration before cancer development. In 2009, Dorgan et al. reported a strong positive association between AMH concentration and risk of breast cancer in a case-control study nested within the Columbia, Missouri Serum Bank 14. Subsequently, two other reports from prospective studies (the Sister Study 15 and the Nurses' Health Studies (NHS and NHSII) 16) also reported a positive, though weaker, association. Confirming the AMH-risk association is important because of the paucity of biomarkers in premenopausal women: while sex hormones (estrogens and androgens) measured in postmenopausal women are strongly associated with breast cancer risk 17, they show only weak associations when measured in premenopausal women 18, 19.
We report here on a collaborative study that had for objectives to confirm the AMH-breast cancer risk association in a large study and to examine this association in relevant subgroups (i.e., by invasiveness, tumor receptor status, menopausal status at diagnosis, and various baseline characteristics). Ten prospective cohorts participated, including the four cohorts that previously published on this topic.
Methods
Study Design and Case and Control Selection
The ten participating cohorts are: Breakthrough Generations Study (BGS); Campaign Against Cancer and Heart Disease (CLUE II); Columbia, Missouri Serum Bank (CSB); Guernsey cohort (Guernsey); Nurses' Health Study (NHS); Nurses' Health Study II (NHSII); Northern Sweden Mammography Screening Cohort (NSMSC); New York University Women's Health Study (NYUWHS); Hormones and Diet in the Etiology of Breast Cancer (ORDET); and the Sister Study. These cohorts are briefly described in Table 1. Each cohort was approved by its institutional review board.
Table 1. Participating cohorts, sample collection and storage, and number and characteristics of cases and controls.
| Cohort1 | Country | Source population | Years of blood draw | Sample type used in study | Storage temperature | Effective cohort size2 | Cases/ Controls | Median age at blood donation in controls yr (min-max) | Median time to diagnosis, yr (min-max) |
|---|---|---|---|---|---|---|---|---|---|
| BGS 1 | UK | General population | 2003-2010 | Plasma | -180°C | 46,344 | 439/439 | 44.0 (21.0-57.0) | 3.0 (0.0-9.0) |
| CLUE II 2, 3 | USA | Residents of Washington County, MD | 1989 | Plasma | -70°C | 2,899 | 136/136 | 40.0 (22.0-49.0) | 13.5 (0.7-23.5) |
| CSB 4, 5 | USA | Attendees of breast cancer screening centers in Columbia, Missouri | 1977-1987 | Serum | -70°C | 2,459 | 101/101 | 44.6 (33.3-54.7) | 16.6 (0.2-23.3) |
| Guernsey 6, 7 | UK | General population | 1977-1990 | Serum | -20°C | 3,120 | 176/176 | 40.1 (32.0-53.5) | 16.7 (0.6-30.4) |
| NHS 8 | USA | Nurses | 1989-1990 | Plasma | -130°C | 6,926 | 136/136 | 46.7 (43.0-53.8) | 4.6 (0.1-13.8) |
| NHSII 9, 10 | USA | Nurses | 1996-1999 | Plasma | -130°C | 22,000 | 395/395 | 42.8 (33.1-52.2) | 4.9 (0.1-13.3) |
| NSMSC 11, 12 | Sweden | Attendees of a population-based screening program in Västerbotten | 1995-2006 | Plasma | -80°C | 3,569 | 66/66 | 49.5 (39.6-53.3) | 6.1 (0.0-13.6) |
| NYUWHS 13, 14 | USA | Attendees of a breast cancer screening center, NYC | 1985-1991 | Serum | -80°C | 7,222 | 749/749 | 44.2 (34.3-56.5) | 12.8 (0.6-24.5) |
| ORDET 15 | Italy | Residents in Varese Province | 1987-1992 | Serum | -80°C | 5,942 | 263/263 | 44.4 (35.2-54.1) | 9.7 (0.3-19.2) |
| Sister Study 16 | USA | Sisters of women with breast cancer | 2003-2009 | Serum | -180°C | 14,772 | 374/661 | 46.5 (35.1-54.6) | 2.8 (0.0-8.4) |
Cohort abbreviations: BGS: Breakthrough Generations Study; CLUE II: Campaign Against Cancer and Heart Disease; CSB: Columbia, Missouri Serum Bank; NHS: Nurses' Health Study; NHSII: Nurses' Health Study II; NSMSC: Northern Sweden Mammography Screening Cohort; NYUWHS: New York University Women's Health Study; ORDET: Hormones and Diet in the Etiology of Breast Cancer.
Participants who would have been eligible if diagnosed with breast cancer during follow-up (i.e. female participants with blood collected prior to menopause).
A nested case-control design was used. With the exception of the Sister Study, which joined this collaborative effort later 15, all cohorts used the same general selection procedures. Eligibility criteria for cases and controls were: 1) premenopausal women of any age (or age <50 years if menopausal status was unknown, for example due to hysterectomy) at blood donation; 2) no prior diagnosis of cancer (except non-melanoma skin cancer); 3) no history of bilateral oophorectomy; and 4) no current or prior use of hormone therapy. Incident cases of invasive or in situ breast cancer were included. Within each cohort, one control was selected for each case using incidence density sampling; matching factors included age and date at blood donation (age-matching criteria for different cohorts ranged from age ±6 mo to ±2 yrs, except ORDET which matched on age ±3 yrs and CSB which used ±5 yrs). Only 162 (6%) had a difference in age ≥ 2 years and only 30 case-control pairs (1%) had a difference in age ≥ 3 years. Some cohorts had additional matching criteria (appendix Table 1 14-16, 19-27). In the NHS and NHSII, cases diagnosed after menopause were not included 16. The differences in procedures for the Sister Study 15 were: 1) in addition to being premenopausal at blood donation, women had to be between the ages of 35 and 54; 2) two controls were selected for each case; and 3) women reporting use of hormone therapy were included in the initial study but are excluded from this report.
Laboratory Assays
With the exception of the Sister Study, AMH concentration was measured using a picoAMH enzyme-linked immunoabsorbent assay (Ansh Labs, Webster, TX). NYUWHS samples were measured at Massachusetts General Hospital (MGH) and samples from the other eight cohorts were subsequently measured at Ansh Labs due to the closure of the MGH laboratory. Each batch (up to 70 samples per batch) contained 2-4 blinded quality control samples. Samples from a case and her matched control(s) were assayed together in the same batch. The samples were labeled in such a way that the laboratory was blinded with respect to case/control or quality control status. The overall cohort-specific coefficients of variation (CVs) were <10%, except for the NYUWHS (CV = 17%). The Sister Study samples were measured at the University of Southern California using an Ultrasensitive ELISA (Ansh Labs, Webster, TX), and samples below the lower limit of detection of this assay (0.5 pmol/l) were re-measured using the picoAMH ELISA. The inter-batch CVs in the Sister Study were 14.5% 15.
We conducted a calibration study to examine how NYUWHS and Sister Study measurements compared to measurements performed at Ansh Labs, where the samples from the 8 other cohorts were analyzed. Excellent agreement (intraclass as well as Pearson correlations > 0.98, Appendix Figure 1) was found for both cohorts. Thus, we did not calibrate the AMH measurements.
Testosterone had been measured previously for 70% of the matched sets using methods described in 15, 19, 24, 25, 28-30 (see also Supplementary Methods). For the remaining 30% (all sets from CLUE II, NHS, and NSMSC plus a subset of sets from Guernsey, NYUWHS, and ORDET cohorts), testosterone was newly measured at the Mayo Clinic Endocrine Laboratory using LC-MS/MS. Intra- and inter-batch CVs were <7% and <9%, respectively. Previous testosterone measurements were calibrated to the Mayo Clinic LC-MS/MS assay (see Supplementary Methods).
Covariate Data
Each cohort sent individual data on breast cancer risk factors and factors possibly related to AMH concentration to NYU, where data harmonization was conducted. Data collected closest to blood draw were used. Data on subsequent age at menopause were also obtained (except for CSB and NSMSC which did not send follow-up questionnaires).
Statistical Analysis
Subjects whose AMH concentration was below the lowest detectable value (range <2%-18% depending on cohort, Table 3) were assigned the lowest detectable value (LDV) for their cohort (LDV differed by cohort due to different dilution factors) divided by √2. Samples with AMH above the highest detectable value (n=14) were set to the highest detectable value. AMH concentration was log2-transformed to normalize its distribution.
Table 3. AMH assay, lowest detected value (LDV) and AMH geometric means (95% CIs) for cases and controls.
| Cohort1 | Assay2 | LDV3, pmol/l | < LDV, % | Geometric mean4 (95% CI), pmol/l | Age-adjusted geometric mean4 (95% CI), pmol/l | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Cases | Controls | Cases | Controls | Cases | Controls | |||
| BGS | picoAMH ELISA | 0.0165 | 4.1 | 5.9 | 2.57 (2.12, 3.11) | 2.33 (1.91, 2.86) | 2.31 (2.00,2.67) | 1.95 (1.68,2.27) |
| CLUE II | picoAMH ELISA | 0.0165 | 3.7 | 2.9 | 4.71 (3.29, 6.75) | 4.14 (2.91, 5.90) | 1.85 (1.41,2.42) | 1.52 (1.14,2.01) |
| CSB | picoAMH ELISA | 0.0330 | 5.0 | 12.9 | 2.52 (1.67, 3.81) | 1.39 (0.91, 2.13) | 2.90 (2.15,3.92) | 1.61 (1.17,2.20) |
| Guernsey | picoAMH ELISA | 0.0264 | 5.7 | 2.8 | 3.12 (2.33, 4.17) | 3.68 (2.84, 4.78) | 1.29 (1.03,1.63) | 1.36 (1.07,1.73) |
| NHS | picoAMH ELISA | 0.0165 | 4.4 | 10.3 | 2.03 (1.45, 2.83) | 1.03 (0.71, 1.52) | 4.21 (3.24,5.46) | 2.22 (1.69,2.92) |
| NHSII | picoAMH ELISA | 0.0165 | 1.5 | 1.5 | 6.77 (5.83, 7.87) | 5.21 (4.47, 6.06) | 4.55 (3.90,5.30) | 3.15 (2.68,3.70) |
| NSMSC | picoAMH ELISA | 0.0165 | 6.1 | 7.6 | 1.00 (0.58, 1.70) | 0.71 (0.43, 1.18) | 2.98 (2.05,4.33) | 2.23 (1.51,3.31) |
| NYUWHS | picoAMH ELISA | 0.143 | 15.4 | 15.6 | 2.54 (2.21, 2.92) | 2.32 (2.02, 2.67) | 2.76 (2.47,3.08) | 2.40 (2.14,2.70) |
| ORDET | picoAMH ELISA | 0.0264 | 3.8 | 9.5 | 2.84 (2.25, 3.58) | 1.93 (1.48, 2.51) | 2.79 (2.31,3.36) | 1.93 (1.59,2.34) |
| Sister Study | Ultrasensitive & picoAMH ELISA5 | 0.0214 | 16.0 | 18.5 | 1.20 (0.93, 1.54) | 1.03 (0.85, 1.25) | 2.30 (1.96,2.70) | 1.80 (1.59,2.05) |
Cohort abbreviations: BGS: Breakthrough Generations Study; CLUE II: Campaign Against Cancer and Heart Disease; CSB: Columbia, Missouri Serum Bank; NHS: Nurses' Health Study; NHSII: Nurses' Health Study II; NSMSC: Northern Sweden Mammography Screening Cohort; NYUWHS: New York University Women's Health Study; ORDET: Hormones and Diet in the Etiology of Breast Cancer.
Assays were conducted at Ansh Labs, except for the NYUWHS (Core Laboratory, Massachusetts General Hospital Pathology Service) and the Sister Study (Reproductive Endocrinology Laboratory, University of Southern California).
LDV varied depending on the dilution factor used.
Subjects with AMH measurement below the LDV were assigned the value of LDV divided by the square root of 2. Age-adjusted means adjusted for age and age-squared. Samples with AMH above the highest detectable value (n=14 total, 3 from CLUE II and 11 from NYUWHS) were set to the highest detectable value.
All samples were measured using the Ultrasensitive assay; samples with AMH concentration < the LDV of the ultrasensitive assay (0.500 pmol/l) were re-measured using the picoAMH ELISA assay.
Conditional logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (95% CIs) for the association of AMH with breast cancer risk. Our main analyses were based on cohort-specific quartiles, defined using the controls' distribution. We also conducted analyses using consortium-wide quartiles. Restricted cubic splines were used to assess deviation from linearity 31.
Because the number of cases in some cohorts was fairly small, which was a concern for subgroup analyses, our main analyses are based on the aggregated data, i.e. combining individual data from all cohorts. We also conducted an analysis using a two-stage approach, estimating ORs within each cohort prior to pooling using a random-effects model 32.
Potential confounders included in the multivariate model were: race, education, BMI, age at menarche, parity, age at first full-term pregnancy (FTP), oral contraceptive use, partial/unilateral oophorectomy, family history of breast cancer, history of benign breast biopsy, and smoking. For all continuous variables, only a small proportion (< 3%) of data was missing and we used the cohort-specific median for imputation. For categorical variables with missing data, an ‘unknown’ category was created. We also conducted analyses adjusting for total testosterone (ordered cohort-specific quartiles) in addition to these factors.
Stratified analyses were conducted to examine whether the AMH-breast cancer risk association varied according to participant or tumor characteristics. All tests for heterogeneity and effect modification were performed by comparing models with/without an interaction term between the covariate and ordered categorical AMH. The Wald test was used to assess the statistical significance of the interactions. All tests for interaction used cohort-specific AMH quartiles (coded as ordered categories 1, 2, 3, 4) and each of the other variables as categorical variables with unordered levels (as shown in the tables). For analyses stratified by age-related covariates (age at blood draw, age at diagnosis/index date (for controls, the date of diagnosis of the matched case), and menopausal status at diagnosis/index date), we used AMH quartiles based on the controls' distribution within each of four age-at-blood-draw categories (≤40, 41-44, 45-49, ≥50) within each cohort. The unconditional logistic regression model, adjusted for age at blood draw and cohort, gave results very similar to the conditional model; therefore, we used unconditional logistic regression, adjusting for age and cohort, in analyses stratified by characteristics which were not matching variables, in order to include the maximum number of subjects in the analysis.
All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). All tests were two-sided and were considered statistically significant if p < 0.05.
Results
A total of 2,835 breast cancer cases and 3,122 controls were included in the study. Participant characteristics are described in Table 2 for the whole consortium, and in Appendix Table 2 for each cohort. The majority of subjects (>65%) were between the ages of 40 and 49 at blood draw. Overall, the differences between cases and controls were as expected. Controls had a higher proportion of obese women than cases, as expected in premenopausal women. More cases than controls were nulliparous or had their first FTP after age 30. Cases were more likely to have a first-degree family history of breast cancer and a history of benign breast biopsy. The proportion of current users of oral contraceptives was small (cases: 6.2%, controls: 5.7%), reflecting the fact that this was an exclusion criterion in several cohorts.
Table 2. Baseline characteristics of cases and controls.
| Characteristic1 | Cases (N = 2835) | Controls (N = 3122) | P-value2 |
|---|---|---|---|
|
| |||
| N (%) | N (%) | ||
| Age at blood draw, years | Matched | ||
| <35 | 108 (3.8%) | 111 (3.6%) | |
| 35-39 | 534 (18.8%) | 535 (17.1%) | |
| 40-44 | 897 (31.6%) | 999 (32.0%) | |
| 45-49 | 966 (34.1%) | 1117 (35.8%) | |
| 50-54 | 318 (11.2%) | 349 (11.2%) | |
| 55+ | 12 (0.4%) | 11 (0.4%) | |
| Race/ethnicity1 | 0.75 | ||
| White | 2562 (93.7%) | 2800 (93.9%) | |
| Black/African American | 118 (4.3%) | 120 (4.0%) | |
| Other | 53 (1.9%) | 61 (2.0%) | |
| Education1 | 0.02 | ||
| High school or less | 759 (30.2%) | 873 (30.8%) | |
| Some college/university, vocational training or more | 1758 (69.8%) | 1963 (69.2%) | |
| BMI1, kg/m2 | 0.043 | ||
| <18.5 | 51 (1.8%) | 57 (1.8%) | |
| 18.5-24.9 | 1702 (60.4%) | 1779 (57.4%) | |
| 25-29.9 | 710 (25.2%) | 777 (25.0%) | |
| 30+ | 353 (12.5%) | 489 (15.8%) | |
| Age at menarche, years | 0.443 | ||
| <12 | 603 (21.7%) | 659 (21.6%) | |
| 12 | 788 (28.3%) | 803 (26.3%) | |
| 13 | 786 (28.2%) | 903 (29.5%) | |
| 14+ | 606 (21.8%) | 692 (22.6%) | |
| Parity1 | 0.053 | ||
| 0 | 680 (24.6%) | 710 (23.3%) | |
| 1 | 400 (14.5%) | 435 (14.3%) | |
| 2 | 1028 (37.2%) | 1138 (37.4%) | |
| 3+ | 653 (23.7%) | 758 (24.9%) | |
| Age at first full-term pregnancy1, years | 0.0033 | ||
| <20 | 161 (7.5%) | 226 (9.4%) | |
| 21-24 | 696 (32.4%) | 825 (34.4%) | |
| 25-29 | 784 (36.5%) | 834 (34.8%) | |
| ≥30 or nulliparous | 506 (23.6%) | 515 (21.5%) | |
| Oral contraceptive use1 | 0.15 | ||
| Never user | 736 (26.9%) | 772 (25.5%) | |
| Former user | 1830 (66.9%) | 2083 (68.8%) | |
| Current user | 171 (6.2%) | 174 (5.7%) | |
| Partial oophorectomy1 | 0.02 | ||
| No | 2747 (97.3%) | 2989 (96.1%) | |
| Yes | 76 (2.7%) | 120 (3.9%) | |
| Family history of breast cancer4 | <0.001 | ||
| No | 1984 (80.6%) | 2143 (87.1%) | |
| Yes | 477 (19.4%) | 318 (12.9%) | |
| Benign breast biopsy1 | <0.001 | ||
| No | 2096 (75.8%) | 2511 (82.3%) | |
| Yes | 669 (24.2%) | 541 (17.7%) | |
| Smoking status1 | 0.02 | ||
| Never | 1576 (58.8%) | 1847 (62.5%) | |
| Former | 752 (28.1%) | 751 (25.4%) | |
| Current | 352 (13.1%) | 359 (12.1%) | |
Missing data: race/ethnicity: 4.1%; education: 10.1%; BMI: 0.7%; age at menarche: 2.0%; parity: 2.6%; age at first full-term pregnancy: 0.2%; oral contraceptive use: 3.2%; partial oophorectomy: 0.4%; benign breast biopsy: 2.4%; smoking status: 5.4%.
p-value from conditional logistic regression model
p for trend from conditional logistic regression model for ordered categorical variable
Calculated after excluding the Sister Study (all participants in this study have a family history of breast cancer).
The AMH assay results are shown in Table 3. The geometric mean AMH for controls varied by cohort, with a >4-fold difference between the lowest and highest values (0.71 pmol/l in the NSMSC and 5.21 pmol/l in NHSII). Adjusting for age, which was strongly related to AMH (Spearman correlation coefficient = -0.67), reduced these differences (2.3-fold difference: 1.36 pmol/l in Guernsey to 3.15 pmol/l in NHSII), though they remained statistically significant. In all cohorts except Guernsey, the age-adjusted geometric mean for cases was higher than for controls.
The ORs for breast cancer in relation to AMH quartiles are shown in Table 4. In univariate analysis, there was a statistically significant trend of increasing risk with increasing AMH concentration (ORQ4-Q1 = 1.64 (95% CI= 1.35-1.98); ptrend < 0.0001). Results were similar after adjustment for potential confounders (ORQ4-Q1 = 1.60, 95% CI= 1.31-1.94; ptrend < 0.0001). Further adjusting for testosterone did not substantially alter the ORs, nor did removing one cohort at a time (data not shown). The ORs remained statistically significant after simultaneously excluding the four cohorts that published previously (ORQ4-Q1= 1.38, 95% CI= 1.07-1.79). Odds ratios were not appreciably different in analyses using consortium-wide AMH quartiles (Appendix Table 3). The spline analysis showed no evidence of deviation from linearity (p = 0.13). Results were similar in the two-stage analysis (multivariate-adjusted ORQ4-Q1 = 1.66, 95% CI= 1.30-2.12; Figure 1), which showed no evidence of heterogeneity by cohort (I2 = 22.7%, p = 0.23).
Table 4. Odds ratios (ORs) and 95% confidence intervals (95% CIs) for breast cancer associated with AMH concentration.
| AMH quartiles1 | Ptrend5 | ||||
|---|---|---|---|---|---|
|
| |||||
| Q1 | Q2 | Q3 | Q4 | ||
| Cases/Controls | 631/789 | 684/777 | 711/779 | 809/777 | . |
| Unadjusted OR2 (95% CI) | 1.00 (Referent) | 1.20 (1.02, 1.41) | 1.35 (1.14, 1.61) | 1.64 (1.35, 1.98) | <.0001 |
| Adjusted OR3 (95% CI) | 1.00 (Referent) | 1.18 (1.00, 1.39) | 1.32 (1.10, 1.58) | 1.60 (1.31, 1.94) | <.0001 |
| Adjusted OR3 (95% CI), among women with testosterone measurements | 1.00 (Referent) | 1.18 (0.99, 1.40) | 1.34 (1.11, 1.61) | 1.62 (1.32, 1.98) | <.0001 |
| Adjusted OR4 (95% CI), including adjustment for testosterone | 1.00 (Referent) | 1.17 (0.99, 1.40) | 1.33 (1.10, 1.60) | 1.58 (1.29, 1.93) | <.0001 |
Defined using cohort-specific cutpoints.
Estimated using conditional logistic regression (cohort and age are adjusted for through matching).
Estimated using conditional logistic regression and adjusting for race/ethnicity (white, black, other or unknown), education (high school or less, some college or higher, unknown), BMI (ordered categorical, <18.5, 18.5-25, 25-30, 30+ kg/m2), age at menarche (ordered categorical, <12, 12, 13, 14+ years), parity (ordered categorical, 0, 1, 2, 3+), age at 1st FTP (ordered categorical, <=20, 21-25, 26-30, 30+ years or nulliparous), oral contraceptive use (never, former, current, unknown), partial oophorectomy (no, yes, unknown), family history of breast cancer (no, yes), benign breast biopsy (no, yes, unknown), and smoking status (never, former, current, unknown).
Estimated using conditional logistic regression and adjusting for variables in footnote 2 and testosterone (cohort-specific quartiles, with measurements from previous studies calibrated to the Mayo LC-MS/MS assay).
Ptrend was calculated using ordered-categorical AMH.
Figure 1. Cohort-specific associations between AMH and breast cancer risk (ORs and 95% CIs for the 4th quartile vs. 1st quartile)1.
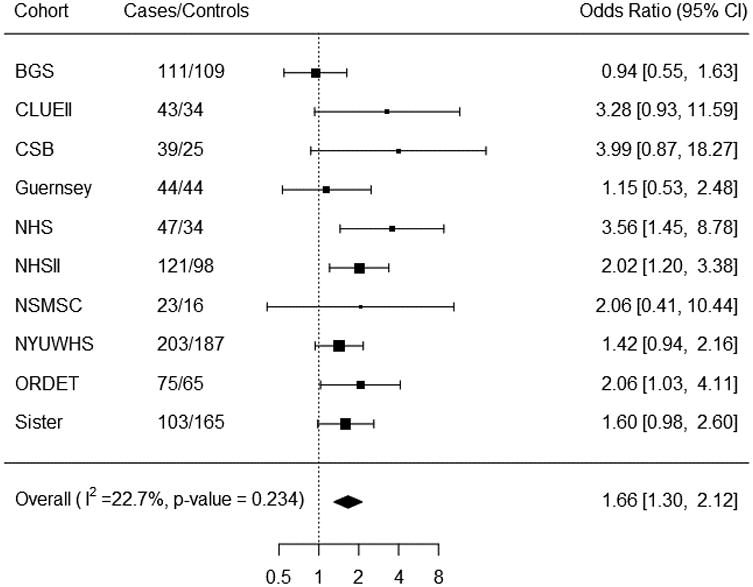
Analyses stratified by tumor characteristics are shown in Table 5. We did not see evidence of heterogeneity by invasive/in situ status. While several assessments of joint receptor status have supported the idea that ER-/PR+ tumors occur infrequently 33-36, others did not find this joint receptor subtype to be reproducible 37-39. Because there has not yet been a resolution and we did not have the tumor tissues to re-assess receptor status with current IHC methods, we show analyses both by single and joint ER/PR receptor status. Although the interaction test was not statistically significant (pinteraction = 0.21), the association between AMH and risk was statistically significant for ER+ (ORQ4-Q1 = 1.74, 95% CI = 1.33-2.28; ptrend <0.0001) but not for ER- tumors (ORQ4-Q1 = 1.17, 95% CI = 0.68-2.01; ptrend = 0.54). Heterogeneity was observed for PR status (pinteraction = 0.02), with a statistically significant association for PR+ tumors (ORQ4-Q1 = 1.97, 95% CI = 1.48-2.64; ptrend <0.0001) but no association for PR- tumors (ORQ4-Q1 = 1.00, 95% CI = 0.65-1.55; ptrend = 0.95). Though there was no statistically significant heterogeneity (pinteraction = 0.15) in the analysis by combined ER/PR status, the trend test was significant only for ER+/PR+. No statistically significant heterogeneity was observed between HER2+ and HER2- tumors (pinteraction = 0.37). No association was seen for triple negative (ER-/PR-/HER2-) tumors (ptrend = 0.95).
Table 5. Odds ratios1 (ORs) and 95% confidence intervals (95% CIs) for breast cancer associated with AMH concentration by tumor characteristics.
| AMH quartiles2 | Ptrend3 | Pinteraction4 | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Q1 | Q2 | Q3 | Q4 | ||||
| Invasiveness | 0.41 | ||||||
| Invasive | Cases/Controls | 508/636 | 547/619 | 564/595 | 636/606 | ||
| Adjusted OR (95% CI) | 1.00 (Referent) | 1.19 (0.99, 1.43) | 1.39 (1.14, 1.70) | 1.67 (1.34, 2.09) | <.0001 | ||
| In situ | Cases/Controls | 122/153 | 136/156 | 147/184 | 172/169 | ||
| Adjusted OR (95% CI) | 1.00 (Referent) | 1.19 (0.79, 1.79) | 1.10 (0.72, 1.69) | 1.35 (0.85, 2.13) | 0.25 | ||
| ER status | 0.21 | ||||||
| ER+ | Cases/Controls | 324/438 | 353/424 | 377/411 | 441/439 | ||
| Adjusted OR (95% CI) | 1.00 (Referent) | 1.27 (1.01, 1.60) | 1.52 (1.19, 1.96) | 1.74 (1.33, 2.28) | <.0001 | ||
| ER- | Cases/Controls | 84/90 | 93/108 | 91/109 | 112/110 | ||
| Adjusted OR (95% CI) | 1.00 (Referent) | 0.95 (0.60, 1.52) | 1.02 (0.62, 1.69) | 1.17 (0.68, 2.01) | 0.54 | ||
| PR status | 0.02 | ||||||
| PR+ | Cases/Controls | 266/374 | 304/372 | 334/369 | 405/390 | ||
| Adjusted OR (95% CI) | 1.00 (Referent) | 1.29 (1.00, 1.65) | 1.61 (1.23, 2.11) | 1.97 (1.48, 2.64) | <.0001 | ||
| PR- | Cases/Controls | 142/154 | 142/160 | 134/151 | 148/159 | ||
| Adjusted OR (95% CI) | 1.00 (Referent) | 0.96 (0.67, 1.39) | 0.99 (0.66, 1.49) | 1.00 (0.65, 1.55) | 0.95 | ||
| HER2 status | 0.37 | ||||||
| HER2+ | Cases/Controls | 44/60 | 44/55 | 38/62 | 80/57 | ||
| Adjusted OR (95% CI) | 1.00 (Referent) | 1.11 (0.58, 2.11) | 1.17 (0.57, 2.44) | 3.39 (1.55, 7.42) | 0.002 | ||
| HER2- | Cases/Controls | 182/275 | 227/280 | 244/263 | 266/279 | . | |
| Adjusted OR (95% CI) | 1.00 (Referent) | 1.36 (1.01, 1.83) | 1.80 (1.31, 2.48) | 2.05 (1.45, 2.92) | <.0001 | ||
| Joint receptor status | |||||||
| ER+/PR+ | Cases/Controls | 259/360 | 288/358 | 317/354 | 386/371 | 0.15 | |
| Adjusted OR (95% CI) | 1.00 (Referent) | 1.26 (0.97, 1.62) | 1.58 (1.20, 2.08) | 1.96 (1.46, 2.64) | <.0001 | ||
| ER+/PR- | Cases/Controls | 65/78 | 65/66 | 60/57 | 55/68 | ||
| Adjusted OR (95% CI) | 1.00 (Referent) | 1.25 (0.68, 2.28) | 1.13 (0.58, 2.19) | 0.82 (0.40, 1.68) | 0.51 | ||
| ER-/PR+ | Cases/Controls | 7/14 | 16/14 | 17/15 | 19/19 | ||
| Adjusted OR (95% CI) | 1.00 (Referent) | 3.10 (0.60, 15.9) | 3.53 (0.60, 20.8) | 3.23 (0.48, 21.9) | 0.26 | ||
| ER-/PR- | Cases/Controls | 77/76 | 77/94 | 74/94 | 93/91 | ||
| Adjusted OR (95% CI) | 1.00 (Referent) | 0.83 (0.50, 1.39) | 0.90 (0.51, 1.58) | 1.15 (0.63, 2.09) | 0.60 | ||
| Triple-negative (ER-/PR-/HER2-) tumors | |||||||
| Cases/Controls | 29/28 | 25/35 | 28/29 | 33/42 | |||
| Adjusted OR (95% CI) | 1.00 (Referent) | 0.84 (0.31, 2.28) | 1.17 (0.41, 3.37) | 1.02 (0.34, 3.04) | 0.95 | ||
Estimated using conditional logistic regression model and adjusting for race/ethnicity (white, black, other or unknown), education (high school or less, some college or higher, unknown), BMI (<18.5, 18.5-25, 25-30, 30+ kg/m2), age at menarche (ordered categorical, <12, 12, 13, 14+ years), parity (ordered categorical, 0, 1, 2, 3+), age at 1st FTP (ordered categorical, <=20, 21-25, 26-30, 30+ years or nulliparous), oral contraceptive use (never, former, current, unknown), partial oophorectomy (no, yes, unknown), family history of breast cancer (no, yes), benign breast biopsy (no, yes, unknown), and smoking status (never, former, current, unknown).
Defined using cohort-specific cutpoints.
Ptrend was calculated using ordered categorical AMH.
Pinteraction was calculated by including an interaction term between AMH (ordered categorical) and each tumor characteristic.
No statistically significant heterogeneity of the AMH-risk association was found in analyses stratified by age at blood donation, age at diagnosis, or baseline characteristics (Appendix Tables 4 and 5), though the association appeared stronger among women ages ≥45 years at blood donation than for younger women.
Table 6 shows the results by menopausal status at diagnosis/index date. No statistically significant heterogeneity was detected (pinteraction = 0.34). The OR comparing top vs. bottom AMH quartiles was 1.35 (95% CI= 1.05-1.73; ptrend = 0.03) for the premenopausal subgroup and 1.61 (95% CI= 1.03-2.53; ptrend = 0.03) for the postmenopausal subgroup. Further adjusting for subsequent age at menopause hardly altered the ORs in the postmenopausal subgroup.
Table 6. Odds ratios (ORs) and 95% confidence intervals (95% CIs) for breast cancer associated with AMH concentration by menopausal status at diagnosis.
| AMH quartiles1 | Ptrend4 | Pinteraction5 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Q1 | Q2 | Q3 | Q4 | |||
| Matched sets with both case and control(s) pre-menopausal at diagnosis/index date | 0.34 | |||||
| Cases/Controls | 222/292 | 282/339 | 327/369 | 369/374 | ||
| Adjusted OR2 (95% CI) | 1.00 (Referent) | 1.21 (0.93, 1.56) | 1.17 (0.91, 1.50) | 1.35 (1.05, 1.73) | 0.03 | |
| Matched sets with both case and control(s) post-menopausal at diagnosis/index date | ||||||
| Cases/Controls | 161/176 | 90/116 | 96/94 | 100/75 | ||
| Adjusted OR2 (95% CI) | 1.00 (Referent) | 0.88 (0.60, 1.30) | 1.14 (0.74, 1.76) | 1.61 (1.03, 2.53) | 0.03 | |
| Adjusted OR3 (95% CI) | 1.00 (Referent) | 0.88 (0.59, 1.30) | 1.13 (0.72, 1.79) | 1.59 (0.96, 2.63) | 0.06 | |
Defined using cohort- and age-specific cutpoints.
Estimated using conditional logistic regression model, adjusting for race/ethnicity (white, black, other or unknown), education (high school or less, some college or higher, unknown), BMI (<18.5, 18.5-25, 25-30, 30+ kg/m2), age at menarche (ordered categorical, <12, 12, 13, 14+ years), parity (ordered categorical, 0, 1, 2, 3+), age at 1st FTP (ordered categorical, <=20, 21-25, 26-30, 30+ years or nulliparous), oral contraceptive use (never, former, current, unknown), partial oophorectomy (no, yes, unknown), family history of breast cancer (no, yes), benign breast biopsy (no, yes, unknown), and smoking status (never, former, current, unknown). Analyses were performed among women with known age at menopause.
Estimated using conditional logistic regression model and adjusting for variables in footnote 2 and age at menopause.
Ptrend was calculated using ordered categorical AMH.
Pinteraction was calculated by including an interaction term between AMH (ordered categorical) and menopausal status at diagnosis.
Stratified analyses were not appreciably different in analyses using consortium-wide quartiles (data not shown).
Discussion
In this prospective study including 2,835 cases and 3,122 matched controls from ten cohorts, we found a positive association between circulating AMH concentration and breast cancer risk. Compared with women in the lowest AMH quartile, women in the top quartile had a 60% higher risk of breast cancer in analyses adjusting for potential confounders. The association appeared limited to ER+/PR+ tumors. It was observed for both premenopausal and postmenopausal breast cancer.
Our study included six new cohorts in addition to the four that previously reported a positive association between AMH and breast cancer risk. Cases from these six cohorts represented 64% of the cases included in the study. Excluding one cohort at a time did not significantly alter the results and the association was still statistically significant when the four cohorts that published previously were simultaneously excluded (ORQ4-Q1= 1.38, 95% CI= 1.07-1.79). Thus, and given the dose-response observed, we feel confident that our results are not due to random variation.
A statistically significant trend of increasing risk with increasing AMH was observed for ER+, PR+, and ER+/PR+ tumors. This suggests that estrogens and progesterone, whose binding to their respective receptors results in increased breast epithelial cell proliferation 11, 40, are involved in the mechanism underlying the AMH-breast cancer association. AMH is not strongly correlated with estradiol (follicular r = 0.02; luteal r = 0.17; untimed r = 0.12) 14, 16, but is strongly predictive of age at menopause and is thus an indicator of remaining duration of exposure to the high levels of estrogens and progesterone observed prior to menopause. We also observed that ORs and dose-response trends were strongest for women who were ≥45 years of age at blood draw and thus approaching menopause. This suggests that AMH concentration during perimenopause may be particularly informative regarding breast cancer risk. Perimenopause is characterized by an increase in the number of anovulatory cycles, which lack the surge in progesterone observed in the luteal phase of ovulatory cycles, in addition to changes in patterns of estrogen concentrations. AMH concentration, as a marker of perimenopausal progression, would be expected to reflect ovarian sex hormone exposure during this life stage. These observations support the hypothesis that the AMH-breast cancer risk association may be explained, in part, by AMH acting as a marker of time to menopause.
However, other observations from our study suggest that the association of AMH with risk is not explained entirely by its role as a marker of remaining years before menopause. First, we observed a positive association for premenopausal breast cancer. Also, the association of AMH with postmenopausal breast cancer was not attenuated by adjusting for age at menopause. We therefore cannot exclude an effect of AMH through other mechanisms, including a direct action of AMH, given the presence of AMH receptors in the breast 41.
Because experimental studies have shown a protective effect of AMH against breast tumors related to basal-like histology, Nichols et al. hypothesized that AMH could protect against this tumor subtype 15. We did not observe a positive association with AMH for triple-negative tumors, a subgroup that substantially overlaps with the subgroup of basal-like tumors 42, 43. The number of cases in this subgroup, was small (115 cases) though, and additional studies specifically in the basal-like subgroup would be of interest.
Besides its prospective design and large sample size, another strength of our study was that detailed data on breast cancer risk factors were available. Odds ratios were not much altered when we adjusted for these factors, suggesting that they do not confound the AMH-risk association. We also adjusted for testosterone, which has been consistently associated with risk of breast cancer in both pre- and post-menopausal women 19, 44. These two hormones were not correlated (age-adjusted Spearman correlation coefficient = 0.12) and ORs did not change substantially, suggesting that these two hormones act through different mechanisms. We used only one blood sample per participant, but AMH has been shown to vary little both within 45-47 and between 48, 49 menstrual cycles and also for repeat measurements (intra-class correlation coefficients of 0.88 for measurements 1 year apart, 0.67 for measurements taken 2-3 years apart, and correlation of 0.66 for measurements taken 4 years apart) 16, 50, 51. Further, using only one measurement in biomarker studies usually tends to attenuate true associations 52. Because neither biological/lifestyle variables (e.g. age, smoking, parity), nor the technical factors on which we had data (time in storage, type of sample (serum/plasma), time between collection and processing, and storage temperature) explained the differences in AMH concentrations we observed between cohorts, we do not know whether these differences reflect true differences between populations or technical artifacts. This is why we chose to conduct our analyses using cohort-specific quartiles, and our results should be interpreted on the relative scale (i.e. risk associated with levels in a specific quartile relative to women of the same age with levels in the lowest quartile) and not on the absolute scale (risk associated with absolute AMH concentration).
We note some implications of our results. First, the protective effect of AMH against breast and gynecological cancers in laboratory studies has led to the suggestion that AMH could be used in the treatment of these cancers 53. Our results, however, indicate an opposite effect of AMH in women than observed in laboratory studies, which may be due to the use of supraphysiologic doses of recombinant AMH in those studies 7, 9, 41, 54. The second implication regards breast cancer risk prediction models. Information on absolute risk is needed for younger women because guidelines regarding the age to start mammographic screening are not consistent 55-57 and because younger women tend to benefit most from preventive pharmacologic intervention 58. Current risk prediction models, though, have shown limited discriminatory accuracy 59. Our results suggest that AMH could improve breast cancer risk prediction models for younger women.
In conclusion, we found that women with high AMH concentrations were at higher risk of breast cancer than women of the same age with lower AMH concentrations in a large prospective study. The association was statistically significant only for ER+/PR+ tumors, which suggests that the association is due, at least in part, to the role of AMH as an indicator of exposure to estrogens and progesterone. The association with postmenopausal breast cancer is also consistent with AMH reflecting remaining time to menopause; however, because this association was not attenuated with adjustment for age at menopause and because we also observed an association of AMH with pre-menopausal breast cancer, our results suggest that additional mechanisms are at play.
Supplementary Material
Novelty and Impact.
Information on their individual risk of breast cancer can help women make decisions about breast cancer screening and prevention but current risk prediction models lack discriminatory accuracy. In this large prospective study, premenopausal women with AMH concentration in the top quartile had a 60% greater risk of breast cancer than women of the same age with AMH concentration in the bottom quartile. AMH is thus a candidate for inclusion in breast cancer risk prediction models for younger women.
Acknowledgments
This work was supported by grant NIH R01 CA178949. Support for the individual cohorts included:
Breakthrough Generations Study (BGS): This work was supported by Breast Cancer Now and The Institute of Cancer Research. We acknowledge NHS funding to the Royal Marsden and The Institute of Cancer Research NIHR Biomedical Research Centre. We thank the study participants, study staff, and the doctors, nurses and other health care providers and health information sources who have contributed to the study.
Guernsey cohort (Guernsey): Cancer Research UK C570/A16491 Availability of data and materials: Data access policies for the Guernsey study are available on the Cancer Epidemiology Unit website at https://www.ceu.ox.ac.uk/policies2
Nurses' Health Study (NHS): NCI UM1 CA186107; R01 CA49449
Nurses' Health Study II (NHSII): NCI UM1 CA176726; R01 CA67262
New York University Women's Health Study (NYUWHS): NIH R01 CA098661, UM1 CA182934 and center grants P30 CA016087 and P30 ES000260
Sister Study: This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES044005) to D.P. Sandler and the Avon Foundation (02-2012-085) to H.B. Nichols and D.P. Sandler.
Abbreviations
- AMH
Anti-Müllerian Hormone
- BGS
Breakthrough Generations Study
- BMI
body mass index
- CLUE II
Campaign Against Cancer and Heart Disease
- CSB
Columbia, Missouri Serum Bank
- CV
coefficient of variation
- FTP
full-term pregnancy
- NHS
Nurses' Health Study
- NHSII
Nurses' Health Study II
- NSMSC
Northern Sweden Mammography Screening Cohort
- NYUWHS
New York University Women's Health Study
- ORDET
Hormones and Diet in the Etiology of Breast Cancer
References
- 1.Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, Grootegoed JA. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–62. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- 2.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS ONE. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monniaux D, Clement F, Dalbies-Tran R, Estienne A, Fabre S, Mansanet C, Monget P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link? Biol Reprod. 2014;90:85. doi: 10.1095/biolreprod.113.117077. [DOI] [PubMed] [Google Scholar]
- 4.Dolleman M, Verschuren WM, Eijkemans MJ, Broekmans FJ, van der Schouw YT. Added value of anti-Mullerian hormone in prediction of menopause: results from a large prospective cohort study. Hum Reprod. 2015;30:1974–81. doi: 10.1093/humrep/dev145. [DOI] [PubMed] [Google Scholar]
- 5.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–80. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. Predicting age at menopause from serum antimullerian hormone concentration. Menopause. 2011;18:766–70. doi: 10.1097/gme.0b013e318205e2ac. [DOI] [PubMed] [Google Scholar]
- 7.Segev DL, Hoshiya Y, Stephen AE, Hoshiya M, Tran TT, MacLaughlin DT, Donahoe PK, Maheswaran S. Mullerian inhibiting substance regulates NFkappaB signaling and growth of mammary epithelial cells in vivo. J Biol Chem. 2001;276:26799–806. doi: 10.1074/jbc.M103092200. [DOI] [PubMed] [Google Scholar]
- 8.Hoshiya Y, Gupta V, Segev DL, Hoshiya M, Carey JL, Sasur LM, Tran TT, Ha TU, Maheswaran S. Mullerian Inhibiting Substance induces NFkB signaling in breast and prostate cancer cells. Mol Cell Endocrinol. 2003;211:43–9. doi: 10.1016/j.mce.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Gupta V, Carey JL, Kawakubo H, Muzikansky A, Green JE, Donahoe PK, MacLaughlin DT, Maheswaran S. Mullerian inhibiting substance suppresses tumor growth in the C3(1)T antigen transgenic mouse mammary carcinoma model. Proc Natl Acad Sci U S A. 2005;102:3219–24. doi: 10.1073/pnas.0409709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collaborative Group on Hormonal Factors in Breast C. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–51. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Key TJ, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. European journal of cancer & clinical oncology. 1988;24:29–43. doi: 10.1016/0277-5379(88)90173-3. [DOI] [PubMed] [Google Scholar]
- 12.McCoy AC, Kliethermes B, Zhang K, Qin W, Sticca R, Bouton M, Sauter ER. Serum Mullerian inhibiting substance levels are lower in premenopausal women with breast precancer and cancer. BMC Res Notes. 2011;4:152. doi: 10.1186/1756-0500-4-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su HI, Flatt SW, Natarajan L, DeMichele A, Steiner AZ. Impact of breast cancer on anti-mullerian hormone levels in young women. Breast Cancer Res Treat. 2013;137:571–7. doi: 10.1007/s10549-012-2361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorgan JF, Stanczyk FZ, Egleston BL, Kahle LL, Shaw CM, Spittle CS, Godwin AK, Brinton LA. Prospective case-control study of serum mullerian inhibiting substance and breast cancer risk. Journal of the National Cancer Institute. 2009;101:1501–9. doi: 10.1093/jnci/djp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols HB, Baird DD, Stanczyk FZ, Steiner AZ, Troester MA, Whitworth KW, Sandler DP. Anti-Mullerian Hormone Concentrations in Premenopausal Women and Breast Cancer Risk. Cancer prevention research (Philadelphia, Pa) 2015 doi: 10.1158/1940-6207.CAPR-14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliassen AH, Zeleniuch-Jacquotte A, Rosner B, Hankinson SE. Plasma anti-Mullerian hormone concentrations and risk of breast cancer among premenopausal women in the Nurses' Health Studies. Cancer Epidemiol Biomarkers Prev. 2016 doi: 10.1158/1055-9965.EPI-15-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. Journal of the National Cancer Institute. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 18.Endogenous H, Breast Cancer Collaborative G. Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Dowsett M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoff man-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, Peeters PH, Riboli E, Rinaldi S, Rollison DE, Stanczyk FZ, Trichopoulos D, Tworoger SS, Vineis P. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14:1009–19. doi: 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeleniuch-Jacquotte A, Afanasyeva Y, Kaaks R, Rinaldi S, Scarmo S, Liu M, Arslan AA, Toniolo P, Shore RE, Koenig KL. Premenopausal serum androgens and breast cancer risk: a nested case-control study. Breast Cancer Res. 2012;14:R32. doi: 10.1186/bcr3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swerdlow AJ, Jones ME, Schoemaker MJ, Hemming J, Thomas D, Williamson J, Ashworth A. The Breakthrough Generations Study: design of a long-term UK cohort study to investigate breast cancer aetiology. Br J Cancer. 2011;105:911–7. doi: 10.1038/bjc.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helzlsouer KJ, Alberg AJ, Huang HY, Hoffman SC, Strickland PT, Brock JW, Burse VW, Needham LL, Bell DA, Lavigne JA. Serum concentrations of organochlorine compounds and the subsequent development of breast cancer. Cancer Epidemiology Biomarkers & Prevention. 1999;8:525–32. [PubMed] [Google Scholar]
- 22.Visvanathan K, Crum RM, Strickland PT, You X, Ruczinski I, Berndt SI, Alberg AJ, Hoffman SC, Comstock GW, Bell DA, Helzlsouer KJ. Alcohol dehydrogenase genetic polymorphisms, low-to-moderate alcohol consumption, and risk of breast cancer. Alcohol Clin Exp Res. 2007;31:467–76. doi: 10.1111/j.1530-0277.2006.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fentiman I, Hanby A, Allen D, Key T, Meilahn E. Hormone dependency of breast tumours developing in the Guernsey Cohort Study. Breast cancer research and treatment. 2006;97:205–8. doi: 10.1007/s10549-005-9113-8. [DOI] [PubMed] [Google Scholar]
- 24.Thomas HV, Key TJ, Allen DS, Moore JW, Dowsett M, Fentiman IS, Wang DY. A prospective study of endogenous serum hormone concentrations and breast cancer risk in premenopausal women on the island of Guernsey. Br J Cancer. 1997;75:1075–9. doi: 10.1038/bjc.1997.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Micheli A, Muti P, Secreto G, Krogh V, Meneghini E, Venturelli E, Sieri S, Pala V, Berrino F. Endogenous sex hormones and subsequent breast cancer in premenopausal women. Int J Cancer. 2004;112:312–8. doi: 10.1002/ijc.20403. [DOI] [PubMed] [Google Scholar]
- 26.Scarmo S, Afanasyeva Y, Lenner P, Koenig KL, Horst RL, Clendenen TV, Arslan AA, Chen Y, Hallmans G, Lundin E, Rinaldi S, Toniolo P, Shore RE, Zeleniuch-Jacquotte A. Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: a nested case-control study. Breast Cancer Res. 2013;15:R15. doi: 10.1186/bcr3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, Shore RE, Krogh V, Koenig KL, Riboli E, Berrino F, Hallmans G, Stattin P, Toniolo P, Kaaks R. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol. 2004;150:161–71. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 28.Dorgan JF, Stanczyk FZ, Kahle LL, Brinton LA. Prospective case-control study of premenopausal serum estradiol and testosterone levels and breast cancer risk. Breast Cancer Res. 2010;12:R98. doi: 10.1186/bcr2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoemaker MJ, Folkerd EJ, Jones ME, Rae M, Allen S, Ashworth A, Dowsett M, Swerdlow AJ. Combined effects of endogenous sex hormone levels and mammographic density on postmenopausal breast cancer risk: results from the Breakthrough Generations Study. Br J Cancer. 2014;110:1898–907. doi: 10.1038/bjc.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortner RT, Eliassen AH, Spiegelman D, Willett WC, Barbieri RL, Hankinson SE. Premenopausal endogenous steroid hormones and breast cancer risk: results from the Nurses' Health Study II. Breast Cancer Res. 2013;15:R19. doi: 10.1186/bcr3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in Medicine. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed SS, Thike AA, Zhang K, Lim JC, Tan PH. Clinicopathological characteristics of oestrogen receptor negative, progesterone receptor positive breast cancers: re-evaluating subsets within this group. J Clin Pathol. 2017;70:320–6. doi: 10.1136/jclinpath-2016-203847. [DOI] [PubMed] [Google Scholar]
- 34.Shen T, Brandwein-Gensler M, Hameed O, Siegal GP, Wei S. Characterization of estrogen receptor-negative/progesterone receptor-positive breast cancer. Hum Pathol. 2015;46:1776–84. doi: 10.1016/j.humpath.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Itoh M, Iwamoto T, Matsuoka J, Nogami T, Motoki T, Shien T, Taira N, Niikura N, Hayashi N, Ohtani S, Higaki K, Fujiwara T, Doihara H, Symmans WF, Pusztai L. Estrogen receptor (ER) mRNA expression and molecular subtype distribution in ER-negative/progesterone receptor-positive breast cancers. Breast Cancer Research and Treatment. 2014;143:403–9. doi: 10.1007/s10549-013-2763-z. [DOI] [PubMed] [Google Scholar]
- 36.Chan M, Chang MC, Gonzalez R, Lategan B, del Barco E, Vera-Badillo F, Quesada P, Goldstein R, Cruz I, Ocana A, Cruz JJ, Amir E. Outcomes of Estrogen Receptor Negative and Progesterone Receptor Positive Breast Cancer. PLoS One. 2015;10:e0132449. doi: 10.1371/journal.pone.0132449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hefti MM, Hu R, Knoblauch NW, Collins LC, Haibe-Kains B, Tamimi RM, Beck AH. Estrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtype. Breast Cancer Res. 2013;15:R68. doi: 10.1186/bcr3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Maeyer L, Van Limbergen E, De Nys K, Moerman P, Pochet N, Hendrickx W, Wildiers H, Paridaens R, Smeets A, Christiaens MR, Vergote I, Leunen K, Amant F, Neven P. Does estrogen receptor negative/progesterone receptor positive breast carcinoma exist? J Clin Oncol. 2008;26 doi: 10.1200/JCO.2007.14.8411. [DOI] [PubMed] [Google Scholar]
- 39.Foley NM, Coll JM, Lowery AJ, Hynes SO, Kerin MJ, Sheehan M, Brodie C, Sweeney KJ. Re-Appraisal of Estrogen Receptor Negative/Progesterone Receptor Positive (ER-/PR+) Breast Cancer Phenotype: True Subtype or Technical Artefact? Pathol Oncol Res. 2017 doi: 10.1007/s12253-017-0304-5. [DOI] [PubMed] [Google Scholar]
- 40.Hilton HN, Graham JD, Clarke CL. Minireview: Progesterone Regulation of Proliferation in the Normal Human Breast and in Breast Cancer: A Tale of Two Scenarios? Mol Endocrinol. 2015;29:1230–42. doi: 10.1210/me.2015-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segev DL, Ha TU, Tran TT, Kenneally M, Harkin P, Jung M, MacLaughlin DT, Donahoe PK, Maheswaran S. Mullerian inhibiting substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J Biol Chem. 2000;275:28371–9. doi: 10.1074/jbc.M004554200. [DOI] [PubMed] [Google Scholar]
- 42.Allott EH, Cohen SM, Geradts J, Sun X, Khoury T, Bshara W, Zirpoli GR, Miller CR, Hwang H, Thorne LB, O'Connor S, Tse CK, Bell MB, Hu Z, Li Y, Kirk EL, Bethea TN, Perou CM, Palmer JR, Ambrosone CB, Olshan AF, Troester MA. Performance of Three-Biomarker Immunohistochemistry for Intrinsic Breast Cancer Subtyping in the AMBER Consortium. Cancer Epidemiol Biomarkers Prev. 2016;25:470–8. doi: 10.1158/1055-9965.EPI-15-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bastien RR, Rodriguez-Lescure A, Ebbert MT, Prat A, Munarriz B, Rowe L, Miller P, Ruiz-Borrego M, Anderson D, Lyons B, Alvarez I, Dowell T, Wall D, Segui MA, Barley L, Boucher KM, Alba E, Pappas L, Davis CA, Aranda I, Fauron C, Stijleman IJ, Palacios J, Anton A, Carrasco E, Caballero R, Ellis MJ, Nielsen TO, Perou CM, Astill M, Bernard PS, Martin M. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics. 2012;5:44. doi: 10.1186/1755-8794-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Endogenous Hormones Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 45.La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Human Reproduction. 2006;21:3103–7. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- 46.Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy C, Englert Y. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Human reproduction. 2007;22:1837–40. doi: 10.1093/humrep/dem101. [DOI] [PubMed] [Google Scholar]
- 47.Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, De Ziegler D. Serum antimüllerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertility and sterility. 2008;90:395–400. doi: 10.1016/j.fertnstert.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 48.Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–63. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 49.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–7. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 50.Dorgan JF, Spittle CS, Egleston BL, Shaw CM, Kahle LL, Brinton LA. Assay reproducibility and within-person variation of Mullerian inhibiting substance. Fertil Steril. 2010;94:301–4. doi: 10.1016/j.fertnstert.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979–87. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 52.de Klerk NH, English DR, Armstrong BK. A review of the effects of random measurement error on relative risk estimates in epidemiological studies. Int J Epidemiol. 1989;18:705–12. doi: 10.1093/ije/18.3.705. [DOI] [PubMed] [Google Scholar]
- 53.Kim JH, MacLaughlin DT, Donahoe PK. Mullerian inhibiting substance/anti-Mullerian hormone: A novel treatment for gynecologic tumors. Obstet Gynecol Sci. 2014;57:343–57. doi: 10.5468/ogs.2014.57.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoshiya Y, Gupta V, Kawakubo H, Brachtel E, Carey JL, Sasur L, Scott A, Donahoe PK, Maheswaran S. Mullerian inhibiting substance promotes interferon gamma-induced gene expression and apoptosis in breast cancer cells. J Biol Chem. 2003;278:51703–12. doi: 10.1074/jbc.M307626200. [DOI] [PubMed] [Google Scholar]
- 55.Final Recommendation Statement: Breast Cancer: Medications for Risk Reduction. U.S. Preventive Services Task Force. 2016 Dec; [Google Scholar]
- 56.ACOG Statement on Breast Cancer Screening Guidelines. 2016 [Google Scholar]
- 57.Oeffinger KC, Fontham EH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the american cancer society. JAMA. 2015;314:1599–614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, Vogel V. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. Journal of the National Cancer Institute. 1999;91:1829–46. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 59.Meads C, Ahmed I, Riley RD. A systematic review of breast cancer incidence risk prediction models with meta-analysis of their performance. Breast Cancer Res Treat. 2012;132:365–77. doi: 10.1007/s10549-011-1818-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


