Abstract
Stimulant use rates are higher among men who have sex with men (MSM) than the general population. Contingency management (CM) may be an effective intervention for reducing stimulant use in this population. To specify both the mechanism and temporal effects of contingent reward on behavior change, logistic growth trajectory modeling (LGTM) was used to contrast a non-contingent matched rewards condition (i.e., non-contingent yoked controls; NCYC) to a voucher-based CM intervention (maximum= $430) to reduce stimulant use among MSM. Stimulant-using MSM were randomized to either a CM intervention (n=70) or a NCYC condition (n=70). Results from a LGTM (analytical sample n=119; nCM=61; nNCYC=58) indicated four distinct intervention response patterns: responders (i.e., predicted > 90% stimulant metabolite-free urinalyses; 64.7% of sample); worsening intervention response (14.3%); non-responders (12.6%); and, single-positive (8.4%); all estimated trajectory coefficients were significant at p < 0.03 (2-tailed). Participants receiving CM were significantly overrepresented in the responder (64%) and single-positive (80%) categories (χ2(3) = 29.04; p < 0.001); all non-responders and 76.5% of the worsening intervention response category were in the NCYC condition. Results demonstrate the utility of trajectory modeling and further support the contingent application of reward as the operative mechanism associated with patterns of stimulant abstinence with CM applied to a sample of stimulant-using MSM outside the context of formal drug treatment.
Keywords: men who have sex with men (MSM), stimulants, contingency management, methamphetamine, HIV
1. Introduction
Among men who have sex with men (MSM) in the United States (U.S.), reported use of stimulants, including methamphetamine, is 10-19 times higher than the general population (Finlayson et al., 2011; SAMHSA, 2013). Among communities of MSM who misuse stimulants, a series of health conditions co-occur (Beyrer et al., 2012; Mimiaga et al., 2015) with corresponding and increased rates of condomless sex (Freeman et al., 2011; Boone, Cook and Wilson, 2013, Benotsch, Lance, Nettles, and Koester, 2012) and risks for HIV infection (Koblin et al., 2006; Plankey et al., 2007; Ostrow et al., 2009).
1.1 Contingency Management to Reduce Stimulant Use in MSM
Contingency management (CM) is currently the most effective treatment for stimulant use disorder among individuals seeking treatment, with an effect size averaging d= 0.6 (Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwall, and Roll, 2006; Dutra et al., 2008). CM interventions are effective at reducing methamphetamine use in MSM seeking drug treatment (Carrico et al., 2015, Shoptaw et al., 2005). Superior outcomes during CM treatment compared to standard cognitive behavioral therapy are observed, with methamphetamine use reductions maintained to one-year follow-up evaluations (Shoptaw et al., 2005). Methamphetamine use reductions produced by CM also produce significant reductions in reported unprotected sex behaviors for both HIV-positive and HIV-negative MSM, signaling one way that efficacious behavioral drug treatment may be used to limit HIV transmission risk behaviors in a highly HIV prevalent group.
Our group conducted a study in which stimulant-using, non-treatment-seeking MSM were enrolled in a post-exposure prophylaxis (PEP) study designed to reduce HIV seroconversion after a high-risk exposure. Because ongoing stimulant use is likely to interfere with uptake and completion of PEP, a CM voucher incentive protocol was included as a randomized intervention to examine impact of stimulant use reduction on response to the PEP protocol. The study found that participants randomly assigned to contingent incentives submitted significantly more stimulant negative urines than those assigned to a non-contingent yoked control (NCYC) condition (8.9 vs 6.1, p = .035; Landovitz et al., 2015). The CM intervention for stimulant use was also associated with higher rates of initiation and completion of the PEP protocol, which was the primary outcome of the study.
The present paper presents a secondary analysis of stimulant use data from that prior study using a novel logistic growth trajectory modeling analysis. The first goal was to extend previous observations by providing more detailed information about individual differences in response to CM. The second goal was to provide further evidence from the trajectory analysis that the contingency between reward and stimulant abstinence is a critical determinant of efficacy in an abstinence incentive intervention.
2. Material and Methods
2.1 Participants
Recruitment occurred through both on-line and print advertising, as well as through venue-based outreach at clubs, bars, commercial sex venues, and service locations frequented by MSM stimulant users. Participants (N = 140; enrolled June 2010 – June 2012) were HIV-negative (verified at baseline using Oraquick) men who reported engaging in sex with other men, who self-reported any stimulant use in the past 30 days, and who reported condomless anal intercourse with an HIV-positive or serostatus-unknown partner in the past three months. All participants were active stimulant users who agreed to enroll in a study that offered random assignment to a contingent or non-contingent CM intervention focused on stimulant use, and free access for all to a 28-day PEP treatment course to be initiated within 72 hours of a high-risk exposure (or potential exposure) to HIV. Findings, related to PEP initiation/adherence, and participants’ sexual risk behaviors, have been published previously (Landovitz et al., 2015).
2.2 Procedures
Eligible participants were randomized into either a voucher-based CM condition providing rapid-reset escalating voucher rewards for biomarker-confirmed stimulant metabolite-free urinalyses, or a non-contingent yoked control (NCYC) condition providing voucher rewards unrelated to urine metabolite outcomes but identical to those of a yoked partner (Silverman & Robles, 1999). Participants randomized to CM could earn voucher-based incentives thrice-weekly (Mondays, Wednesdays, Fridays) for presenting urine samples that were stimulant metabolite free. The first negative sample was worth $2.50, with successive negative urine samples increasing in value by $1.25 until the value of a stimulant negative urine sample reached $20, after which all subsequent negative urine samples were also valued at $20. A rapid reset procedure was applied in case of relapse. A $10 bonus was provided each time participants provided three consecutive negative samples. The maximal CM payout for 24 stimulant-metabolite free urine samples was $430. Psychiatric or other counseling was not provided to the participants. Additional information related to the study design, procedures, and primary outcomes can be found in a prior publication (Landovitz et al., 2015). The institutional review boards of Friends Research Institute, Inc. and University of California, Los Angeles approved all procedures and provided oversight for all study activities.
2.3 Measures
2.3.1 Admission Form
Administered at baseline only, the form collects demographic information, detailed alcohol and other drug use history, family and social history, legal status, HIV status, and general health and psychiatric status.
2.3.2 Urine Drug Screening
Participants provided one urine sample during the baseline period. Following randomization, participants provided thrice-weekly (M/W/F) urine drug screens for eight weeks (max 24 samples). Urine samples were screened for methamphetamine, cocaine, and amphetamine metabolites through a rapid, self-timing, qualitative immunoassay (Phamatech, San Diego, CA). Missing urine screens were coded as stimulant positive.
2.4 Statistical Analyses
Descriptive statistics were provided for all variables. Bivariate contrasts between nominal variables was carried out using Pearson’s χ2 analyses whenever possible, and Fisher’s Exact analyses when necessary. Contrasts of continuous variables were carried out using student’s t-tests.
Maximum likelihood growth trajectory models (Jones & Nagin, 2013) employing the binary family and logistic family function were used to analyze intervention response patterns across all participants (i.e., data collapsed across CM and NCYC groups) using a repeated measures binary describing the stimulant use outcome for each participant on any given urinalysis day. The analysis was used to a) characterize typical temporal patterns of intervention response, and b) estimate the associations between these temporal patterns and randomization to receive CM. The analytical outcome of the logistic growth trajectory models (LGTMs) was a set of statistically determined “categories,” whereby each participant was assigned to a category based on their observed temporal pattern of stimulant abstinence during the eight-week intervention (as determined by a polynomial of best fit). All models were estimated and tested for significance using Stata v.13SE (Statacorp), and results were flagged as significant beginning at α ≤ 0.05, two-tailed.
2.4.1 Missing data
There was a considerable amount of missing data; average number of samples submitted was 7 out of 24. However, amount of missing data did not differ across a) primary users of cocaine versus methamphetamine, b) participants assigned to contingent versus non-contingent control. In addition, 21 participants were dropped from these analyses as they contributed no evaluable data following their randomization visit.
3. Results
3.1 Sociodemographics
Participants (n = 119) were Caucasian/white (40%), African American/black (35%), or Hispanic/Latino (17%); the mean age was 38 years old (SD = 7). Participants self-identified as gay (46%) or bisexual (50%). Most reported having a high school diploma or less (58%), and earning less than $15,000/year (83%). Mean self-reported frequency of methamphetamine use over the past 30 days at baseline was 8.0 days (SD = 8.3 days; interquartile range = 2 – 12; n = 107). Examining primary stimulant of abuse at intake, 87% of the sample reported methamphetamine use in the past 12 months, whereas 54% reported cocaine use in the past 12 months. Only 18 participants (i.e., 13%) reported using cocaine in the past 12 months but not methamphetamine. At baseline, 24% had methamphetamine metabolites in their urine, while 13% presented with cocaine metabolites in their urine.
3.2 Time-based Intervention Response Patterns
Maximum likelihood multivariate LGTM analysis identified four unique response patterns, hereafter labeled as “responders” (constant response pattern; i.e., > 90% predicted probability of providing a stimulant metabolite-free urine sample on any given day of the intervention; 64.7% of sample), “non-responders” (constant response pattern; i.e., < 15% predicted probability of providing stimulant metabolite-free urine sample on any given day of the intervention; 12.6%), “worsening response” (negative linear response pattern; i.e., declining probability of providing a stimulant metabolite-free urine sample during the course of the intervention; 14.3%), and “single-positives” (positive cubic response pattern; i.e., estimated ≥ 90% probability of providing a stimulant metabolite-positive urine sample during days 0-5 (i.e., during week one) and days 15-28 (i.e., during weeks three and five) of the intervention, estimated ≥ 95% probability of a clean sample otherwise; 8.4%). As demonstrated in Table 1, there were no significant sociodemographic differences across these four observed patterns of intervention response.
Table 1.
Participant Sociodemographics by Intervention Response Pattern (N = 119)
| Characteristic Category | Non-Responders (n = 15) | Worsening Response (n = 17) | Responders (n = 77) | Single Positive (n = 10) | Total | Sig.a |
|---|---|---|---|---|---|---|
| Racial/Ethnic Identity | ||||||
| Caucasian/white | 7 (46.7%) | 7 (41.2%) | 29 (37.7%) | 4 (40.0%) | 47 (39.5%) | ns |
| African American/black | 5 (33.3%) | 7 (41.2%) | 26 (33.8%) | 4 (40.0%) | 42 (35.3%) | |
| Native American | 1 (6.7%) | 0 (0.0%) | 2 (2.6%) | 1 (10.0%) | 4 (3.4%) | |
| Asian/Pacific Islander | 0 (0.0%) | 0 (0.0%) | 3 (3.9%) | 0 (0.0%) | 3 (2.5%) | |
| Hispanic/Latino | 2 (13.3%) | 2 (11.8%) | 15 (19.5%) | 1 (10.0%) | 20 (16.8%) | |
| Multiracial/Other | 0 (0.0%) | 1 (5.9%) | 2 (2.6) | 0 (0.0%) | 3 (2.5%) | |
| Age | ||||||
| Mean (SD) | 39.0 (11.9) | 37.5 (10.6) | 36.8 (11.9) | 41.7 (7.3) | 37.6 (7.3) | ns |
| Sexual Identity | ||||||
| Gay | 5 (33.3%) | 8 (47.1%) | 41 (53.3%) | 1 (10.0%) | 55 (46.2%) | Ns |
| Bisexual | 9 (60.0%) | 8 (47.1%) | 33 (42.9%) | 9 (90.0%) | 59 (49.6%) | |
| Heterosexual | 1 (6.7%) | 0 (0.0%) | 1 (1.3%) | 0 (0.0%) | 2 (1.7%) | |
| Other | 0 (0.0%) | 1 (5.9%) | 2 (2.6%) | 0 (0.0%) | 3 (2.52%) | |
| Educational Attainment | ||||||
| Less than HS | 6 (40.0%) | 2 (11.8%) | 14 (18.2%) | 3 (30.0%) | 25 (21.0%) | ns |
| HS Diploma | 6 (40.0%) | 6 (35.3%) | 30 (39.0%) | 2 (20.0%) | 44 (37.0%) | |
| More than HS | 3 (20.0%) | 9 (52.9%) | 33 (42.9%) | 5 (50.0%) | 50 (42.0%) | |
| Income (yearly) | ||||||
| Less than $15k | 14 (93.3%) | 16 (94.1%) | 59 (77.6%) | 9 (90.0%) | 98 (83.1%) | ns |
| $15k - $30k | 1 (6.7%) | 0 (0.0%) | 7 (9.2%) | 1 (10.0%) | 9 (7.6%) | |
| $30k - $60k | 0 (0.0%) | 1 (5.9%) | 5 (6.6%) | 0 (0.0%) | 6 (5.1%) | |
| More than $60k | 0 (0.0%) | 0 (0.0%) | 5 (6.6%) | 0 (0.0%) | 5 (4.2%) |
Fisher’s Exact (except age; student’s t-test); α ≤ 0.05, two-tailed
All estimated linear or polynomial coefficients were significant at p ≤ 0.03. Figure 1 provides the estimated probabilities for a given member of each category to provide a stimulant metabolite-free urine sample on any given day of the 8-week (56-day) intervention.
Figure 1.
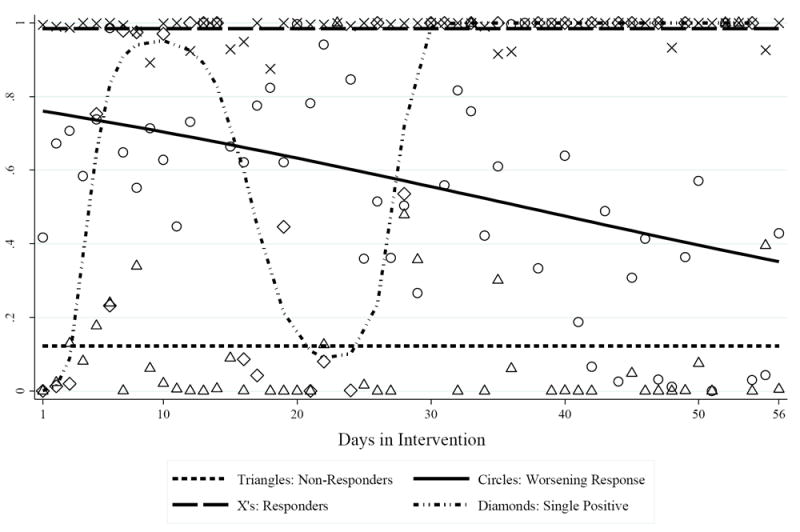
Logistic Growth Trajectory Model Analysis (N = 119). Point estimates represent the calculated probability of providing a stimulant metabolite-free urine sample on a given day of the intervention (triangles = non-responders; circles = worsening response pattern; X’s = responders, diamonds = single-positive); trend lines represent the overall pattern of intervention response for each category (short dash = non-responders; solid = worsening response; long dash = responders; short dash dot dot = single positive).
Chi-square analysis (df = 3) estimated the association between random assignment to receive CM and LGTM category membership. All non-responders (n = 15; 100%) and over three-quarters of all worsening response members (n = 13; 76.5%) were in the NCYC group, whereas 64% of the responders (n = 49) and 80% of the single-positive members (n = 8) were in the CM group (χ2(3) = 29.04; p ≤ 0.001). Additionally, post-hoc analyses revealed that start-of-intervention stimulant use status (i.e., whether each participant provided a stimulant-metabolite free urine sample at baseline) was highly predictive of observed LGTM category membership; though a majority of the non-response, worsening response, and single positive members provided urine samples positive for stimulant metabolites at baseline, only 22% of the responders provided a stimulant-positive urine sample at baseline (Fisher’s Exact p ≤ 0.001; Table 2).
Table 2.
Intervention response patterns: group size, distribution across conditions, and baseline urinalysis result
| Intervention Response Pattern | Condition | Stimulant-Positive Urinalysis at Baseline | ||
|---|---|---|---|---|
| N | NCYC | CM | ||
| Responder | 77 | 36% | 64% | 22% |
| Single Positive | 10 | 20% | 80% | 70% |
| Worsening | 17 | 77% | 23% | 60% |
| Non responder | 15 | 100% | 0% | 50% |
4. Discussion
In this sample of high-risk MSM, the contingent application of voucher rewards was associated with more favorable patterns of stimulant abstinence than patterns seen when a similar magnitude of rewards was delivered without the contingency. Pitting the rewarding nature of voucher-based incentives against the euphoric rewards of stimulant use (i.e., the “substitutability” of rewards) produced two distinct patterns of participant response. These were participants who responded consistently to CM (i.e., consistent responders or single positives) and those who did not (worsening or non-responders). Of note, no participants assigned to the CM condition were represented in either temporal pattern of non-responders. Importantly, no racial, ethnic, age, sexual identity, educational attainment, or income differences in participants across intervention response patterns were observed. Nor were there differing response patterns for the two primary stimulants of abuse (i.e., methamphetamine and cocaine), implying that the effect of the contingent application of rewards on patterns of response was broad. As shown, the LGTM approach characterizes temporal patterns over time, which extends analyzes of static differences in overall treatment response.
The link previously identified between consistent abstinence from methamphetamine use and ability to complete biomedical HIV prevention using PEP regimens (Landovitz et al., 2015) provides compelling evidence for CM as one element of combination HIV prevention. It is the sustained stimulant abstinence in the responder patterns (consistent and single positive responders) from the contingent delivery of rewards that is the mechanism linking improved medication outcomes in HIV-PEP for MSM following potential HIV exposure. This expands previously reported drug use outcomes and adds an important individual differences perspective. The non-contingent yoked control is a sophisticated study design that equates both amount and temporal distribution of monetary payments across groups. The design allows conclusions about the mechanism of action of CM, namely the linchpin being the contingent relationship between target behavior and receipt of monetary incentives. While other studies have used the yoked control design (Higgins, Wong, Badger, Ogden, Dantona, 2000; Schottenfeld, Moore, Pantalon, 2011), this is the first to use the design with MSM and adds support to the literature from this key population on the importance of the contingency for impacting stimulant use. This demonstration is further impactful in highlighting success in using CM to produce reductions in stimulant-linked HIV transmission risk in the setting of high HIV prevalence and incidence.
It is encouraging that participants receiving CM were more likely to achieve abstinence from stimulants after an instance of use than participants receiving non-contingent rewards. In this study, in the group that received non-contingent rewards, patterns of stimulant use continued or worsened, showing no evidence of a spontaneous improvement through noncontingent provision of financial resources to the participants. This finding contrasts with one trial of incentives as HIV prevention conducted in a resource-limited country showing no significant differences for contingent and non-contingent delivery of incentives for reducing HIV-incidence (Baird et al., 2014). When basic survival needs are met, however, as is the case for most stimulant-using MSM in the U.S., incentives (not financial resource) work when delivered contingent on stimulant abstinence.
Findings from this study may also provide suggestions for the context of CM efficacy when used in settings with stimulant-using MSM who are not seeking formal drug treatment. Menza et al. (2010) reported CM showed no efficacy over a standard comparison in reducing methamphetamine use among non-treatment-seeking, stimulant-using MSM in Seattle. In spite of our finding of superior outcomes for CM, key parallels exist with ours and the Menza et al. (2010) study. In both samples, stimulant-using MSM were not seeking formal drug treatment. As well, the numbers of negative urine samples provided in both studies were less than that observed when CM was applied as a stand-alone and as a combined treatment with cognitive behavioral therapy for stimulant-dependent MSM in treatment (Shoptaw et al., 2005). One factor that may explain limited CM efficacy in the Menza et al. (2010) study was poor compliance with the demanding thrice-weekly CM visit schedule, which suggests low underlying motivation toward reducing stimulant use. Indeed, the study team had to relax the testing schedule to twice weekly to populate the trial. The provision of free PEP in our study may have attracted a more motivated group and/or provided a sufficiently engaging platform for the contingencies to operate effectively and produce stimulant abstinence. In San Francisco a community-level, low-cost CM program has been implemented as a motivation booster for over a decade (“Project PROP”; Shoptaw et al., 2006) as part of the public health response to links between methamphetamine use and infectious disease among high-risk MSM (Strona et al., 2006). Project PROP frequently facilitates referral of participants to a range of treatment options. These findings suggest that CM may work to instill stimulant abstinence in high-risk MSM who are willing to consider treatment, but that efficacy for CM erodes in parallel fashion with the men’s motivation for behavior change regarding stimulant use.
That temporal responders were more likely to be observed among those who presented a stimulant metabolite-free urine sample at intake is consistent with prior analyses of outcomes in trials of methamphetamine treatments that show strong correlations between start-of-intervention drug use status and intervention response (Brensilver et al., 2012; Cochran, Stitzer, Nunes, Hu, & Campbell, 2014). Patterns of intervention response observed in this study also are consistent with findings from early studies of CM used in methadone maintenance clinics (Morral et al., 1997), though the identification of a single positive responder pattern is unique to this study. Our findings also identified a group that worsened through time. Yet both sets of findings are consistent in underscoring the point that for individuals whose methamphetamine use disorder at baseline is severe, the odds of achieving the three consecutive days of abstinence needed to produce a urine sample free of metabolites and earn a voucher is far lower than for those who have less of the disorder at baseline.
5. Limitations & Conclusions
These findings are limited by the relatively small sample size, the non-blinded nature of the random arm assignment (i.e., participants knew whether they were assigned to the CM or NCYC groups), and by the eligibility criteria of high-risk, HIV-negative, stimulant-using MSM rather than through probabilistic selection of MSM from a broad sampling frame. The eligibility criteria included self-reported recent engagement in both stimulant use (previous 30 days) and condomless sex (previous three months), implying that enrollment may have been more likely to occur at 1) a time of particularly high risk for participants; and/or, 2) a time when participants might have been motivated to reduce their risk behavior, including stimulant use behaviors. As such, regression to the mean, and/or self-selection biases may have contributed to the observed efficacy of the intervention (e.g., 64.7% intervention responders). The convention of coding all missing urine samples as positive for stimulant metabolites is another potential limitation of this study; such a conservative analytical approach to operationalization may overestimate the number of positive samples among participants exhibiting poor attendance (McPherson et al,. 2014). This risk is particularly acute given high rates of missing data. Given the similarity in rates of missing data per condition, however, the bias for responders and non-responder groups may have differentially penalized the NCYC condition. Additionally, robust temporal information on participants’ ongoing stimulant use was only collected for 8-weeks; it is unclear whether temporal patterns of stimulant use across the CM/NCYC groups would have remained divergent across a longer timeframe.
Findings presented here highlight the efficacy of contingency-based rewards in promoting two distinct patterns of stimulant abstinence among sexual risk-taking, active stimulant users. The main finding from these analyses is that growth trajectory modeling provided important insights on patterns of response that favored CM for establishing abstinence in a group of MSM at high risk for HIV infection, even among the minority of MSM who used a stimulant while engaged in the study. Results presented here demonstrate the utility of trajectory modeling and further support the contingent application of reward as the operative mechanism associated with patterns of stimulant abstinence among a sample of stimulant-using MSM.
Acknowledgments
This study was supported by the California HIV Research Program, grant number MC08-LA-710. R. J. L. acknowledges additional support from the National Institute of Drug Abuse at the National Institutes of Health (grant K23DA026308), and R. J. L., S. S., and C. J. R. acknowledge additional support from the National Institute of Mental Health at the National Institutes of Health (grant 5P30MH058107). The authors wish to thank Jesse Fletcher, Ph.D. for his statistical support, his work as project director during the implementation of the study, and his contributions to the development of this manuscript.
Role of the Funders
The funders had no role in the study design, collection/analysis/interpretation of the data, writing of the report, or in the decision to submit the article for publication.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baird SJ, Garfein RS, McIntosh CT, Ozler B. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. Lancet. 2012 Apr 7;379(9823):1320–9. doi: 10.1016/S0140-6736(11)61709-1. Epub 2012 Feb 15. [DOI] [PubMed] [Google Scholar]
- Benotsch EG, Lance SP, Nettles CD, Koester S. Attitudes Toward Methamphetamine Use and HIV Risk Behavior in Men Who Have Sex with Men. The American Journal on Addictions. 2012;21:S35–S42. doi: 10.1111/j.1521-0391.2012.00294.x. [DOI] [PubMed] [Google Scholar]
- Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, Brookmeyer R. Global epidemiology of HIV infection in men who have sex with men. The Lancet. 2012;380(9839):367–377. doi: 10.1016/S0140-6736(12)60821-6. http://dx.doi.org/10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone MR, Cook SH, Wilson P. Substance use and sexual risk behavior in HIV- positive men who have sex with men: an episode-level analysis. AIDS and Behavior. 2013;17(5):1883–1887. doi: 10.1007/s10461-012-0167-4. [DOI] [PubMed] [Google Scholar]
- Brensilver M, Heinzerling KG, Swanson A-N, Shoptaw SJ. Placebo-group responders in methamphetamine pharmacotherapy trials: The role of immediate establishment of abstinence. Experimental and Clinical Psychopharmacology. 2012;20(5):430. doi: 10.1037/a0029210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Gómez W, Siever MD, Discepola MV, Dilworth SE, Moskowitz JT. Pilot randomized controlled trial of an integrative intervention with methamphetamine-using men who have sex with men. Arch Sex Behav. 2015;44(7):1861–7. doi: 10.1007/s10508-015-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran G, Stitzer M, Nunes EV, Hu M-C, Campbell A. Clinically relevant characteristics associated with early treatment drug use versus abstinence. Addict Sci Clin Pract. 2014;9:6. doi: 10.1186/1940-0640-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A Meta-Analytic Review of Psychosocial Interventions for Substance Use Disorders. American Journal of Psychiatry. 2008;165(2):179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol. 2000;68(1):64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Finlayson TJ, Le B, Smith A, Bowles K, Cribbin M, Miles I, DiNenno E, et al. HIV risk, prevention, and testing behaviors among men who have sex with men: National HIV Behavioral Surveillance System, 21 US cities, United States, 2008. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [PubMed] [Google Scholar]
- Jones BL, Nagin DS. A Note on a Stata Plugin for Estimating Group-based Trajectory Models. Sociological Methods & Research. 2013 doi: 10.1177/0049124113503141. [DOI] [Google Scholar]
- Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, Barresi PJ, Coates TJ, Chesney MA, Buchbinder S. Risk factors for HIV infection among men who have sex with men. AIDS. 2006 Mar 21;20(5):731–9. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- Landovitz RJ, Fletcher JB, Shoptaw S, Reback CJ. Contingency Management Facilitates the Use of Postexposure Prophylaxis Among Stimulant-Using Men Who Have Sex With Men. Open Forum Infectious Diseases. 2015;2(1) doi: 10.1093/ofid/ofu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McPherson S, Barbosa-Leiker C, Mamey MR, McDonell M, Enders CK, Roll J. A ’missing not at random’ (MNAR) and ’missing at random’ (MAR) growth model comparison with a buprenorphine/naloxone clinical trial. Addiction. 2015;110(1):51–8. doi: 10.1111/add.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menza TW, Jameson DR, Hughes JP, Colfax GN, Shoptaw S, Golden MR. Contingency management to reduce methamphetamine use and sexual risk among men who have sex with men: a randomized controlled trial. BMC Public Health. 2010;10:774. doi: 10.1186/1471-2458-10-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga MJ, O’Cleirigh C, Biello KB, Robertson AM, Safren SA, Coates TJ, Koblin BA, Chesney MA, Donnell DJ, Stall RD, Mayer KH. The effect of psychosocial syndemic production on 4-year HIV incidence and risk behavior in a large cohort of sexually active men who have sex with men. J Acquir Immune Defic Syndr. 2015;68(3):329–36. doi: 10.1097/QAI.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morral AR, Iguchi MY, Belding MA, Lamb RJ. Natural classes of treatment response. Journal of Consulting and Clinical Psychology. 1997;65(4):673. doi: 10.1037//0022-006x.65.4.673. [DOI] [PubMed] [Google Scholar]
- Ostrow DG, Plankey MW, Cox C, Li X, Shoptaw S, Jacobson LP, Stall RC. Specific sex drug combinations contribute to the majority of recent HIV seroconversions among MSM in the MACS. J Acquir Immune Defic Syndr. 2009 Jul 1;51(3):349–55. doi: 10.1097/QAI.0b013e3181a24b20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plankey MW, Ostrow DG, Stall R, Cox C, Li X, Peck JA, Jacobson LP. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007 May 1;45(1):85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Moore B, Pantalon MV. Contingency management with community reinforcement approach or twelve-step facilitation drug counseling for cocaine dependent pregnant women or women with young children. Drug Alcohol Depend. 2011;118(1):48–55. doi: 10.1016/j.drugalcdep.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, Hucks-Ortiz C. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug and Alcohol Dependence. 2005;78:125–134. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Klausner JD, Reback CJ, Tierney S, Stansell J, Hare CB, Gibson S, Siever M, King WD, Kao U, Dang J. A public health response to the methamphetamine epidemic: the implementation of contingency management to treat methamphetamine dependence. BMC Public Health. 2006;6:214. doi: 10.1186/1471-2458-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Robles E. The Economic Analysis of Substance Use and Abuse: An Integration of Econometrics and Behavioral Economic Research. University of Chicago Press; 1999. Employment as a drug abuse treatment intervention: A behavioral economic analysis; pp. 279–310. [Google Scholar]
- Strona FV, McCright J, Hjord H, Ahrens K, Tierney S, Shoptaw S, Klausner JD. The acceptability and feasibility of the Positive Reinforcement Opportunity Project, a community-based contingency management methamphetamine treatment program for gay and bisexual men in San Francisco. J Psychoactive Drugs. 2006;(Suppl 3):377–83. doi: 10.1080/02791072.2006.10400601. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration [SAMHSA] NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: SAMHSA; 2013. Results from the 2012 National Survey on Drug Use and Health: Detailed Tables—Index by Section. [Google Scholar]


