Abstract
Background
Childhood Attention Deficit Hyperactivity Disorder (ADHD) persists into adulthood in around half of those affected (1), constituting a major public health challenge(1). No known demographic, clinical or neuropsychological factors robustly explain clinical course, directing our focus to the brain. Herein, we link the trajectories of cerebral cortical development during childhood and adolescence with the severity of adult ADHD.
Methods
Using a longitudinal study design, 92 participants with AHD had childhood (mean 10.7 years, SD 3.3) and adult clinical assessments (mean 23.8 years, SD 4.3) with repeated neuroanatomic magnetic resonance imaging. Contrast was made against 184 matched typically developing volunteers.
Results
ADHD persisted in 34 (37%) subjects and adult symptom severity was linked to cortical trajectories. Specifically as the number of adult symptoms increased, particularly inattentive symptoms, so did the rate of cortical thinning in the medial and dorsolateral prefrontal cortex. For each increase of one symptom of adult ADHD the rate of cortical thinning increased by 0.0018mm (SE 0.0004, t=4.2, p<0.0001), representing a 5.6% change over the mean rate of thinning for the entire group. These differing trajectories resulted in a convergence towards typical dimensions among those who remitted and a fixed, non-progressive deficit in persistent ADHD. Notably, cortical thickening or minimal thinning (greater than -0.007mm/year) was found exclusively among individuals who remitted.
Conclusion
Adult ADHD status is linked with the developmental trajectories of cortical components of networks supporting attention, cognitive control and the default mode network. This informs our understanding of the developmental pathways to adult ADHD.
Keywords: Attention, Cerebral cortex, Recovery, Neuroimaging, Development, Cogntion
Introduction
Many children with ADHD do not simply grow out of their ADHD; around half of affected children will continue to meet full criteria for ADHD as adults (2). Deficits in attention are more persistent than hyperactivity and impulsivity (3, 4) and are strongly linked with academic underachievement, underemployment and problems with interpersonal relationships (5, 6). The public health impact of ADHD that persists into adulthood is substantial given that ∼2.5% of adults have the disorder (7, 8). The costs arising from direct health-care and loss of productivity lie between $3,000 to $11,000 each year for every affected individual, an estimate which does not include less quantifiable factors such as the adverse effects on quality of life, self-esteem, and impact on family members (9, 10).
Understanding the pathophysiological mechanisms underpinning variable clinical outcome of ADHD and other neuropsychiatric disorders is thus a public health priority. It is also a precursor for eventually developing tools to assist in the early identification of those who are likely to show persistence as opposed to remission of ADHD symptoms. Epidemiological, clinical and neuropsychological studies have generally not yielded findings that robustly explain clinical outcome: this includes variables such as gender, ethnicity, socio-economic class and childhood symptom severity (11-15). Indeed, only the presence of other comorbid childhood diagnoses emerges as a consistent predictor of persistence of ADHD into adulthood, and the link is not strong (16, 17). This prompts a search for changes in brain structure that may more directly drive clinical outcome.
We focus on cerebral cortical structure, specifically its thickness, given that studies have found that the cortical mantle is thinner in adults with ADHD in regions important for cognitive control and attention- principally the cingulate cortex, the dorsolateral prefrontal cortex (18-21). A thinner cortex has also been reported in the more posterior cortical regions, particularly midline regions such as the precuneus and cuneus (Proal et al 2011). While highly informative, previous imaging studies have been exclusively cross-sectional, which limits the delineation of the neurodevelopmental trajectories that might characterize ADHD, particularly its variable clinical outcome.
We thus examined the association between trajectories of cerebral cortical development during childhood and adolescence and adult ADHD symptoms. Based on our previous preliminary work, we reasoned that clinical improvement would be associated with a convergence towards typical cortical dimensions, and persistence with a divergence away from typical dimensions (22, 23). To examine this question we use a unique data set that combines repeated clinical assessment from childhood into adulthood with neuroanatomic magnetic resonance imaging to link the trajectories of cerebral cortical development with adult symptoms of ADHD.
Methods
All participants are part of a longitudinal study into the neurobiology of ADHD at the intramural program of NIH. The principal inclusion criterion for initial study entry was childhood ADHD, diagnosed using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria and determined using the Parent Diagnostic Interview for Children and Adolescents (24). All participants additionally had a Conners' Teacher Hyperactivity rating greater than 2SD above age and sex specific means. Exclusion criteria were a full-scale IQ of less than 80, evidence of medical or neurological disorders, or any other axis I psychiatric disorder requiring treatment with medication at study entry. All participants had at least one magnetic resonance image (MRI) of the brain acquired below the age of 17 years. Socioeconomic status was defined using the Hollingshead Scales (25) and intelligence quotient was estimated using age appropriate version of the Wechsler intelligence scales.
Inclusion into this follow-up study was based on age: eligible individuals had reached the age of 17 years or over at the time of the study. Assessment of adult ADHD (at age l7 years or older) was obtained through clinical interviews (either PS or WS) using the clinician administered ADHD-RS-IV, providing examples and prompts appropriate for late adolescent and young adult group (26). This interviewer rates each of the 9 possible DSM-IV-R symptoms of inattention and 9 symptoms of hyperactivity/impulsivity on a 4–point Likert scale: 0=none, 1=mild; 2=moderate=3=severe. In the primary analysis we used a rating of ‘severe’ as the cut-off to define symptom presence. We also repeated analyses using the ‘moderate’ cut-off for symptoms (and obtained very similar results). ADHD, combined type, is diagnosed when an individual has both six or more symptoms of inattention and six or more symptoms hyperactivity/impulsivity. The inattentive or hyperactive/impulsive subtypes are diagnosed when symptoms are confined to these domains. Inter-rater reliability was high (kappa>0.92). Presence of other axis 1 psychiatric diagnoses was established through the Structured Clinical Interview for DSM Disorders. Also obtained was the Conners Adult ADHD Rating Scale long version (CAARS), which provides self-assessment of severity of ADHD symptoms and has a well-established factor structure, reliability, and validity (27). The proportion of time that subjects were treated with psychostimulants throughout the study was determined from patient records.
Comparison was made against typically developing controls, drawn from a study of typical brain development. Two comparison subjects were drawn for each ADHD individual. All comparison subjects remained free of all axis 1 DSM-IV mental disorders throughout the duration of the study. The ADHD and typically developing control groups were matched on the sex, age of assessments, socio-economic status, and intelligence.
Two hundred and three individuals from the original cohort of children with ADHD were 17 years or older at the time of the follow-up study (2006-2011) and thus eligible for follow-up. At study entry 191 (94%) of these individuals had combined type ADHD; 8 (4%) had inattentive subtype and 3 (2%) had predominately hyperactive-impulsive subtype. Of these 203 individuals, reassessment in adulthood was completed in 111 (55%). The reasons for loss to follow-up are given in Figure S1 in the Supplement. Of these 111 subjects, 19 had MRI data which showed movement or others artifacts. This left 92 individuals with both clinical assessments and neuroimaging acquired in both in childhood and adulthood. This group of 92 individuals was the basis for the primary longitudinal analyses. At study entry the mean age of this group was 10.7 years (SD 3.3). Of these 92 individuals, at study entry 87 had combined type ADHD, 3 had predominately inattentive subtype and 2 had predominately hyperactive-impulsive subtype. This group of 92 individuals had a higher socio-economic status than those lost to follow-up and a trend to higher estimated intelligence, but did not differ significantly in sex, age of study entry or baseline clinical symptom severity- See Table S1 in the Supplement. The mean age of final assessment of these 92 individuals was 23.8 years, (SD 4.3) and the mean duration of follow-up was 13.1 years (SD 3.6 years). Scans in the different outcome groups were well balanced in terms of the date of acquisition.
The institutional review board of the National Institutes of Health approved the research protocol, and written informed consent and assent to participate in the study were obtained from parents and children, respectively.
Neuroanatomic methods
Tl-weighted images with contiguous 1.5-mm axial slices were obtained using three-dimensional spoiled gradient recalled echo in the steady state on a 1.5-Tesla GE Signa scanner (General Electric Co., Milwaukee). Imaging parameters were as follows: echo time=5 msec, repetition time-24 msec, flip angle-45, acquisition matrix=256×192, number of excitations=1, field of view=24 cm. The same scanner was utilized throughout the study. Native magnetic resonance imaging (MRI) scans were masked using the Brain Extraction Tool (28), registered into standardized stereotaxic space using a nine-parameter linear transformation (29), corrected for nonuniformity artifacts (30) and segmented (31, 32). The Constrained Laplacian Anatomic Segmentation using Proximity surface extraction procedure generated surface meshes representing white and gray matter interfaces (33). The root mean square thickness between corresponding nodes on the surface meshes was calculated in native space. A 30-mm surface blurring algorithm, which preserves cortical topologic features, was used to reduce noise in thickness measurements (34). Thickness measurements were aligned using surface registration to maximize thickness value correspondence between participants in terms of gyral patterning (35).
Analyses
In the primary analysis we determined where the trajectory of cortical development from childhood into adulthood was associated with the number of ADHD symptoms in adulthood. We treated symptom scores as a continuous variable in this analysis. Symptom scores provide more variance as an outcome measure than categories and thus may augment the detection of outcome related cortical changes. Additionally, there is ongoing discussions on the appropriate number of symptoms required to make a diagnosis of adult ADHD in the forthcoming revision of the DSM (36). We thus regressed cortical thickness against symptom score, age, and the interaction between symptom score and age using mixed-model regression. This approach was taken as our data contains different numbers of observations in participants, measured at different and irregular time periods (37). A random effect for each individual was included to account for within-person dependence. Thus, for cortical points, the jth cortical thickness of the ith individual was modeled using the following equation,
where di is a random effect modeling within-person dependence; the intercept and β terms are fixed effects, and eij represents the residual error. The interaction between age and symptoms is given by the β3 fixed effect. This slope parameter denotes how the cortical thickness changes with age as a function of final adult symptom score. T tests were used to test the significance of each parameter in the mixed model regression and the results projected onto a brain template. We also applied a false discovery rate procedure to adjust for the multiple comparisons. A linear age model was used previous studies suggest that the dominant effect of age is linear decline over this age range ((38-41). Graphs illustrating the developmental trajectories of clusters were generated using fixed-effects parameter estimates. The false discovery rate procedure was applied to control for multiple comparisons with a q value of 0.05 (42). In a subsidiary, illustrative analysis we used ordinary least square regression to calculate the slopes (or cortical trajectories) in regions that were linked to outcome in the primary mixed model analysis for each individual. This was done purely for illustrative purposes and these individual level slopes were not used in any further analyses.
Comparison was made against a group of 184 typically developing youth (providing a 2 to 1 match) with a total of 498 neuroanatomic scans- see Table S1 in the Supplement. In these analyses, the ADHD subjects were grouped into those who showed clinical remission versus those who had persistent ADHD. Remission in these analyses was defined as having fewer than 6 symptoms of inattention and fewer than 6 of hyperactivity/impulsivity. The ‘persistent’ ADHD group combined the three forms of ADHD (inattentive/ hyperactive-impulsive and combined) allowing for a more robust estimate of group trajectory. It should be noted that the persistent group thus contains individuals who improve in the sense of moving from combined type ADHD in childhood to predominately inattentive or predominately hyperactive-impulsive subtype by adulthood. Nonetheless, all individuals in the ‘persistent’ group meet current DSM-IV criteria for one of the subtypes of ADHD.
To determine whether any cortical regions in the baseline childhood scan were associated with later adult outcome we regressed baseline cortical thickness values against adult ADHD symptom counts.
Results
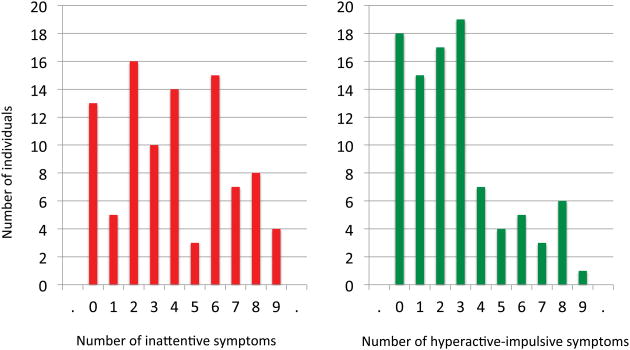
By adulthood, the mean number of total symptoms for the ADHD group was 6.4 (SD 4.3) and the distribution of symptoms is shown in Figure 1. Inattentive symptoms were more persistent (mean 3.8, SD 2.6) than hyperactive and impulsive symptoms (mean 2.6, SD 2.3). This was reflected in adult DSM-IV diagnoses: of the 92 subjects with longitudinal neuroimaging data, 21 (23%) had inattentive subtype, 9 (10%) had combined type and 4 (4%) had hyperactive-impulsive subtype ADHD. Fifty-eight subjects (63%) no longer met DSM-IV criteria for ADHD by adulthood.
Figure 1.
Distributions of the number of inattentive and hyperactive-impulsive symptoms in adulthood.
The rate of persistent ADHD was higher in females but the outcome groups did not differ significantly in estimated intelligence, baseline clinical characteristics, socio-economic status ethnicity- Table 1. There was a trend to higher rates of psychiatric current comorbidities in those with persistent ADHD. As would be expected, psychostimulant treatment at the time of final assessment was significantly more common among those with the persisting disorder (χ2=ll, p<0.001). However, the proportion of the time on psychostimulant medication during the entire study did not differ significantly between the outcome groups – see Table 1b and Supplement 1.
Table 1.
Demographic and clinical characteristics of the ADHD group who remitted or persistence at the time of study entry and at the time of final assessment.
| STUDY ENTRY | |||
|---|---|---|---|
|
| |||
| ADHD: remitted | ADHD persistent | ||
|
| |||
| Age (yrs) Mean (SD) |
10.5 (3.3) | 10.8 (3.4) | t(90)=0.45, p=0.65 |
|
| |||
| Sex Male: Female |
37:18 | 16:21 | χ(l)2=5.2, p=0.03 |
|
| |||
| IQ Mean (SD) |
109 (13) | 112 (17) | t(84)=0.73, p=0.46 |
|
| |||
| Socioeconomic status Mean (SD) |
45 (21) | 42 (21) | t(88)=0.73, p=0.46 |
|
| |||
| CBCL attention problems Mean (SD) |
72 (10) | 73(6) | t(88)=0.77, p=0.44 |
|
| |||
| CBCL externalizing problems | 66 (11) | 65 (11) | t(88)=0.97, p=0.33 |
|
| |||
| FINAL ASSESSMENT | |||
|
| |||
| ADHD: remitted | ADHD: persistent | ||
|
| |||
| Age (yrs) Mean (SD) | 24.2 (4.4) | 23.1 (4.0) | t(90)=1.27, p=0.21 |
|
| |||
| Symptoms | |||
|
| |||
| DSM IV symptoms: | |||
| Inattention | 2.2 (1.5) | 6.7 (1.5) | t(90)=14.0, p<0.0001 |
| Hyperactivity-impulsivity | 1.8 (1.4) | 4.4 (2.7) | t(90)=5.83, p<0.001 |
|
| |||
| CAARS | |||
| Inattention | 53 (12) | 68(11) | t(81)=5.4, p<0.001 |
| Hyperactivity | 52 (10) | 61(11) | t(81)=3.3, p=0.001 |
| Impulsivity | 49 (10) | 59 (12) | t(81)=3.7, p<0.001 |
| Self-concept | 49 (11) | 60 (12) | t(81)=1.9, p=0.06 |
| DSM inattentive | 60 (14) | 75 (11) | t(81)=4.7 p<0.001 |
| DSM hyperactive | 53(11) | 63(13) | t(81)=3.7, p=0.001 |
| DSM total | 59 (13) | 73 (11) | t(81)=4.7, p<0.001 |
|
| |||
| Comorbidity | |||
|
| |||
| Any comorbidity Mood: | 9 (16%) | 12 (32%) | Any comorbidity vs none, exact p=0.08 |
| Depression | 3 | 6 | |
| Dysthymia | 1 | 3 | |
|
| |||
| Anxiety: | |||
| GAD | 4 | 2 | |
| Anxiety NOS | 0 | 3 | |
| OCD | 0 | 1 | |
| PTSD | 1 | 1 | |
|
| |||
| Psychostimulant medication use | |||
|
| |||
| Proportion of study on medication | 50% (33%) | 59% (29%) | t(88)=1.13, p=0.26 |
|
| |||
| Psychostimulant use at follow-up | Medicated vs unmedicated χ2=11, p<0.001 | ||
| Daily | 1 (2%) | 9 (24%) | |
| Intermittent (<l/wk) | 4 (7%) | 5 (14%) | |
| None | 50 (91%) | 23 (62%) | |
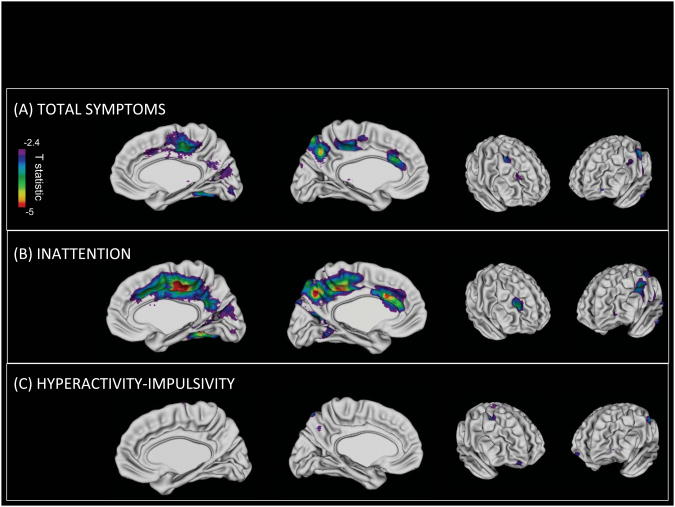
In the primary analysis we determined where trajectories of cortical development from childhood into adulthood were significantly associated with ADHD symptoms in adulthood. In the right hemisphere, cortical slopes in the medial cortical wall and dorsolateral prefrontal cortex were associated with the total number of adult symptoms, following adjustment for multiple comparisons – Figure 2. The medial regions centered on the cingulate gyrus, in its posterior regions with extension into the caudal regions and isthmus. Additionally, there was extension along the medial prefrontal cortex and posteriorly into the precuneus, cuneus and fusiform gyri. In these regions, the rate of cortical thinning increased by 0.0009mm/year (SE0.00023mm/year) for each increase of one symptom. Details for each region are given in Table 2. In the left hemisphere, adult ADHD symptoms were associated with the cortical trajectories in similar medial regions, specifically the caudal anterior cingulate, the paracentral lobule, precuneus and the postcentral gyrus. The magnitude of the increase in cortical thinning associated with increasing adult ADHD symptoms was -0.0010mm/year (SE 0.00024mm/year). To place these results in perspective, the rate of cortical thinning in the regions shown in Figure 2 for the entire group with ADHD was -0.017 mm/year (SE 0.0011mm/year). The increasing rate of thinning accompanying each increase of one symptom represents a 5.6% change over this group estimate. There was also a nominally significant association between trajectories in the right inferior parietal lobule and adult ADHD status, although this did not survive the adjustment for multiple comparisons.
Figure 2.
The top panel shows regions where the total number of ADHD symptoms in adulthood are significantly associated (p<0.05, adjusted for multiple comparisons) with the cortical trajectories from childhood into adulthood. The association is stronger for inattentive (B) than hyperactive-impulsive symptoms (C).
Table 2.
The slope parameter indicates the change in cortical thinning accompanying each increase of one symptom of ADHD. The negative values indicate that increasing numbers of ADHD symptoms are accompanied by an increase in the pace of cortical thinning. The standard error of the slope parameter and associated t value are given along with the spatial extent of the regions shown in Figure 2.
| Slope parameter (mm/year) |
Standard error (mm/year) |
t | P | Cluster extent (number of vertices) |
|
|---|---|---|---|---|---|
| TOTAL SYMPTOMS | |||||
| Right hemisphere | |||||
| Medial wall | -0.00090 | 0.0002 | -3.4 | 0.0007 | 1373 |
| Dorsolateral prefrontal cortex | -0.0010 | 0.0003 | -2.9 | 0.004 | 365 |
| Inferior parietal lobule | -0.00084 | 0.0002 | -2.3 | 0.02 | 81 |
| Left hemisphere | |||||
| Medial wall | -0.0010 | 0.0002 | -4.1 | 0.0001 | 2249 |
| Postenctral gyrus | -0.0087 | 0.0002 | -3.3 | 0.0009 | 359 |
| INATTENTIVE SYMPTOMS | |||||
| Right hemisphere | |||||
| Medial wall | -0.0018 | 0.0004 | -4.1 | 0.0001 | 3131 |
| Dorsolateral prefrontal cortex | -0.0019 | 0.0006 | -3.1 | 0.002 | 415 |
| Inferior parietal lobule | -0.0014 | 0.0005 | -2.2 | 0.01 | 50 |
| Left hemisphere | |||||
| Medial wall | -0.0019 | 0.0004 | -4.4 | 0.0001 | 4694 |
| Postcentral gyrus | -0.0017 | 0.0004 | -4.1 | 0.0001 | 1353 |
The link between cortical trajectories and ADHD outcome was largely driven by inattentive symptoms. The magnitude of the change in cortical thinning associated with increasing inattentive symptom counts is given in Table 2b. No region remained significantly associated with adult hyperactive-impulsive symptoms following adjustment for multiple comparisons.
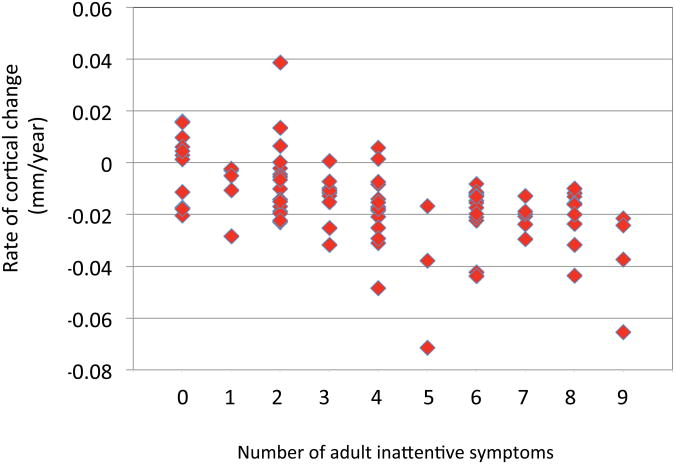
The slopes for every individual within the regions shown in Figure 2 was calculated and plotted against adult inattentive symptom counts. This illustrates that either thickening or minimal thinning of the cortex was associated with adult remission – Figure 3. Ranking the slope estimates for the right hemispheric region in Figure 2, the highest 24 values, indicating either cortical increase or a minimal cortical thinning rates of greater than -0.007mm/year were found among those who had fewer than six inattentive symptoms by adulthood. Similarly for the left hemispheric regions linked outcome, the top 17 values (rates greater than -0.01mm/year) were all found in those who remitted.
Figure 3.
Scatterplot of individual slope estimates for the medial prefrontal/cingulate regions where the primary mixed model analysis showed trajectories to be associated with adult outcome.
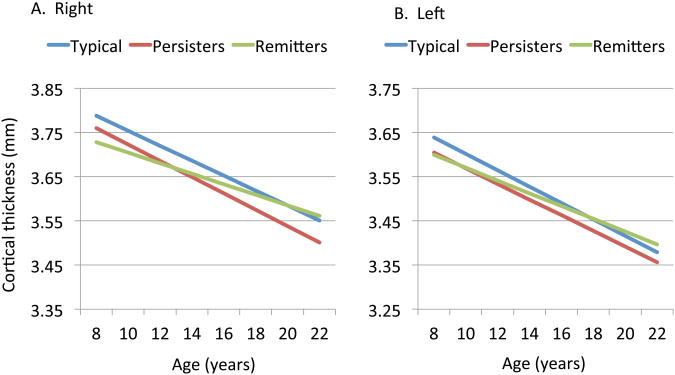
To elucidate further the impact of these dynamics of cortical change, we contrasted the cortical trajectories of the persisting and remitted ADHD groups against a typically developing group – Figure 4. For the regions linked with adult total ADHD symptoms score (shown in Figure 3) the remitted group showed a less negative slope (i.e. slower rate of cortical thinning) in the medial/cingulate cortex (right -0.013mm/year, SE 0.001; left -0.018mm/year SE 0.0016) than the persistent ADHD group (right hemisphere, -0.021 mm/year, SE 0.002, t=3.28, p=0.001; left hemisphere, -0.026 mm/year, SE 0.002; t=3.37, p=0.0008). This result would be expected as this is a categorical re-description of the earlier symptom score findings. The remitted group also showed a slower estimated rate of cortical thinning than the typically developing group (right -0.019mm.year SE 0.0009, t=3.05, p=0.002; left -0.024mm/year SE 0.001, t=3.32, p=0.0009). The typically developing and persistent ADHD group did not differ significantly in cortical trajectory in this region (all p>0.1). As a result of these different slopes, the remitted ADHD group converged to typical dimensions with age whereas the persistent ADHD group showed a fixed, non-progressive deficit. Slope estimates for each group for the remaining cerebral cortical regions are given in Table S2 in the Supplement.
Figure 4.
Cortical thickness in regions linked to adult ADHD status for the right (A) and left (B) hemispheres. The trajectories differed significantly between the typically developing controls and remitted ADHD group (all p<0.001), but not between the typically developing and persistent ADHD groups (p>0.1). The two ADHD groups also had significantly different trajectories (p<0.001).
Finally, we examined whether any cortical region in the baseline scans were associated with adult ADHD symptoms. No regions were found.
We found no higher order interactions between sex, age and symptoms in the determination of cortical thickness - see Figure S2 in the Supplement. The slopes of cortical trajectories also did not correlate significantly with any measure of baseline symptom severity (all p>0.1). Results also held when the values of cortical thickness at the time of the last scan were entered as a covariate. The pattern of results held when the 21 subjects with any comorbid conditions were excluded – see Figure S3A in the Supplement.
Slope estimates in the cortical regions linked with clinical outcome also did not correlate significantly with the proportion of time on psychostimulants during the study (for the regions linked with total symptom count, r= -0.17, p=0.11; for regions linked with inattentive symptom count r=-.14, p=0.19). Results were largely unchanged when the 23 subjects who were taking psychostimulants at the time of the final assessment were excluded- see Figure S3B in the Supplement.
Discussion
Trajectories of cerebral cortical development from childhood into adulthood are associated with the adult outcome of ADHD. In predominately medial cortical regions, higher rates of cortical thinning are associated with higher levels of adult ADHD symptoms.
The differing trajectories were centered bilaterally on the cingulate gyrus and medial prefrontal cortex extending to the precuneus and the right dorsolateral prefrontal cortex. These regions span several of the brain networks most closely linked with ADHD. Firstly, the cingulate is a key hub of the cingulo-operculum network that maintains task performance and monitors for behaviorally salient stimuli (43). Additionally, we link outcome with components of the fronto-parietal control network, specifically the anterior cingulate, dorsolateral prefrontal cortex and inferior parietal lobule. These richly interconnected regions guide much goal-directed behavior, controlling cognitive processes such as the inhibition inappropriate responses and the adjustment of behavior in the response to feedback (44, 45). Decreased activation of these cortical regions found in ADHD during tasks requiring cognitive control and working memory and may underpin the symptoms of the disorder (45, 46). These functional anomalies are complemented by reports of compromised structural integrity in these structures and interconnecting white matter tracts (18-21, 47). Finally, clinical outcome was also linked with the developmental trajectory of the posterior cingulate gyrus. This is a central hub in the network of brain regions that show decreased activity during cognitive tasks but increased activity during rest, the so-called the ‘default mode network’ (48, 49). Abnormal activity in this default mode network has been found in ADHD and may lead to disruptions in task-related brain activity, manifesting as lapses in attention that are a cardinal feature of ADHD (50-52). The clinical course of ADHD is thus linked with the developmental trajectory of key cortical components of the executive control, attentional and default mode networks. In this context, it is unsurprising that our findings are driven more by the course of inattentive than hyperactive-impulsive symptoms.
We find that thickening or minimal thinning (greater than -0.007mm/year) of these cortical regions occurred exclusively among those whose symptoms, particularly those of inattention, decreased by adulthood to a level below the current threshold for diagnosis. However, it is important to note that clinical improvement was also associated with cortical thinning. This observational study was not designed to provide prognostic biomarkers, however, the associations we find between cortical trajectories and the clinical endpoint of adult ADHD status suggest that cortical trajectories are a promising tool for future study.
It has been argued that structural and functional normalization of the cortex is the mechanism underpinning symptomatic remission in ADHD (22, 53). Ideally this hypothesis should be tested using neuroimaging within the context of a clinical trial but in the absence of this data, our observational supports this concept. We find that the remitted ADHD group tends to converge toward typical dimensions whereas the persistent ADHD group shows more fixed, non-progressive deficits. The trajectory differences associated with adult outcome remained significant following adjustment for multiple comparisons only in the medial prefrontal/cingulate and dorsolateral prefrontal cortical regions shown in Figure 2, but were present at a nominal level of significance in other lateral cortical regions. In an earlier study into the cortical correlates of adolescent outcome (mean age of 15 years) with a shorter follow-up period of ∼6 years, we likewise found links between right parietal cortical trajectories and clinical course (23). Those who showed clinical improvement showed convergence towards more typical cortical dimensions and this link held in the current study, albeit at a nominal level of significance. The current study, which raises the mean age of follow-up to 23.5 years, reports more extensive links between clinical outcome and trajectories, particularly in medial prefrontal cortical regions. One possibility is that an adolescent endpoint is too ‘early’ to capture fully the cortical correlates of improving inattention. The current study also uses a version of neuroimaging software that affords a more precise definition of the medial cortical wall than was available at the time of the earlier study(35). While we find stronger associations between cortical trajectories and inattentive symptoms this could in part reflect the lack of variance in hyperactive-impulsive symptoms at the adult endpoint. A larger study would be needed to test this possibility, and would also allow us to contrast the trajectories of the different subtypes of adult ADHD – combined, inattentive and hyperactive-impulsive. We treated symptom scores as a continuous rather than ordinal variable in this study, although note the paucity of evidence supporting this assumption.
The study benefitted from the use throughout the entire study of the same neuroimaging sequences acquired on the same magnetic resonance scanner. While the integration of data sets collected using different scanners and sequences is possible (54), it presents challenges. The inclusion criteria for the study resulted in a homogenous, severely affected phenotype at baseline: nearly all subjects had combined type ADHD and were relatively free of other major psychiatric comorbidities beyond oppositional defiant disorder. While this enhances the applicability of the results to the ‘pure’ syndrome it limits the generalizability of the findings to children with either inattentive or hyperactive-impulsive subtype ADHD. Our subjects were also drawn from a relatively socio-economically affluent area and a priority for future work is the inclusion of a more diverse sample. This factor may also contribute to the high IQ in our participants, an important limitation as intelligence might be relevant to the brain phenotype in ADHD and lower intelligence and ADHD may share genetic factors (55, 56)(57, 58).
As would be expected, the rates of psychostimulant use at the time of final assessment were higher in those with persistent ADHD, which raises the possibility that medication may contribute to the findings. However, the results held when analyses were confined to those who were unmedicated at the time of final assessment. Additionally, we found no significant difference between the outcome groups in the proportion of time on psychostimulants during the study, although this measure did not include estimates of total lifetime medication dose and relied on participant report. We also do not have systematic data on non-pharmacological interventions received by the individuals. Attrition rates were around 45%, and while those lost to follow-up did not differ systematically in most measured baseline variables they did have a lower socio-economic status than those in the follow-up study. We note however that our finding that 37% of individuals still meet diagnostic criteria for ADHD in adulthood is in line with previous estimates of rates of persistence and further emphasizes that ADHD can no longer be considered a disorder confined to childhood (3, 17).
Using longitudinal data we delineate individual differences in cortical development that are associated with adult ADHD. We find that these differences localize to the candidate neural systems most strongly implicated in the disorder, throwing light onto the developmental pathways to adult ADHD.
Supplementary Material
Acknowledgments
This study was funded by the Intramural Programs of the National Human Genome Research Institute and the National institute of Mental Health.
Footnotes
Financial disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halperin J, W T, J C, DJ M, J N. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry. 2008;49:958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lara C, Fayyad J, de Graaf R, Kessler RC, Aguilar-Gaxiola S, Angermeyer M, et al. Childhood Predictors of Adult Attention-Deficit/Hyperactivity Disorder: Results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiatry. 2009;65:46–54. doi: 10.1016/j.biopsych.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 4.Martel MM, von Eye A, Nigg J. Developmental differences in structure of attention-deficit/hyperactivity disorder (ADHD) between childhood and adulthood. International Journal of Behavioral Development. 2012;36:279–292. doi: 10.1177/0165025412444077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polderman TJC, Boomsma DI, Bartels M, Verhulst FC, Huizink AC. A systematic review of prospective studies on attention problems and academic achievement. Acta Psychiatrica Scandinavica. 2010;122:271–284. doi: 10.1111/j.1600-0447.2010.01568.x. [DOI] [PubMed] [Google Scholar]
- 6.Pingault JB. Childhood trajectories of inattention and hyperactivity and prediction of educational attainment in early adulthood: a 16-year longitudinal population-based study. The American journal of psychiatry. 2011;168:1164. doi: 10.1176/appi.ajp.2011.10121732. [DOI] [PubMed] [Google Scholar]
- 7.Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. The British Journal of Psychiatry. 2009;194:204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- 8.Vetter VL, Elia J, Erickson C, Berger S, Blum N, Uzark K, et al. Cardiovascular Monitoring of Children and Adolescents With Heart Disease Receiving Medications for Attention Deficit/Hyperactivity Disorder. Circulation. 2008;117:2407–2423. doi: 10.1161/CIRCULATIONAHA.107.189473. [DOI] [PubMed] [Google Scholar]
- 9.Bernfort L, Nordfeldt S, Persson J. ADHD from a socio-economic perspective. Acta Pædiatrica. 2008;97:239–245. doi: 10.1111/j.1651-2227.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaum HG, Kessler RC, Lowe SW, Secnik K, Greenberg PE, Leong SA, et al. Costs of attention deficit, Äìhyperactivity disorder (ADHD) in the US: excess costs of persons with ADHD and their family members in 2000. Current Medical Research and Opinion. 2005;21:195–205. doi: 10.1185/030079904X20303. [DOI] [PubMed] [Google Scholar]
- 11.Bussing R, Mason DM, Bell L, Porter P, Garvan C. Adolescent Outcomes of Childhood Attention-Deficit/Hyperactivity Disorder in a Diverse Community Sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:595–605. doi: 10.1016/j.jaac.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauermeister JJ, Bird HR, Shrout PE, Chavez L, Ramirez R, Canino G. Short-Term Persistence of DSM-IV ADHD Diagnoses: Influence of Context, Age, and Gender. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:554–562. doi: 10.1016/j.jaac.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biederman J, FS MS, et al. A prospective 4-year follow-up study of attention-deficit hyperactivity and related disorders. Archives of General Psychiatry. 1996;53(437):446. doi: 10.1001/archpsyc.1996.01830050073012. [DOI] [PubMed] [Google Scholar]
- 14.Lahey BB, Pelham WE, Loney J, Kipp H, Ehrhardt A, Lee SS, et al. Three-Year Predictive Validity of DSM-IV Attention Deficit Hyperactivity Disorder in Children Diagnosed at 4, Äì6 Years of Age. Am J Psychiatry. 2004;161:2014–2020. doi: 10.1176/appi.ajp.161.11.2014. [DOI] [PubMed] [Google Scholar]
- 15.Monuteaux MC, Mick E, Faraone SV, Biederman J. The influence of sex on the course and psychiatric correlates of ADHD from childhood to adolescence: A longitudinal study. Journal of Child Psychology and Psychiatry. 2010;51:233–241. doi: 10.1111/j.1469-7610.2009.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biederman J, Petty CR, Dolan C, Hughes S, Mick E, Monuteaux MC, et al. The long-term longitudinal course of oppositional defiant disorder and conduct disorder in ADHD boys: findings from a controlled 10-year prospective longitudinal follow-up study. Psychol Med. 2008;38:1027–1036. doi: 10.1017/S0033291707002668. [DOI] [PubMed] [Google Scholar]
- 17.Lara C, Fayyad J, de Graaf R, Kessler RC, Aguilar-Gaxiola S, Angermeyer M, et al. Childhood Predictors of Adult Attention-Deficit/Hyperactivity Disorder: Results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiatry. 2009;65:46–54. doi: 10.1016/j.biopsych.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, et al. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- 19.Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, et al. Brain Gray Matter Deficits at 33-Year Follow-up in Adults With Attention-Deficit/Hyperactivity Disorder Established in Childhood. Arch Gen Psychiatry. 2011;68:1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amico F, Stauber J, Koutsouleris N, Frodl T. Anterior cingulate cortex gray matter abnormalities in adults with attention deficit hyperactivity disorder: A voxel-based morphometry study. Psychiatry Research: Neuroimaging. 2011;191:31–35. doi: 10.1016/j.pscychresns.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Shaw P, Gogtay N, Rapoport J. Childhood Psychiatric Disorders as Anomalies in Neurodevelopmental Trajectories. Hum Brain Mapp. 2010;31:917–925. doi: 10.1002/hbm.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 24.Reich W. Diagnostic interview for children and adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Hollingshead A. Four Factor Index of Social Status. New Haven: Yale University Department of Sociology; 1975. [Google Scholar]
- 26.DuPaul GJ, Power JD, Anastopouls AA, Reid R. ADHD Rating Scale-IV: Checklists, Norms and Clinical Interpretation. New York: The Guilford Press; 1998. [Google Scholar]
- 27.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 28.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18:192–205. [PubMed] [Google Scholar]
- 30.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 31.Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Transactions on Medical Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 32.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–93. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 34.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 35.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 36.Association AP. DSM-5 development. ADHD; 2012. [Google Scholar]
- 37.Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- 38.Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- 39.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. NeuroImage. 2005;24:948–954. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Raznahan A, Lerch JP, Lee N, Greenstein D, Wallace GL, Stockman M, et al. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 43.Petersen SE, Posner MI. The Attention System of the Human Brain: 20 Years After. Annual Review of Neuroscience. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Posner MI, DiGirolamo GJ. Executive attention: conflict, target detection and cognitive control. In: Parasuraman R, editor. The attentive brain. Cambridge, MA: MIT press; 1998. [Google Scholar]
- 45.Bush G. Cingulate, Frontal, and Parietal Cortical Dysfunction in Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2011;69:1160–1167. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubia K. Cool Inferior Frontostriatal Dysfunction in Attention-Deficit/Hyperactivity Disorder Versus Hot Ventromedial Orbitofrontal-Limbic Dysfunction in Conduct Disorder: A Review. Biol Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 47.Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, et al. Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cereb Cortex. 2008;18:1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- 48.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 49.Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain's Default Network. Ann N YAcadSci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 50.Tian L, Jiang T, Liang M, Zang Y, He Y, Sui M, et al. Enhanced resting-state brain activities in ADHD patients: A fMRI study. Brain and Development. 2008;30:342–348. doi: 10.1016/j.braindev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Uddin LQ, Kelly AMC, Biswal BB, Margulies DS, Shehzad Z, Shaw D, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 52.Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132:560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- 54.Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodman R, Simonoff E, Stevenson J. The Impact of Child IQ, Parent IQ and Sibling IQ on Child Behavioural Deviance Scores. Journal of Child Psychology and Psychiatry. 1995;36:409–425. doi: 10.1111/j.1469-7610.1995.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 56.Mariani MA, Barkley RA. Neuropsychological and academic functioning in preschool boys with attention deficit hyperactivity disorder. Developmental Neuropsychology. 1997;13:111–129. [Google Scholar]
- 57.Kuntsi J, Eley TC, Taylor A, Hughes C, Asherson P, Caspi A, et al. Co-occurrence of ADHD and low IQ has genetic origins. Am J Med Genet B Neuropsychiatr Genet. 2004;124:41–47. doi: 10.1002/ajmg.b.20076. [DOI] [PubMed] [Google Scholar]
- 58.de Zeeuw P, Schnack HG, van Belle J, Weusten J, van Dijk S, Langen M, et al. Differential Brain Development with Low and High IQ in Attention-Deficit/Hyperactivity Disorder. PLoS ONE. 2012;7:e35770. doi: 10.1371/journal.pone.0035770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.