Abstract
In rhesus macaques, previous studies have shown that episodic exposure to allergen alone or combined with ozone inhalation during the first 6 months of life results in a condition with many of the hallmarks of asthma. This exposure regimen results in altered development of the distal airways and parenchyma (Avdalovic et al., 2012). We hypothesized that the observed alterations in lung parenchyma would be permanent following a long-term recovery in filtered (FA) housing. Forty-eight infant rhesus macaques (30 days old) sensitized to house dust mite (HDM) were treated with two week cycles of FA, house dust mite allergen (HDMA), ozone (O3) or HDMA/ozone (HDMA + O3) for five months. At the end of the five months, six animals from each group were necropsied. The other six animals in each group were allowed to recover in FA for 30 more months at which time they were necropsied. Design-based stereology was used to estimate volumes of lung components, number of alveoli, size of alveoli, distribution of alveolar volumes, interalveolar capillary density. After 30 months of recovery, monkeys exposed to HDMA, in either group, had significantly more alveoli than filtered air. These alveoli also had higher capillary densities as compare with FA controls. These results indicate that early life exposure to HDMA alone or HDMA + O3 alters the development process in lung alveoli.
Keywords: Lung development, alveoli, stereology, ozone, and asthma
Introduction
Asthma is one of the most ubiquitous chronic disorders affecting nearly 7.1 million children in the United States under the age of 18 years (“Asthma and Children Fact Sheet.” American Lung Association. N.p., Oct. 2012. Web. 29 July 2013). Ozone and other air pollutants may have increased adverse effects on the lungs of children compared with adults. Research indicates that early life exposure to air pollution or aeroallergens may promote the asthma phenotype and the foundation of asthma occurs in the first year of life1–4. It well known that air pollution affects lung function and growth in developing lungs5–8. Epidemiological studies in California have shown pulmonary function decrements in young adults based on early life residence in areas of high ozone levels9. However, it is difficult to study the effects of ozone and other air pollutants in human epidemiological studies due to differences in design, methodology, and populations studies6, 10.
Children with asthma sometimes outgrow their asthma in the second decade of life leading to clinical remission of symptoms, but reports vary and it appears to be roughly 50% of asthmatic children outgrow their symptoms11, 12. However, very few studies have investigated aspects of asthma remission. Asthma during development may still lead to a loss of lung function in adulthood despite symptom remission13. In a study of adult asthma patients that were asymptomatic asthma for at least 2 years, there was evidence of airway hyperresponsiveness after methacholine inhalation tests with or without mild airflow obstruction14. In addition, asthma patients in asymptomatic remission have an increased density of eosinophils and mast cells in the bronchial mucosa when compared to normal patients15. These findings suggest that asymptomatic patients may still retain underlying characteristics of the disease.
Previous studies have shown that episodic exposure to ozone exacerbates the allergic response in a monkey model of allergic airway disease7. These studies began exposure at 1 month of age with cyclic exposure periods extending to 6 months of age. At the end of the exposure period, studies showed that airway and parenchymal morphology had been remodeled5,6. We investigated the effect of early-life 6 month episodic exposure to allergen and/or ozone on parenchymal morphology, including alveolar number and capillary density in sensitized rhesus macaques at the end of exposure These parameters were measured in another group of macaques after 30 months of recovery from the same exposure. We hypothesized that the changes in the first 6 months of life from cyclic exposure to ozone and HDMA will continue to have structural changes indicative of altered development following exposure throughout the early years of life.
Materials and Methods
Animals, exposure, and tissue collection
Forty-eight male infant rhesus monkeys (Macaca Mulatta) were selected from the outdoor breeding colony at the CNPRC, selected randomly, assigned to groups, and placed in a nursery for bottle-feeding with 24 hr care at 2 d of age. Body weight between groups at this age was similar. The infants were housed in a 4.2m3 capacity exposure chambers with open temperature controlled incubators. Each chamber housed three animals. Chamber ventilation cycled at a rate of 30 changes per hr with filtered air (FA). Three animals were housed in each exposure chamber during the study. Experimental protocols were reviewed and approved by the University of California, Davis Institutional Animal Care and Use Committee. Care and housing of animals complied with the provisions of the Institute of Laboratory Animal resources and conformed to practices established by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Animal studies conformed to applicable provisions of the Animal Welfare Act and other federal statutes and regulations relating to animals (Guide for the Care and Use of Laboratory Animals; National Institutes of Health, revised 1985).
The 48 rhesus monkeys were divided into two cohorts of 24 animals each. Within each cohort, there were 4 groups with six animals per group. The control group was exposed to filtered air (FA) alone. All animals were sensitized to house dust mite (Dermatophagoides pteronyssinus) by subcutaneous injection with alum adjuvant on the back on weeks 1, 2, and 4 as well as by intranasal instillation on weeks 1, 3, and 5. This house dust mite allergen was chosen over previously researched HDMA due to a reduction in impurities and was also used in the aerosol administration. HDMA sensitization was confirmed via skin testing with intradermal HDMA on Day 38 of the exposure protocol. The second group was exposed to house dust mite allergen (HDMA) aerosol in the exposure chambers (days 1, 3 and 5 of a 14 day cycle) for 2 hours. The third group was exposed to cycles of ozone with ozone being delivered for 8 hours a day at 0.5ppm for the first 5 days in a 14 day cycle. Ozone was generated as previously described and concentration was monitored using a Dasibi 1003-AH ozone analyzer (Dasibi Environmental Corportation, Glendale, CA)5, 8. This level of ozone is higher than the National Air Quality Standard of 0.075ppm and it used to increase the result ozone has on the developing macaque lung. The fourth group was also sensitized to HDM and exposed to both HDMA/ozone. All animals in the exposure groups were exposed to 11 cyclic exposures of 5 days of exposure and 9 days of FA (Figure 1). Exposures had a HDMA mass concentration averaging 7.05 ± 0.73 mg/m3 and a mean O3 concentration of 0.500 ± 0.005 ppm16.
Figure 1.
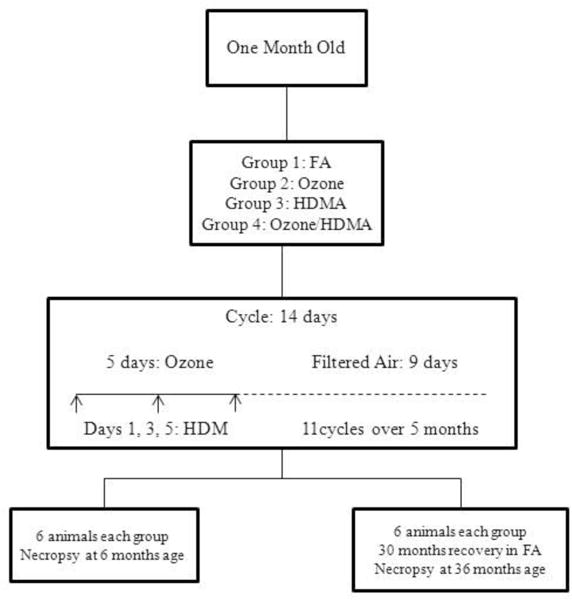
Exposure diagram. Age at beginning of exposure and types of exposure for each group is outlined in the diagram. Timing of HDMA and Ozone exposure is shown for a typical cyclic exposure. All animals were sensitized to house dust mite by subcutaneous injection with alum adjuvant on the back on weeks 1, 2, and 4 as well as by intranasal instillation on weeks 1, 3, and 5. Sensitization to HDM was confirmed via skin testing on Day 38 of the exposure protocol. Ozone exposure was 8hrs/day (midnight to 8 a.m.) and HDMA exposure was 2.5hrs/day on days 1, 3, and 5 in the a.m. HDM concentrations in aerosol were 435± 96 μg/m3.
The first cohort (6 month old cohort) was necropsied at the end of the 11th cycle at 6 months of age. The second cohort (36 month old cohort) remained in exposure rooms receiving FA for 30 months and the animals were necropsied at 36 months of age. Monkeys were weighed and then sedated with Telazol (8 mg/kg im) and anesthetized with Diprivan (0.1–0.2 mg/kg/min iv), with the dose adjusted as necessary by the attending veterinarian. The monkeys were euthanized with an overdose of pentobarbital sodium followed by exsanguinations through the abdominal aorta. The right middle lobe was immediately fixed with 1% glutaraldehyde – 1% paraformaldehyde in cacodylate buffer at 25-cm fluid pressure (adjusted to pH 7.4, 330 mosM). Lung lobe volume measured by fluid weight displacement after fixation17. The left caudal lobe was inflated with Optimal Cutting Temperature (OCT) diluted 1:1 in PBS and cooled to 4 degrees C.
Stereological Estimates
The right middle lobed was embedded in 4% agar-gelatin after fixation, isotropically oriented using an orientator, sliced into 5-mm slabs, and cut into 5mm by 5mm by 15 mm bricks. These bricks were sampled using a smooth fractionator18, 19. The sampled tissue was embedded in paraffin, cut in 5-μm serial sections, and stained with hematoxylin and eosin. Serial sections were scanned via Olympus VS110 whole slide scanner running software (VS-ASW-FL version 2.2 build 7667) and saved as .TIF files. Lenses used were Olympus PlanApo N2x N.A. 0.08 for the overview and Olympus PlanApo 10× N.A. 0.40 for the image. The .TIF files of the images were uploaded to a computer with VIS software version 3.4.1.5 using modules Acquisition, MicroImager, NewCast, Automated Physical Disector, and Scan Imager (Visiopharm, Denmark). All stereological sampling was done using VIS software.
The disector method was used to estimate the number of alveoli in the right middle lobe. Counting the number of entrance rings in paired sections by the disector technique allows estimation of total number of alveoli in the lung19–22. Two images were used from the serial sections that provided a disector height of 10μm. Bridges and islands in the images are used for counting. Bridges are connections of interalveolar septa that appear in one section but not the other. The absence of a bridge represents an opening of the alveolus. Islands are a new isolated portion of septa. The calculations are based on the Euler characteristic of Δn = (I-B)/2 with I representing islands and B representing bridges counted in a disector both directions, hence divided by 2. The fractionator principle was used in conjunction with the Euler characteristic to determine the total number of alveoli in the lung (Nalv,lung) where n is the number of alveolar openings per sample and SF is total sampling fraction, the product of the fractions of tissue bars, bricks, heights, and areas that are sampled at each level19.
The height sampling fraction was estimated as a ratio of the disector height (10 μm) to the block width that was cut equal to the block height. The area sampling fraction was estimated as the ratio of the sampling field area to the field displacement to the next sampled field in x-y axis using the VIS software.
One section from the serial sections of each block was sampled into a montage of images at 1650 μm by 1320 μm to ensure no overlapping of images. Each image was evaluated in Stereology Toolbox using a double lattice test system of 25 course/100 fine points23. The lungs were evaluated for parenchymal and non-parenchymal components using point counting. Fine points were used to count rare features such as arteries, veins, terminal bronchioles and respiratory bronchioles while course points were used to count common features such as alveoli, alveolar duct, and interalveolar septa. The volumes of parenchyma (Vpar), alveoli (Valv), interalveolar septa(Vias), alveolar duct core air (Vad), and nonparenchyma (Vnp) were determined by multiplying their volume densities (Vv) by lobe volume (VL) in units of cm3 19.
Capillary density of parenchymal regions of the left caudal lobe was estimated using a fluorescence approach with similar sampling as the right middle lobe. A goat anti-human CD31 (BD Pharmingen) combined with a secondary antibody, anti-goat Alexa 568 (Life Technologies, Grand Island, NY) was used to label capillaries in the parenchyma. The surface density of pulmonary capillaries and inter-alveolar septal tissue in the left caudal lobe were estimated using point and intersection counting and expressed as a ratio of capillary to inter-alveolar surface area22.
Estimation of the volume-weighted mean alveolar volume was determined by using a point-sampled intercept method. The estimation was determined by , where l3ias is the mean of the cubed point-sampled intercepts of alveoli. Fsgv is the factor of shrunken global volume, the units are in μm3 from Hyde et al.19
The number-weighted mean alveolar volume was calculated as follows: . Using the volume-weighted mean alveolar volume and number-weighted alveolar volumes, we calculated the coefficient of variation of the distribution of number-weighted alveolar volumes. This calculation was previously described20 as
Length density of terminal and respiratory bronchioles per volume of right middle lobe (Lv rb or tb,lung mm−2) was measured using IUR sections in a robust sampling from the filtered air and the Ozone/HDMA cohorts from the 30 month recovery group. The formula to calculate length density was
Qa is the number of profiles while a/p is the area per point (mm2) of the point probe and PL is the points hitting the lung24.
Statistical Methods
All data are reported as mean ± standard eror of the mean. We analyzed data for exposure group differences in each cohort using two-way analysis of variance using SPSS Statistics 22 (IBM corporation). Due to the growth of 30 months between the two cohorts; we did not statistically compare the two cohorts. For volume and length of terminal or respiratory bronchioles, a one-way analysis of variance was performed between filtered air and HDMA/ozone exposure groups. Statistical significance was accepted at P ≤ 0.5.
Results
Pathology and Lung Volume
No noticeable changes were observed with gross pathology. As can be seen in Table 1, lung volume as well as all lung compartments continued to increase in all groups. There were no significant differences between lung volumes in the different cohorts (Figure 2).
Table 1.
Mean and standard error for all values for each cohort of filtered air, HDMA, Ozone and Ozone/HDMA. Numbers in bold represent significantly different values than filtered air.
| 6 months | FA | HDMA | Ozone | Ozone/HDMA | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Lobe Volume | 7.62E+00 | 4.04E-01 | 8.38E+00 | 2.06E-01 | 8.10E+00 | 5.21E-01 | 8.18E+00 | 6.46E-01 |
| Nalv, lobe | 1.33E+07 | 2.77E+05 | 1.21E+07 | 7.71E+05 | 1.15E+07 | 5.73E+05 | 1.15E+07* | 4.93E+05 |
| Vv alv | 7.40E+05 | 1.01E+05 | 7.62E+05 | 5.92E+04 | 8.42E+05 | 9.61E+04 | 8.01E+05 | 8.38E+04 |
| Vn alv | 2.94E+05 | 1.94E+04 | 3.59E+05 | 2.47E+04 | 3.66E+05 | 3.00E+04 | 3.67E+05 | 2.69E+04 |
| Valv | 3.91E+00 | 2.49E-01 | 4.27E+00 | 1.86E-01 | 4.18E+00 | 3.01E-01 | 4.21E+00 | 3.00E-01 |
| Vias | 1.45E+00 | 1.10E-01 | 1.57E+00 | 8.20E-02 | 1.47E+00 | 7.31E-02 | 1.53E+00 | 1.81E-01 |
| Vad | 1.56E+00 | 8.55E-02 | 1.91E+00 | 1.24E-01 | 1.65E+00 | 1.50E-01 | 1.77E+00 | 1.98E-01 |
| CVn alv | 1.20E+00 | 1.52E-01 | 1.06E+00 | 1.19E-01 | 1.13E+00 | 1.34E-01 | 1.07E+00 | 1.01E-01 |
| SScap, IAS | 4.25E-01 | 4.80E-03 | 4.19E-01 | 6.25E-03 | 4.05E-01 | 4.26E-03 | 4.19E-01 | 3.45E-03 |
| 36 months | FA | HDMA | Ozone | Ozone/HDMA | ||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Lobe Volume | 3.23E+01 | 1.17E+00 | 3.05E+01 | 3.44E+00 | 2.97E+01 | 3.54E+00 | 2.82E+01 | 1.69E+00 |
| Nalv, lobe | 2.26E+07 | 8.73E+05 | 2.57E+07* | 8.49E+05 | 2.44E+07 | 3.88E+05 | 2.72E+07* | 1.08E+06 |
| Vv alv | 7.89E+05 | 1.17E+05 | 8.12E+06 | 1.59E+05 | 7.91E+06 | 2.61E+05 | 8.11E+06 | 2.56E+05 |
| Vn alv | 6.97E+05 | 3.91E+04 | 6.00E+05 | 7.52E+04 | 6.06E+05 | 6.76E+04 | 4.89E+05 | 2.96E+04 |
| Valv | 1.57E+01 | 9.36E-01 | 1.54E+01 | 1.90E+00 | 1.49E+01 | 1.85E+00 | 1.33E+01 | 7.87E-01 |
| Vias | 6.71E+00 | 3.49E-01 | 6.35E+00 | 6.37E-01 | 6.11E+00 | 8.80E-01 | 6.01E+00 | 5.78E-01 |
| Vad | 7.37E+00 | 5.77E-01 | 6.89E+00 | 1.01E+00 | 7.06E+00 | 1.01E+00 | 6.34E+00 | 3.63E-01 |
| CVn alv | 1.31E+00 | 5.89E-02 | 1.62E+00* | 9.74E-02 | 1.45E+00 | 8.24E-02 | 1.81E+00* | 1.69E-01 |
| SScap, IAS | 4.78E-01 | 9.20E-03 | 5.23E-01* | 1.05E-02 | 5.15E-01 | 4.89E-03 | 5.40E-01* | 1.63E-02 |
Significant difference from FA controls P ≤ 0.05. Nalv lobe, number of alveoli;
, mean volume weighted volume of alveoli; , mean number weighted volume of alveoli; , volume of alveolar space; , volume of interalveolar septa; , volume of alveolar duct; CVn alv, coefficient of variation of the mean number weighted volume of alveoli; SScap, ias, Capillary surface to interalveolar septal surface.
Figure 2.
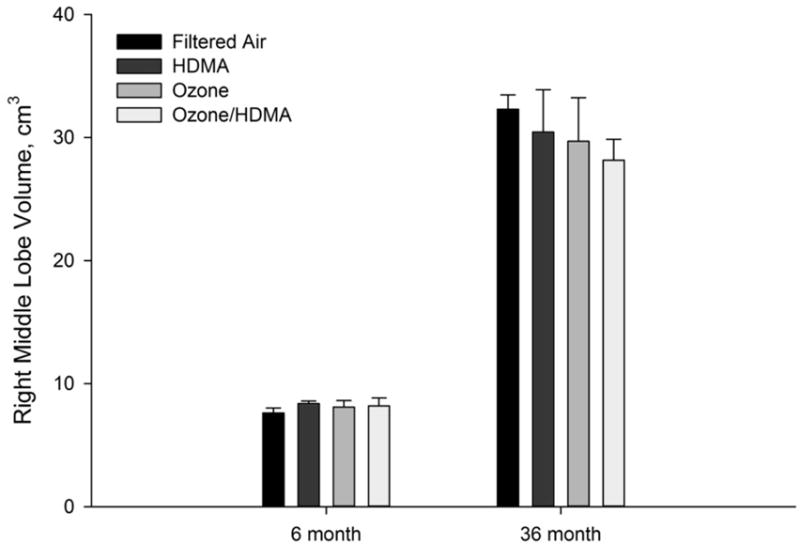
Volume of right middle lobe vs. age (months) in all treatment groups for both 6 month exposure and 36 month exposure and recovery cohorts. There were no statistically significant differences among groups in the respective cohorts.
Changes in Alveolar Number and Size
In the six month cohort, a two-way ANOVA was conducted on the effect of ozone and HDMA on the number of alveoli in the lung. There was a statistically significant decrease in the number of alveoli in the Ozone/HDMA exposure group (P ≤ 0.05) Table 1 and Figure 3). This indicates that macaques exposed to ozone during early age have delayed development in the parenchymal region of the lung.
Figure 3.
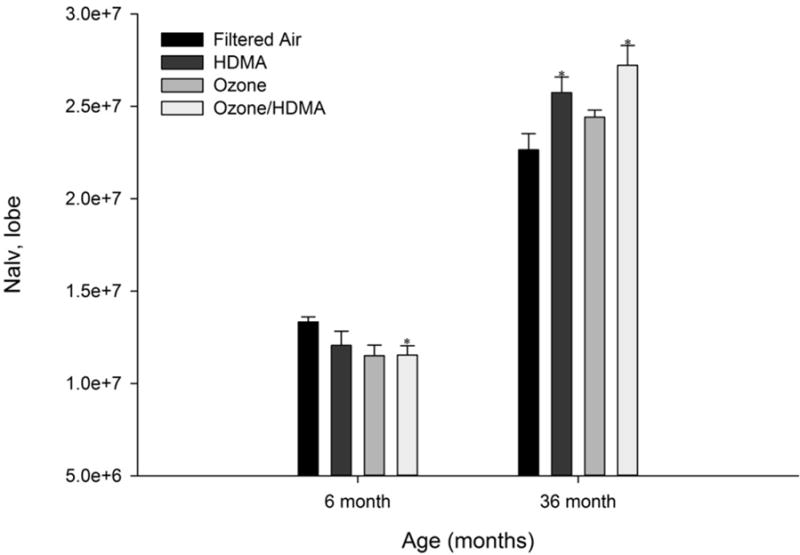
Number of alveoli in the right middle lobe vs. age (months) in all treatment groups for both 6 month exposure and 36 month exposure and recovery cohorts. After 11 cyclic exposures (6 month cohort), there were significantly less alveoli in macaques exposed to Ozone/HDMA (P ≤ 0.05) and those exposed to ozone alone (P = 0.06) in comparison with FA monkeys. While after 30 months of recovery (36 month exposure and recovery cohort), the HDMA and HDMA+O3 groups had significantly more alveoli than the FA group. *Significant difference (P<0.05) from the FA control lobe.
In the 36 month cohort, alveoli increased in number compared to the six month cohort in all exposure groups (Table 1 and Figure 3). This was expected as alveoli increase in number but not size during development with increasing lung volume19. In the 36 month cohort, analysis showed that HDMA had a significant impact on increasing the number of alveoli (P ≤ 0.05) while ozone had no effect.
The coefficient of variation indicates the size differences in alveoli in the lung. A value of 1 would indicate that all the alveoli are of equal size. The higher the value would mean there is more variation in the volume of alveoli. Animals in the 36 month cohort had a significantly increased coefficient of variation due to an effect by HDMA (P ≤ 0.05) (Table 1 and Figure 4). The mean number-weighted volume of alveoli was notably smaller in both the ozone effected (P=0.084) and the HDMA effected (P=0.068) groups which would impact the coefficient of variation.
Figure 4.
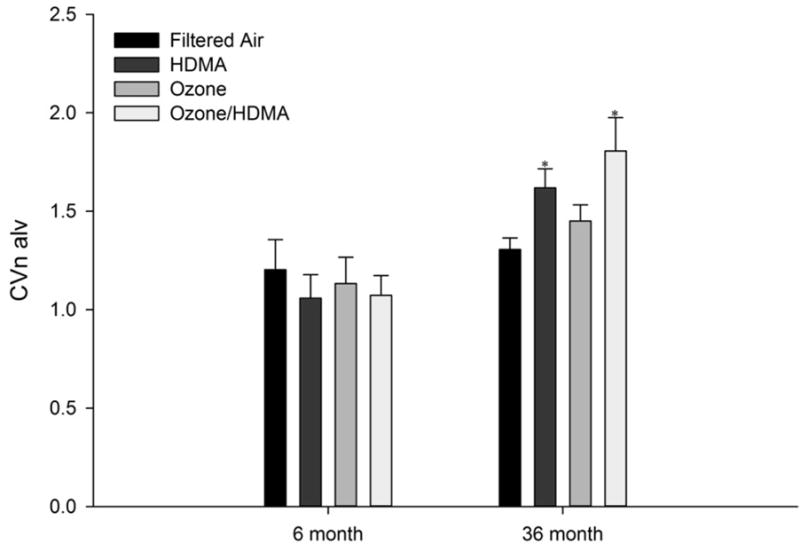
Coefficient of variation of the distribution of mean number-weighted alveolar volumes vs. age (months) in all treatment groups for both 6 month exposure and 36 month exposure and recovery cohorts. There was a significant increase in the CVn alv in the HDMA and HDMA + O3 groups compared to the FA group in the 36 month exposure and recovery cohort. *Significant difference (P<0.05) from the FA control lobe.
Changes in Alveolar Capillary Surface Density
The ratio of the pulmonary capillary to interalveolar septal surface (Ss CAP, IAS) represents morphologically the potential for diffusion capacity25. Figure 5 represents the mean alveolar capillary surface density. In the six month cohort, there was a notably interaction between the effects of HDMA and ozone on capillary density (P=0.054) with ozone being a principal source (P=0.053). This was altered after 30 months of recovery. Age had an effect on capillary density in all groups in the 30 month cohort as they were all significantly increased (P ≤ 0.05) compared with their counterparts in the 6 month cohort. Within the 30 month exposure and recovery cohort, HDMA had a significant effect on capillary density (P ≤ 0.05) with an increased ratio of capillaries per interalveolar septal surface area.
Figure 5.
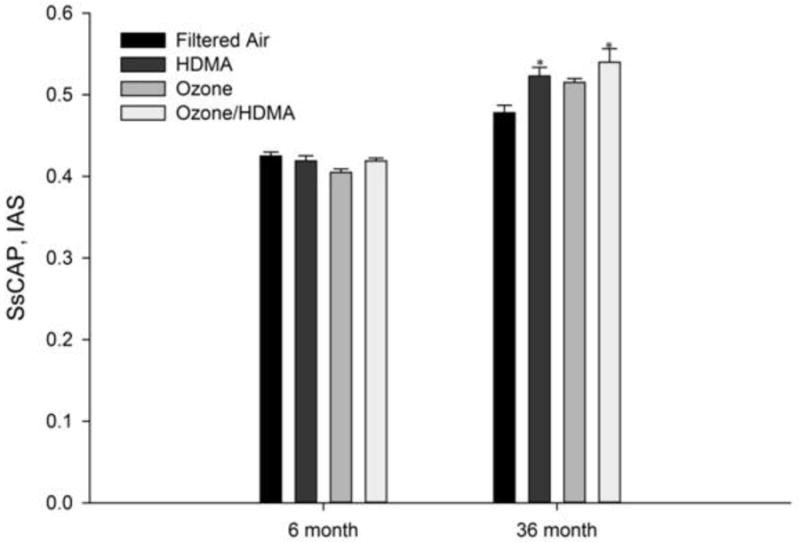
The ratio of the pulmonary capillary to interalveolar septal surface (Ss cap, IAS) in the left cranial lobe vs. age (months) in all treatment groups for both 6 month exposure and 36 month exposure and recovery cohorts. The Ozone group was significantly less than (P ≤ 0.05) the control group exposed only to FA. in the 6 month cohort. In the 36 month cohort, there was a significant increase in the ratio in the HDMA and HDMA + O3 groups compared to the FA group. *Significant difference (P<0.05) from the FA control lobe.
Length and volume of terminal and respiratory bronchioles
There was no significant difference between FA (0.38 cm3 ± 0.09; 91.6cm ± 14.5) and Ozone/HDMA (0.43cm3 ± 0.16; 83.5cm ± 10.6) cohorts from the 30 month recovery group regarding volume or length of respiratory bronchioles, respectively. However, there was a noteworthy difference in length of terminal bronchioles (p=0.06) (FA = 58.9cm ± 7.0; Ozone/HDMA = 85.2cm ± 10.3) without a significant difference in volume (FA = 0.289cm3 ± 0.10; Ozone/HDMA = 0.348cm3 ± 0.13) of terminal bronchioles in these cohorts that indicate a decrease in diameter. Average diameter for terminal bronchioles in FA group was 0.79 mm while in the Ozone/HDMA group it was 0.72 m based on the model of a right circular cylinder (V = π*radius2*length). Only these two cohorts were examined for length difference of bronchioles because only the Ozone/HDMA showed increased airway hyperresponsiveness compared to the FA cohort16.
Discussion
Development of the lung parenchyma in rhesus macaques is very similar to that in humans. Consequently, rhesus macaques serve as an excellent model for studying early postnatal events in the development and growth of interalveolar septa and their alveolar units. Recent evidence has indicated that alveolarization continues through adolescence in humans26. Hence, there is a critical need for developmental research regarding insults to the developing lung past the initial postnatal growth. The contribution of oxidant air pollution and allergen exposure early in life to the development of chronic airway disease in rhesus macaques has previously been documented1, 5, 8, 27. Chronic exposure to injurious agents may result in persistent pulmonary changes that involve both structural and/or functional alterations28, 29. At 6 months of age, alveolar number was similar between treatment groups; however, there was a significant rise in alveolar number from 3 to 6 months of age in the ozone exposed groups. This increase in alveolar number was not associated with any significant increase in microvascular growth as measured by morphometry or changes in angiogenic gene expression5.
Very little is known about the development of the lung after recovery of early life injury or remission of asthma at any point in life. The results presented in this paper are the only morphologic data investigating allergic airway disease recovery in a nonhuman primate model of asthma during postnatal development. Our data indicate that after a prolonged recovery from ozone and HDMA exposure, development continues to be abnormal and the growth of alveoli is altered. The alterations of interalveolar septa that occurred during the 30 month recovery period were observed in the groups exposed to HDMA and Ozone/HDMA and consisted of more, smaller alveoli with a greater capillary density compared with the controls exposed to FA.
Changes in length of terminal bronchioles were not significant but noteworthy. The increase in length with no subsequent change in volume would indicate some airway remodeling continues with a narrowing of the terminal bronchioles along with the lengthening. Airway narrowing of respiratory bronchioles was also noted in adult bonnet monkeys following ozone exposure30.
Alveoli are the basic building blocks of lung parenchyma. In primates, the size of alveoli does not change significantly during development of the lung, but the numbers of alveoli increase proportional to lung volume19. Between the six- and 36-month cohorts, we expected to see similar growth among the different groups if development was normal. In the six-month cohort, we observed a reduction in growth of alveoli between FA and the group exposed to Ozone/HDMA (Table 1 and Figure 3). Abnormal growth persisted in the 36 month cohort in animals exposed to HDMA, especially in the group exposed to both Ozone and HDMA. No research exists investigating remodeling of parenchyma inrecovery from early life lung injury. However, there is evidence of airway remodeling and significant differences in the reticular basement membrane in asymptomatic patients in clinical remission31, but this is the first evidence indicated parenchymal development differences in an asthma model.
Alveolarization in humans by formation of secondary interalveolar septa occurs from 36 weeks of gestation to 1–2 years of age while microvascular maturation, by remodeling of interalveolar septa and restructuring of the capillary bed, occurs from birth to 2–3 years of age32. This process of alveolarization continues in the postnatal period to reach an average of 450 million alveoli in the human adult21. When lung morphology of rhesus macaques and humans is compared, there are similarities in the segmental arrangement, the structure and branching pattern of airways, and arterial structure after birth33. The general developmental stages in the rhesus monkey are as follows: embryo, 21–45 days gestation; fetus, 45–165 days gestation; newborn, 24 h postnatal; neonate, 0–1 month; infant, 1–12 months; juvenile, 12–24 months; adolescent, 2–4 years; and young adult, 4–8 years34. In previous work, we observed a rapid alveolar growth phase during the first year and a slower growth phase that continued until full somatic growth was accomplished at approximately 7 years of age19. During the 5 months of exposure from 1 to 6 months of age, alveolar growth was in the rapid phase, while during most of the recovery phase in filtered air was in the slower growth phase. The most significant alterations in interalveolar septal growth, smaller alveoli with a greater capillary density, were in the groups exposed to HDMA and Ozone/HDMA and recovered in 30 months of filtered air compared with the FA controls.
In a previous exposure study of infant rhesus macaques to the same four exposure conditions Ozone and Ozone/HDMA inhibited alveolarization at 3 months but “caught up” by 6 months of age with the only difference across groups being a significant increase in lobe volume at 6 months5. Similarly capillary surface density was depressed at in the Ozone and Ozone/HDMA groups at 3 months but at 6 months there was no difference across groups5. In contrast, in the Ozone group at 6 months of age in the current study the number of alveoli and capillary density were significantly decreased, while like Avdalovic et al. (2012) there was no other differences across the groups at 6 months of age. Though the exposure conditions were the same, the slight differences between these two studies could be the result of multiple factors associated with sensitization. These factors include: 1) the HDM allergen used, Der f (Dermatophagoides farina) in the previous study5 and Der p (Dermatophagoides pteronyssinus) in the current study; 2) Der f was delivered along with bordetella pertussis as an adjuvant and while Der p was not; 3) the Der f contained a high level of endotoxin while Der P did not; and 4) the filtered air and ozone groups in the previous study were also not sensitized to HDM.
The increase in alveolarization that occurred during the 30 months of growth following exposure in the current study was accompanied by an increase in capillary density and involved the HDMA and Ozone/HDMA groups. It is possible that the post-exposure period may have different mechanisms of injury and repair than during the exposure period. The consequences of these changes in alveolarization may have significant consequences for susceptibility to respiratory disease and altered lung function later in life. The resulting increase in the surface to volume ratio combined with the increase in capillary density would be expected to influence gas exchange in the lung and may explain the observed increase in diffusing capacity observed in human asthmatics (Weitzman and Wilson, 1974). However, diffusing capacity is impacted by several factors including alveolar wall thickness that was not measured in this study. In addition the more variable alveolar size might be expected to increase the risk of alveolar collapse in conditions where surfactant levels are decreased. The increased ratio of capillaries per interalveolar septal surface area may also be a compensatory mechanism related to early life injury to increase diffusing capacity. This compensation may also be related to the smaller and more numerous alveoli seen in the HDMA animals after recovery in that this is a method of counteracting future similar insults or minimizing them. As this remodeling in parenchyma has not been previously documented, it is difficult to ascertain the cause of increased capillary density or increased alveoli in a early life lung injury recovery model.
The number-weighted mean volume of alveoli did not show significant differences in either cohort; however, in the 6 month cohort all exposure groups showed an increase (22–25%), while in the 36 month cohort all exposure groups showed a decrease (13–30%) compared with FA controls. The increased volume in the 6 month cohort was associated with decreases in the number of alveoli in the right middle lobe; conversely, the decreased volume in the 36 month cohort was associated with increases in the number of alveoli in the right middle lobe, the distribution of alveolar volumes, and the capillary to interalveolar septal surface area ratio as compared with FA controls. Since the number-weighted mean volume of alveoli shows a poor relationship with age and the volume of the lung, it appears in normal lungs alveoli are added and do not enlarge to any significant degree during postnatal growth in rhesus monkeys34. The differences in the FA control number-weighted mean volume of alveoli between the 2 cohorts is consistent with the rapid growth phase of alveoli during development in the first 2 years of life34, but unexpected were the smaller lung and alveolar volumes and the increased number of alveoli with higher capillary densities as compared with FA controls in the 36 month cohort. The alveoli that were added during the 30 month post exposure period were smaller and had a greater capillary to alveolar gas exchange surface. This change was reflected in a larger coefficient of variation of the number-weighted mean volume of alveoli (smaller alveoli mixed in with normal sized alveoli). This altered development potentially could be more detrimental to lung structure in adults because a larger distribution of alveolar sizes would mean smaller alveoli collapsing a feature of obstructive lung disease.
The relationship between the severity of asthma and allergy symptoms and allergen and ozone exposure has been documented35. Several studies in humans have indicated that ongoing pulmonary physiological abnormalities persist during asthmatic remission of clinical symptoms36. Very little is known regarding alveolar changes in humans regarding asthma recovery, but airway narrowing tends to be a hallmark during and after asthma. Our data does show some changes occurring in the terminal bronchioles that would suggest ongoing airway narrowing after 30 months of recovery. Hallmarks of airway narrowing are thickening of the wall due to edema, increased connective tissue and smooth muscle37, 38. Rhesus monkeys have been reported to have hyperplastic bronchiolar epithelium and altered smooth muscle bundle orientation in both terminal and respiratory bronchioles after similar cyclic ozone exposure6. Chronic exposure to ozone and allergens followed by 30 months recovery in filtered air resulted in structural changes of small airway narrowing that were associated with airway hyperresponsiveness16. The driving force behind most features of airway remodeling appears to be inflammation with multiple cytokines, chemokines, and growth factors involved from both structural and inflammatory cells39. This creates a dynamic and complex signaling environment that may last months after the initial early life injury based on the results of this study. The mechanism behind the early life remodeling that was seen at 3 months in past experiments by the effects of ozone are potentially different mechanisms than the impact HDMA has on the remodeling seen after 30 months of recovery from early life insult.
The animals utilized in Moore et al., 2014 were the same animals measured in this study. Our results indicate abnormal growth pattern following a 30 month removal from environmental exposure to ozone and HDMA which may contribute to continued airway hyperresponsiveness along with the serotonergic mechanisms examined by Moore et al. (2014). The altered lung architecture may in part explain how asymptomatic adult humans who have “outgrown their disease” retain asthma symptoms. It is not clear whether the greater distribution of alveolar sizes is detrimental to lung growth since most of the alveoli added to the distribution were smaller and with greater capillary density, but a better understanding of the molecular mechanisms responsible for altered alveolar growth would provide valuable biomarkers of this altered growth process.
Highlights.
Abnormal lung development after postnatal exposure to ozone and allergen.
This remodeling is shown as smaller, more numerous alveoli and narrower airways.
Allergen appears to have more of an effect than ozone during recovery.
These animals also have continued airway hyperresponsiveness (Moore et al. 2014).
Acknowledgments
The authors thank the work of Frank Ventimiglia and the Computational Imaging Core at the California National Primate Research Center. This work was supported by: NCRR; Grant number: P51RR00169; Grant sponsor: NIEHS; Grant number: PO1ES00628
The authors thank Laurie Brignolo, D.V.M., Sarah Davis, and Bruce Rodello of the Cailfornia National Primate Research Center for their clinical and organizing expertise. The authors also thank Brian Tarkington, Jessica Arturus, Collette Brown, Carmen Ip, and Amanda Omlar for their technical assistance in data collection.
Abbreviations
- FA
filtered air
- HDM
house dust mite
- HDMA
house dust mite allergen
- O3
ozone
- im
intramuscular
- iv
intravenously
- I
islands
- B
bridges
- Nalv
number of alveoli
- Vpar
volume of parenchyma
- Valv
volume of alveoli
- Vias
volume of interalveolar septa
- Vad
volume of alveolar duct
- Vnp
volume of non-parenchyma
- Vv
volume density
- VL
lobe volume
- Lv rb
length density of respiratory bronchioles
- Lv tb
length density of terminal bronchioles
- Qa
number of profiles
- a/p
area per point
- Pl
points hitting the lung
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller LA, Gerriets JE, Tyler NK, Abel K, Schelegle ES, Plopper CG, Hyde DM. Ozone and allergen exposure during postnatal development alters the frequency and airway distribution of CD25+ cells in infant rhesus monkeys. Toxicology and Applied Pharmacology. 2009;236:39–48. doi: 10.1016/j.taap.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yunginger JW, Reed CE, O’Connell EJ, Melton IIILJ, O’Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma: incidence rates, 1964-1983. American Review of Respiratory Disease. 1992;146:888–894. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 3.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. New England Journal of Medicine. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 4.Mortimer K, Neas L, Dockery D, Redline S, Tager I. The effect of air pollution on inner-city children with asthma. European Respiratory Journal. 2002;19:699–705. doi: 10.1183/09031936.02.00247102. [DOI] [PubMed] [Google Scholar]
- 5.Avdalovic MV, Tyler NK, Putney L, Nishio SJ, Quesenberry S, Singh PJ, Miller LA, Schelegle ES, Plopper CG, Vu T, Hyde DM. Ozone Exposure During thE Early Postnatal Period Alters the Timing and Pattern of Alveolar Growth and Development in Nonhuman Primates. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2012;295:1707–1716. doi: 10.1002/ar.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2006;291:L644–L650. doi: 10.1152/ajplung.00027.2006. [DOI] [PubMed] [Google Scholar]
- 7.Schelegle ES, Walby WF, Alfaro MF, Wong VJ, Putney L, Stovall MY, Sterner-Kock A, Hyde DM, Plopper CG. Repeated episodes of ozone inhalation attenuates airway injury/repair and release of substance P, but not adaptation. Toxicology and Applied Pharmacology. 2003;186:127–142. doi: 10.1016/s0041-008x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- 8.Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, Walby WF, Mitchell V, Tarkington BK, Wong VJ, Baker GL, Pantle LM, Joad JP, Pinkerton KE, Wu R, Evans MJ, Hyde DM, Plopper CG. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicology and Applied Pharmacology. 2003;191:74–85. doi: 10.1016/s0041-008x(03)00218-7. [DOI] [PubMed] [Google Scholar]
- 9.Tager IB, Künzli N, Lurmann F, Ngo L, Segal M, Balmes J. Methods development for epidemiologic investigations of the health effects of prolonged ozone exposure. Part II. An approach to retrospective estimation of lifetime ozone exposure using a questionnaire and ambient monitoring data (California sites) Research report (Health Effects Institute) 1998:27–78. discussion 109-121. [PubMed] [Google Scholar]
- 10.Sousa SIV, Alvim-Ferraz MCM, Martins FG. Health effects of ozone focusing on childhood asthma: What is now known – a review from an epidemiological point of view. Chemosphere. 2013;90:2051–2058. doi: 10.1016/j.chemosphere.2012.10.063. [DOI] [PubMed] [Google Scholar]
- 11.Bronnimann S, Burrows B. A prospective study of the natural history of asthma. remission and relapse rates. CHEST Journal. 1986;90:480–484. doi: 10.1378/chest.90.4.480. [DOI] [PubMed] [Google Scholar]
- 12.Gerritsen J. Follow-up studies of asthma from childhood to adulthood. Paediatric respiratory reviews. 2002;3:184–192. doi: 10.1016/s1526-0542(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 13.Gold DR, Wypij D, Wang X, Speizer FE, Pugh M, Ware JH, Ferris BG, Dockery DW. Gender- and race-specific effects of asthma and wheeze on level and growth of lung function in children in six U.S. cities. American Journal of Respiratory and Critical Care Medicine. 1994;149:1198–1208. doi: 10.1164/ajrccm.149.5.8173760. [DOI] [PubMed] [Google Scholar]
- 14.Boulet LP, Turcotte H, Brochu A. PErsistence of airway obstruction and hyperresponsiveness in subjects with asthma remission. CHEST Journal. 1994;105:1024–1031. doi: 10.1378/chest.105.4.1024. [DOI] [PubMed] [Google Scholar]
- 15.van den Toorn LM, Overbeek SE, Prins J-B, Hoogsteden HC, de Jongste JC. Asthma remission: Does it exist? Current Opinion in Pulmonary Medicine. 2003;9:15–20. doi: 10.1097/00063198-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Moore BD, Hyde DM, Miller LA, Wong EM, Schelegle ES. Persistence of Serotonergic Enhancement of Airway Response in a Model of Childhood Asthma. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2013-0387OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherle WG. A simple method of volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57–60. [PubMed] [Google Scholar]
- 18.Gundersen HJG. The smooth fractionator. Journal of Microscopy. 2002;207:191–210. doi: 10.1046/j.1365-2818.2002.01054.x. [DOI] [PubMed] [Google Scholar]
- 19.Hyde DM, Blozis SA, Avdalovic MV, Putney LF, Dettorre R, Quesenberry NJ, Singh P, Tyler NK. Alveoli increase in number but not size from birth to adulthood in rhesus monkeys. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2007;293:L570–L579. doi: 10.1152/ajplung.00467.2006. [DOI] [PubMed] [Google Scholar]
- 20.Hyde DM, Tyler NK, Putney LF, Singh P, Gundersen HJG. Total number and mean size of alveoli in mammalian lung estimated using fractionator sampling and unbiased estimates of the Euler characteristic of alveolar openings, The Anatomical Record Part A: Discoveries in Molecular. Cellular, and Evolutionary Biology. 2004;277A:216–226. doi: 10.1002/ar.a.20012. [DOI] [PubMed] [Google Scholar]
- 21.Ochs M, Nyengaard JR, Jung A, Knudsen L, Voigt M, Wahlers T, Richter J, Gundersen HJG. The Number of Alveoli in the Human Lung. Am J Respir Crit Care Med. 2004;169:120–124. doi: 10.1164/rccm.200308-1107OC. [DOI] [PubMed] [Google Scholar]
- 22.Hsia CCW, Hyde DM, Ochs M, Weibel ER, on behalf of the ATS/ERS Joint Task Force on the Quantitative Assessment of Lung Structure An Official Research Policy Statement of the American Thoracic Society/European Respiratory Society: Standards for Quantitative Assessment of Lung Structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyde DM, Magliano DJ, Reus E, Tyler NK, Nichols S, Tyler WS. Computer-assisted morphometry: Point, intersection, and profile counting and three-dimensional reconstruction. Microscopy Research and Technique. 1992;21:262–270. doi: 10.1002/jemt.1070210403. [DOI] [PubMed] [Google Scholar]
- 24.Howard V, Reed M. In: Unbiased stereology: three-dimensional measurement in microscopy. Garland Science, editor. 2004. [Google Scholar]
- 25.Weibel ER, Federspiel WJ, Fryder-Doffey F, Hsia CCW, König M, Stalder-Navarro V, Vock R. Morphometric model for pulmonary diffusing capacity I. Membrane diffusing capacity. Respiration Physiology. 1993;93:125–149. doi: 10.1016/0034-5687(93)90001-q. [DOI] [PubMed] [Google Scholar]
- 26.Narayanan M, Owers-Bradley J, Beardsmore CS, Mada M, Ball I, Garipov R, Panesar KS, Kuehni CE, Spycher BD, Williams SE. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. American Journal of Respiratory and Critical Care Medicine. 2012(185):186. doi: 10.1164/rccm.201107-1348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller LA, Plopper CG, Hyde DM, Gerriets JE, Pieczarka EM, Tyler NK, Evans MJ, Gershwin LJ, Schelegle ES, Van Winkle LS. Immune and airway effects of house dust mite aeroallergen exposures during postnatal development of the infant rhesus monkey. Clinical & Experimental Allergy. 2003;33:1686–1694. doi: 10.1111/j.1365-2222.2003.01812.x. [DOI] [PubMed] [Google Scholar]
- 28.Bavis RW, Powell FL, Bradford A, Hsia CC, Peltonen JE, Soliz J, Zeis B, Fergusson EK, Fu Z, Gassmann M. Respiratory plasticity in response to changes in oxygen supply and demand. Integrative and comparative biology. 2007;47:532–551. doi: 10.1093/icb/icm070. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GS, Johnson SM. Invited Review: Neuroplasticity in respiratory motor control. Journal of applied physiology. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 30.Fujinaka LE, Hyde D, Plopper C, Tyler W, Dungworth D, Lollini L. Respiratory bronchiolitis following long-term ozone exposure in bonnet monkeys: a morphometric study. Experimental lung research. 1985;8:167–190. doi: 10.3109/01902148509057520. [DOI] [PubMed] [Google Scholar]
- 31.Grootendorst DC, Sont JK, Willems LNA, Kluin-Nelemans JC, Van Krieken JHJM, Veselic-Charvat M, Sterk PJ. Comparison of inflammatory cell counts in asthma: induced sputum vs bronchoalveolar lavage and bronchial biopsies. Clinical & Experimental Allergy. 1997;27:769–779. [PubMed] [Google Scholar]
- 32.Burri PH. Structural aspects of postnatal lung development–alveolar formation and growth. Neonatology. 2006;89:313–322. doi: 10.1159/000092868. [DOI] [PubMed] [Google Scholar]
- 33.Tyler WS, Julian MD. In: Gross and subgross anatomy of lungs, pleura, connective tissue septa, distal airways, and structural units. P RA, editor. Boco Raton, FL: CRC Press; 1991. [Google Scholar]
- 34.Golub M, ME G. In: Standardized neonatal assessment in the rhesus monkey. Nathanson M, Parer J, editors. Ithaca, NY: Pernatology Press; p. 1984. [Google Scholar]
- 35.Delfino RJ, Zeiger RS, Seltzer JM, Street DH, McLaren CE. Association of Asthma Symptoms with Peak Particulate Air Pollution and Effect Modification by Anti-Inflammatory Medication Use. Environmental health perspectives. 2002;110:A607–A617. doi: 10.1289/ehp.021100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horner CC, Strunk RC. Age-related changes in the asthmatic phenotype in children. Current Opinion in Pediatrics. 2007;19:295–299. doi: 10.1097/MOP.0b013e32809913a2. 210.1097/MOP.1090b1013e32809913a32809912. [DOI] [PubMed] [Google Scholar]
- 37.Tran MU, Weir A, Fanucchi M, Rodriguez A, Pantle L, Smiley-Jewell S, Van Winkle L, Evans M, Miller L, Schelegle E. Smooth muscle hypertrophy in distal airways of sensitized infant rhesus monkeys exposed to house dust mite allergen. Clinical & Experimental Allergy. 2004;34:1627–1633. doi: 10.1111/j.1365-2222.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- 38.Tran M-UT, Weir AJ, Fanucchi MV, Murphy AE, Van Winkle LS, Evans MJ, Smiley-Jewell SM, Miller L, Schelegle ES, Gershwin LJ. Smooth muscle development during postnatal growth of distal bronchioles in infant rhesus monkeys. Journal of applied physiology. 2004;97:2364–2371. doi: 10.1152/japplphysiol.00476.2004. [DOI] [PubMed] [Google Scholar]
- 39.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. Journal of allergy and clinical immunology. 2011;128:451–462. doi: 10.1016/j.jaci.2011.04.047. [DOI] [PubMed] [Google Scholar]


