Abstract
Background
Previous studies indicate epinephrine adversely affects arterial oxygenation when administered in a rat model of local anesthetic overdose. The authors tested whether epinephrine alone exerts similar effects in the intact animal.
Methods
Anesthetized rats received a single intravenous injection of epinephrine (25, 50, or 100 mcg/kg); matched cohorts were pretreated with phentolamine (100 mcg/kg); n = 5 for each of the six treatment groups. Arterial pressure and blood gases were measured at baseline, 1 and 10 min after epinephrine administration. Pulmonary capillary pressures during epinephrine infusion with normal and increased flows were measured in an isolated lung preparation.
Results
Epinephrine injection in the intact animal caused hypoxemia, hypercapnia, and acidosis at all doses. Arterial oxygen tension was reduced within 1 min of injection. Hyperlactatemia occurred by 10 min after 50 and 100 mcg/kg. Rate pressure product was decreased by 10 min after 100 mcg/kg epinephrine. Pretreatment with phentolamine attenuated these effects except at 100 mcg/kg epinephrine. In the isolated lung preparation, epinephrine in combination with increased pulmonary flow increased pulmonary capillary pressure and lung water.
Conclusions
Bolus injection of epinephrine in the intact, anesthetized rat impairs pulmonary oxygen exchange within 1 min of treatment. Effects were blunted by a-adrenergic receptor blockade. Edema occurred in the isolated lung above a threshold pulmonary capillary pressure when epinephrine treatment was coupled with an increase in pulmonary flow. These results potentially argue against using traditional doses of epinephrine for resuscitation, particularly in the anesthetized patient.
Epinephrine is routinely used to treat the pulseless patient because of its potent inotropic and pressor effects. However, clinical evidence does not support the efficacy of this practice in out-of-hospital cardiac arrest1,2 and recent animal studies suggest that epinephrine could exert adverse effects on postarrest outcomes.3 Weinberg et al.4 found that epinephrine administration in the setting of local anesthetic toxicity was associated with cardiovascular and metabolic deterioration. Moreover, Hiller et al.5 reported that epinephrine above a threshold dose of 10 mcg/kg impaired the efficacy of lipid emulsion in a rodent model of lipid infusion for local anesthetic overdose. In both experimental models epinephrine use was nearly uniformly accompanied by overt pulmonary edema, arterial oxygen desaturation, and delayed metabolic deterioration.
Based on these observations we hypothesized that epinephrine might, by itself, exert acute, deleterious effects on pulmonary oxygen exchange. The purpose of the current study was to test this hypothesis by measuring arterial blood gases after epinephrine administration in anesthetized, intact rats. We used a model without coadministration of local anesthetic to avoid the potential confounders of cardiac arrest, chest compressions, or a low output state. We also tested the contribution of vasoconstriction to this phenomenon by determining whether use of an α-adrenergic blocking agent could attenuate any adverse effects. If the role of α-adrenergic activation were confirmed, arterial desaturation caused by pulmonary edema could be due either to increased peripheral vasoconstriction or increased pulmonary venous capillary resistance. Therefore, we also used an isolated lung model to differentiate these by measuring the effect of epinephrine on pulmonary capillary pressures and lung water. These experiments were designed to confirm and gain mechanistic insights into the potential adverse effects of epinephrine on pulmonary gas exchange.
Materials and Methods
Intact Rat Experiments
The following protocol was approved by the Animal Care Committee and Biologic Resources Laboratory at the University of Illinois (Chicago, IL) and the Institutional Animal Care and Utilization Committee of the Jesse Brown Veterans Administration Medical Center (Chicago, IL). Thirty healthy, male Sprague-Dawley rats weighing between 370 and 425 g were anesthetized in a bell jar with isoflurane to allow tracheal intubation. All animals were then placed on a heated stand under a warming lamp and mechanically ventilated with 1.2% isoflurane in 100% oxygen to maintain a constant alveolar concentration of anesthetic during the experiments. A Harvard rodent ventilator model 680 (Harvard Apparatus, South Natick, MA) to deliver a tidal volume of 2.5 ml, at a starting rate of 65–70 breaths/min. Catheters were inserted into the left internal jugular vein and left carotid artery. Electrocardiogram using three subcutaneous needle electrodes and the carotid pressure were recorded continuously throughout the experiment by PowerLab data archiving and retrieval system using Chart 5.2.1 (ADInstruments, Colorado Springs, CO). All animals were allowed to equilibrate for 10 min at 1.2% isoflurane and 100% oxygen, and arterial blood gas measurements (i-STAT1 Analyzer, i-STAT Corporation, Princeton, NJ) were made before the phentolamine and epinephrine injections to confirm a pH between 7.35 and 7.45 and a serum lactate less than 2.0.
Experimental Design
Animals were randomized in advance of the experiment to one of six treatment groups, n = 5 for each group: all animals received a single bolus of epinephrine at one of three doses: 25, 50, or 100 mcg/kg. These epinephrine doses were chosen by virtue of being within a range typically administered in experimental shock models and their approximation of clinically relevant doses according to accepted allometric scaling (using 6 for conversion of human and rat doses by weight). Half of the rats were identified to receive a single intravenous injection of phentolamine 100 mcg/kg, 10 min before the epinephrine injection; this dose is similar on a per kg basis to that recommended for intravenous clinical use. These animals had a second arterial blood gas measurement before receiving epinephrine, which served as their starting (t = 0) values; their cardiovascular parameters just before the epinephrine dose likewise served as the starting point for subsequent measurements. Arterial blood gas samples were analyzed in all animals at 1 and 10 min after the epinephrine injection. Animals were sacrificed by anesthetic overdose after the 10-min time point. Excised lungs were weighed, dried, and weighed again to determine the wet-to-dry ratio. The laboratory personnel were not blinded to the treatment; however, subsequent off-line data compilation was made from archived files of each experiment that were blinded with respect to group.
Isolated Lung Preparation
Eight wild-type mice were used for the study—four mice in each group of isolated lung experiments. According to an approved protocol of the University of Illinois Animal Care Committee, male mice weighing 20–30 g were placed in an anesthesia chamber and anesthetized with 2.5% isoflurane in room air at a flow rate of 2 l/min. After induction, anesthesia was continued by means of a nose cone. The trachea was cannulated with a 19-gauge stainless steel tube for constant positive pressure ventilation at a rate of 120 breaths/min with the anesthetic gas mixture. Heparin (50 U) was injected into the jugular vein. The abdominal cavity was opened to expose the diaphragm, which was ventrally punctured and cut free from the rib cage. Then, a thoracotomy was performed and the two halves of the rib cage were retracted to expose the heart and lungs. To make the pulmonary artery accessible for cannulation, the heart was caudally retracted with a silk suture (6 – 0; Ethicon, Inc., Cornelia, GA) through the apical musculature of the right ventricle. An incision was made in the right ventricle at the base of the pulmonary artery and a polyethylene cannula was maneuvered into the pulmonary artery and secured by means of a suture around the pulmonary artery that included the aorta. A catheter was inserted through an incision made in the left atrium. The lungs were perfused in situ using a peristaltic pump. The heart and the exsanguinated lungs were rapidly excised and transferred en bloc to a perfusion apparatus.
Lung preparations were then suspended from a balanced lever arm coupled to a calibrated displacement transducer for monitoring lung weight. The isolated lungs were perfused at constant flow (2 ml/min), temperature (37°C), and venous pressure (+ 3–4 cm H2O) with bicarbonate-buffered Roswell Park Memorial Institute (RPMI) 1640 medium, supplemented with 3 g/100 ml bovine serum albumin (BSA, Fraction V, 99% pure and endotoxin-free). The lungs were ventilated at 120/min with end expiratory pressure of 2.0 cm H2O using a gas mixture of 5% CO2, 20% O2, 75% N2). Pulmonary arterial pressure was monitored throughout the experiment using a Grass Model P23XL pressure transducer (Astromed, Inc., West Warwick, RI). Lung wet weight was electronically zeroed when the preparation was mounted and subsequent weight changes due to gain or loss of fluid from the lung were recorded. Lung weight and arterial and venous pressure recordings were displayed on a computer video monitor with the aid of amplifiers (Grass CP122 strain gauge amplifier), an analog-to-digital converter, and commercial software that permits acquisition and logging of data (Labtech Notebook Pro for Windows, LabTech Software, Tampa, FL). Brief (5 s), simultaneous occlusions of the arterial inflow and venous outflow paths were implemented at 5-min intervals with the aid of electronic valves (P/N 98301–22; Cole-Parmer Instrument Co., Vernon Hills, IL). Pulmonary capillary pressure (Ppc) was measured by the double-occlusion method.
Assessment of Vascular Pressures and Lung Wet Weight
In one group (n = 4) epinephrine was given to assess changes in vascular pressures and lung wet weight by the described gravimetric method. Inflow and outflow pressures were monitored continuously, and double-occlusion pressures were measured at 5-min intervals. In a separate group (n = 4) epinephrine was infused and the inflow rate was increased to 4 ml/min to simulate the effects of epinephrine on cardiac output. Pulmonary pressures and lung wet weight were simultaneously measured to distinguish the effects of epinephrine with and without increased pulmonary artery flow.
Statistical Analysis
Intact Animal Experiments
Power analysis was based on results of previous experiments comparing rate pressure product (RPP) at 10 min among various treatment groups and yielded a sample size of n = 5 for each group (power was set at 0.8, α at 0.05, and effect size for the metric of arterial oxygen tension was set at 1.2). All in vivo data were analyzed using GraphPad Prism 4 (GraphPad Software, San Diego, CA). Baseline parameters were analyzed by one-way analysis of variance (ANOVA); posttests were not required as there were no intergroup differences in any parameter. All blood gas results and cardiovascular parameters were compared across time by repeated measures two-way ANOVA using Bonferroni posttests when significance was achieved α set at 0.05) for differences over time and between groups. Wet-to-dry ratios were compared by one-way ANOVA using Dunnet multiple comparison posttests. Differences for all analyses were considered significant when posttests indicated P < 0.05.
Isolated Lung Experiments
Lung weights and capillary pressures of each mouse before and 30 min after treatment were compared by paired, two-tailed Student t test.
Results: Intact Animal Experiments
Baseline values: Mean values for all baseline physiologic parameters were interrogated and passed the D’Agostino–Pearson normality test. There were no differences in any parameter among the six groups.
Dose-dependent Effects over 10 Min
Gas Exchange
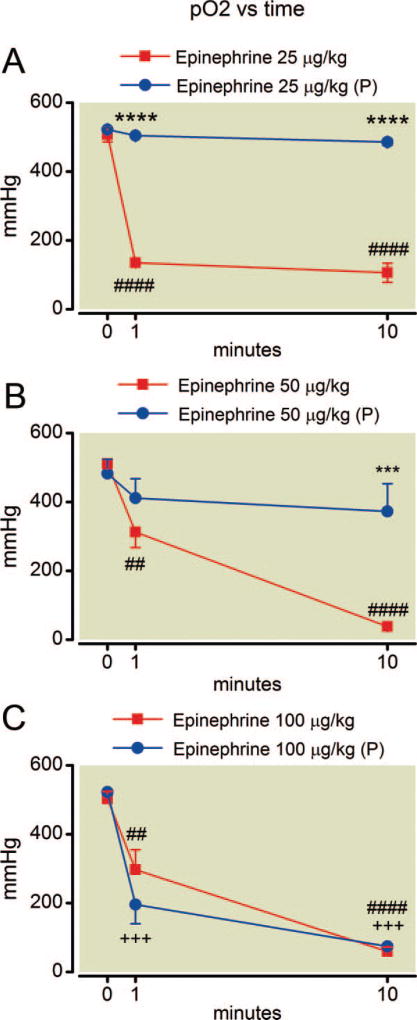
Arterial blood gas measurements were the main focus and arterial oxygen tension was the key metric of this study. Arterial partial pressure of oxygen (fig. 1) declined very rapidly after administration of epinephrine alone at all doses examined. Note the lowest dose of epinephrine reduced mean arterial oxygen tension at 1 min from 507+/−18 mmHg to 249+/−223 mmHg (mean+/−SD) corresponding to an effect size of 1.63 (Cohen d). This effect was attenuated by pretreatment with phentolamine in rats given 25 and 50 mcg/kg epinephrine and oxygen tension values in these treatment groups at 10 min were no different from baseline. However, trends in arterial oxygen tension following 100 µg/kg of epinephrine were unaffected by pretreatment with phentolamine (P = 0.46).
Fig. 1.
(A–C) Time course of oxygen tension after epinephrine administration. Deterioration in oxygen tension occurs within 1 min of epinephrine injection at all doses. Pretreatment with phentolamine, indicated by (P), attenuates this effect at the 25 and 50 µg/kg doses. Mean values are plotted with error bars indicating SEM; # indicates difference following epinephrine alone from baseline value; + indicates difference in the phentolamine pretreatment group from their baseline value; * indicates difference between the two groups. Symbol number indicates significance: two symbols, P less than 0.01; three symbols, P less than 0.01; four symbols, P less than 0.0001.
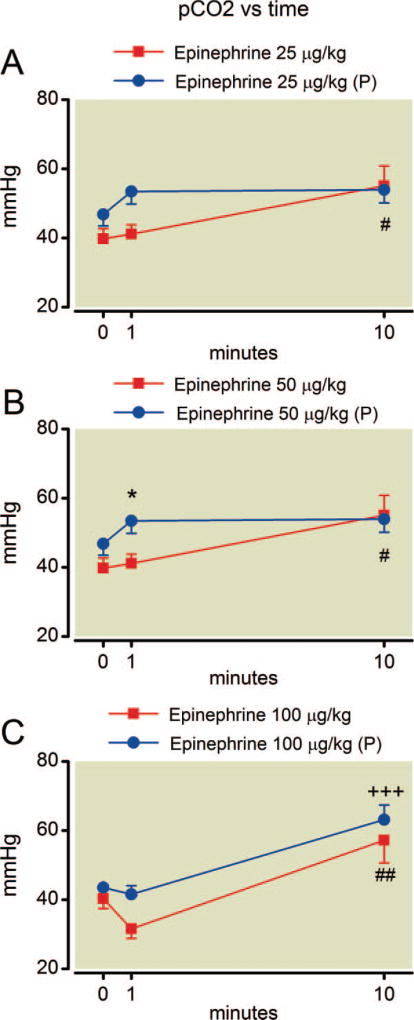
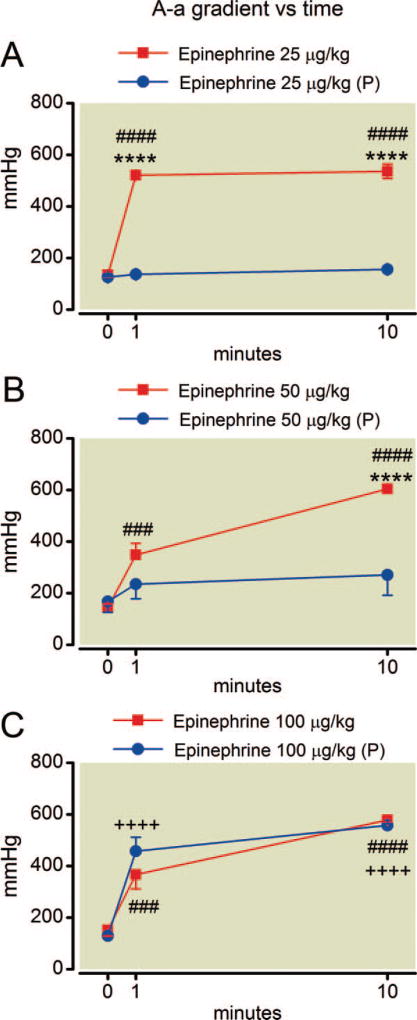
Despite constant settings for mechanical ventilation during the experiment, epinephrine alone caused an increase in arterial carbon dioxide tension by 10 min at all doses (fig. 2). Pretreatment with phentolamine had no overall protective effect on hypercapnia at any epinephrine dose (P = 0.26, F = 1.4; P = 0.26, F = 1.5; and P = 0.19, F = 2.1 for the effect of phentolamine at 25, 50, and 100 µg/kg epinephrine, respectively). The simultaneous measure of arterial oxygen and carbon dioxide tension allowed calculation of the alveolar-arterial oxygen gradient (fig. 3), which rapidly increased after epinephrine treatment at all doses. This effect was also blunted by phentolamine pretreatment at the lower doses of epinephrine (P = 0.0001, F = 571 and P = 0.034, F = 6.5 for the effect of phentolamine at 25 and 50 µg/kg epinephrine, respectively). However, there was no protective effect for phentolamine among rats at 100 µg/kg epinephrine (P = 0.58, F = 0.34) and the plots of alveolar-arterial oxygen gradient with and without phentolamine can be nearly superimposed.
Fig. 2.
(A–C) Time course of arterial carbon dioxide tension after epinephrine administration. Hypercapnia occurs by 10 min at all epinephrine doses without phentolamine pretreatment (P) and by 10 min at the highest epinephrine dose even with pretreatment. Mean values are plotted with error bars indicating SEM; # indicates difference following epinephrine alone from baseline value; + indicates difference in the phentolamine pretreatment group from their baseline value; * indicates difference between the two groups. Symbol number indicates significance: one symbol, P less than 0.05; two symbols, P less than 0.01; three symbols, P less than 0.001.
Fig. 3.
(A–C) Time course of alveolar-arterial oxygen gradient after epinephrine administration. Epinephrine treatment increases the gradient rapidly at all doses examined. Phentolamine pretreatment (P) prevents (25 µg/kg), or blunts (50 µg/kg) this effect except at 100 µg/kg. Mean values are plotted with error bars indicating SEM; # indicates difference following epinephrine alone from baseline value; + indicates difference in the phentolamine pretreatment group from their baseline value; * indicates difference between the two groups. Symbol number indicates significance: three symbols, P less than 0.001; four symbols, P less than 0.0001.
Metabolic Parameters
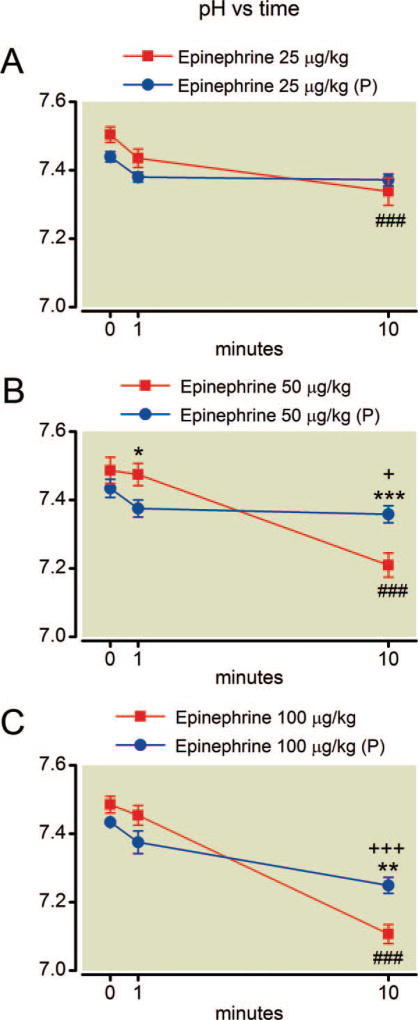
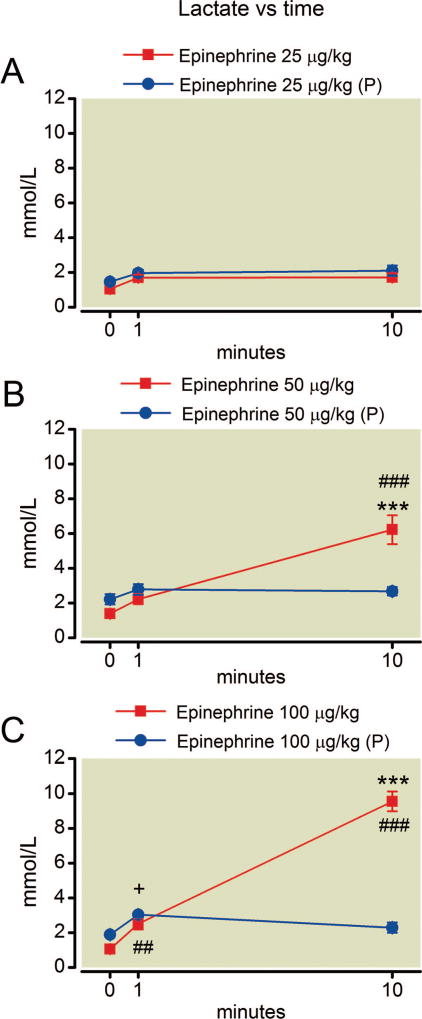
Epinephrine treatment alone resulted in a decline in pH over the course of the experiment at all doses (fig. 4). With the highest dose of epinephrine the mean pH dropped from 7.48 ± 0.05 to 7.11 ± 0.06 (±SD) yielding an effect size of 6.7 (Cohen d). Pretreatment with phentolamine had no overall protective effect on acidosis at any epinephrine dose (P = 0.39, F = 0.81; P = 0.98, F = 0.0008; and P = 0.90, F = 0.02 for the effect of phentolamine at 25, 50, and 100 µg/kg epinephrine, respectively). Epinephrine treatment exerted a potent, overall effect on blood lactate concentration that was increased over baseline values by 10 min after treatment with 50 µg/kg and 100 µg/kg epinephrine (fig. 5). The effect size for the change in lactate concentration 10 min after the highest dose of epinephrine was 9.1 (Cohen d). Phentolamine only protected against hyperlactatemia at the highest epinephrine dose (P = 0.27, F = 1.4; P = 0.079, F = 4.0; and P = 0.0003, F = 36 for the effect of phentolamine at 25, 50, and 100 µg/kg epinephrine, respectively).
Fig. 4.
(A–C) Time course of arterial pH after epinephrine. Epinephrine treatment by itself alters pH by 10 min at all doses. Phentolamine pretreatment (P) is protective at all but the highest dose. Mean values are plotted with error bars indicating SEM; # indicates difference following epinephrine alone from baseline value; + indicates difference in the phentolamine pretreatment group from their baseline value; * indicates difference between the two groups. Symbol number indicates significance: one symbol, P less than 0.05; two symbols, P less than 0.01; three symbols, P less than 0.001.
Fig. 5.
(A–C) Time course of blood lactate after epinephrine. Hyperlactatemia occurs by 10 min after the higher doses of epinephrine. Phentolamine pretreatment (P) is protective at both dose. Mean values are plotted with error bars indicating SEM; # indicates difference following epinephrine alone from baseline value; + indicates difference in the phentolamine pretreatment group from their baseline value; * indicates difference between the two groups. Symbol number indicates significance: one symbol, P less than 0.05; two symbols, P less than 0.01; three symbols, P less than 0.001.
Rate Pressure Product
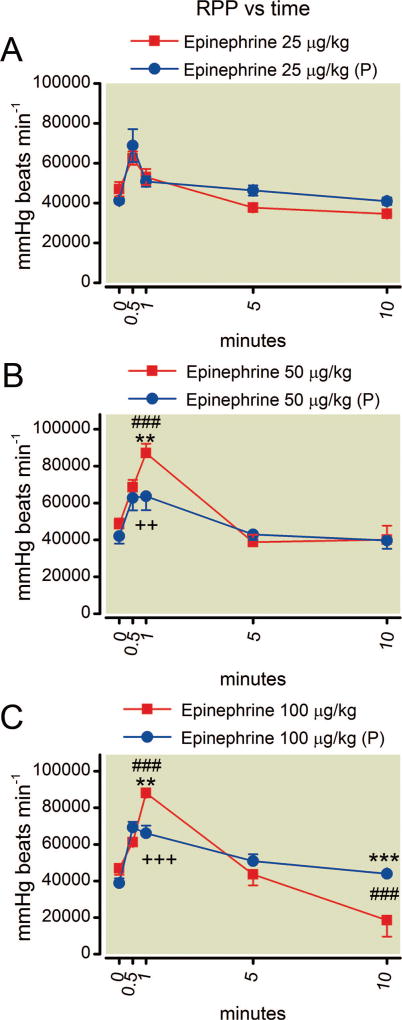
All animals receiving phentolamine pretreatment experienced a transient drop in systolic blood pressure that returned to baseline after 5 min. All animals exhibited an increase in RPP over the first minute after epinephrine treatment (fig. 6). This was blunted at 1 min by phentolamine pretreatment for the 50 and 100 µg/kg epinephrine doses. RPP then returned to baseline values by 10 min after doses of 25 or 50 µg/kg epinephrine. However, animals given 100 µg/kg epinephrine alone showed depressed RPP values by 10 min (P < 0.001 for the difference at 10 min from baseline values; effect size as Cohen d, 1.9) and this effect was prevented by pretreatment with phentolamine (P < 0.001 for the difference between epinephrine alone and epinephrine with phentolamine treatment at 10 min), which resulted in mean RPP returning to baseline values by 10 min (P > 0.05).
Fig. 6.
(A–C) Time course of rate pressure product (RPP) after epinephrine. The higher two doses increase RPP by 1 min but the value returns to baseline by 5 min. The highest dose of epinephrine results in a decreased rate pressure product by 10 min. Phentolamine pretreatment (P) protects against this effect. Mean values are plotted with error bars indicating SEM; # indicates difference following epinephrine alone from baseline value; + indicates difference in the phentolamine pretreatment group from their baseline value; * indicates difference between the two groups. Symbol number indicates significance: two symbols, P less than 0.01; three symbols, P less than 0.001.
Dose-dependent Effects at 10 Min
Blood Gas Values and Metabolism
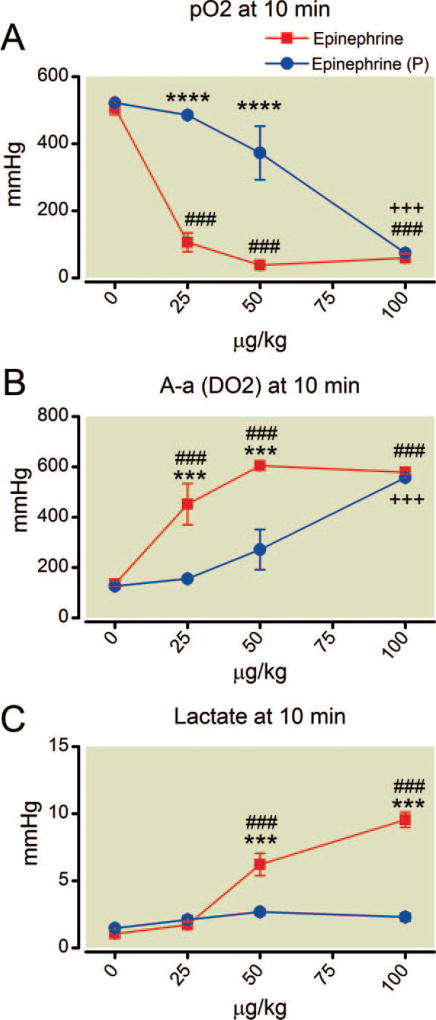
The dose-response curves describing the effects of epinephrine alone on arterial oxygen tension and alveolar-arterial oxygen gradient indicate that by 10 min the same degree of arterial hypoxemia is found at all doses of epinephrine (fig. 7; P > 0.05). Phentolamine pretreatment exerts an overall protective effect for both parameters (P < 0.0001 and F = 61). However, post hoc analysis indicates that this effect specifically occurs only at the lower doses of epinephrine because at 10 min the values of both arterial oxygen tension and alveolar-arterial oxygen gradient for the two groups are virtually identical. Epinephrine alone caused severe, dose-dependent hyperlactatemia but this effect was completely prevented by phentolamine pretreatment (P < 0.0001; F = 98 for the between-group difference in lactate at 10 min).
Fig. 7.
(A–C) Dose-dependent effects on oxygen and lactate 10 min after epinephrine with and without phentolamine. Separation of the plots indicates attenuation or in the case of lactate, prevention, of the epinephrine effect. The intersection of plots for pO2 and (A-a) DO2 indicate that phentolamine is not protective at the highest epinephrine dose. Mean values are plotted with error bars indicating SEM; # indicates difference following epinephrine alone from baseline value; + indicates difference in the phentolamine pretreatment group from their baseline value; * indicates difference between the two groups. Three symbols, P less than 0.001. ****P less than 0.0001. (A-a) DO2= alveolar-arterial oxygen gradient.
Hemodynamics
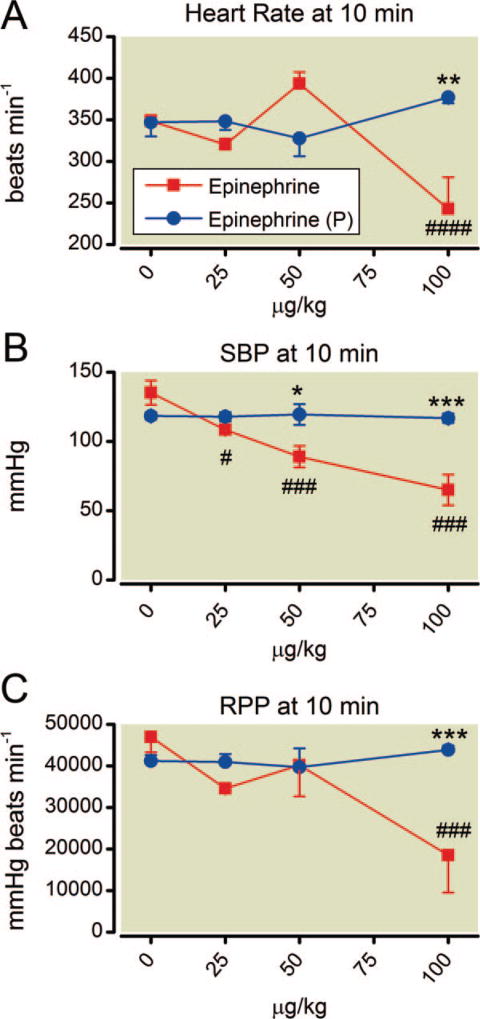
Epinephrine doses less than 50 µg/kg had little effect on hemodynamic parameters at 10 min (fig. 8). However by experiments’ end, the highest dose of epinephrine caused substantial decrements in systolic blood pressure, heart rate, and RPP, except in animals pretreated with phentolamine (which was protective in all cases). Gross pulmonary edema was seen in all animals receiving the highest dose of epinephrine. The wet-to-dry ratio was increased among all animals given epinephrine alone (fig. 9; P values as indicated). Phentolamine was protective at all but the highest epinephrine dose.
Fig. 8.
(A–C) Dose-dependent effects on cardiovascular parameters 10 min after epinephrine with and without phentolamine. Key differences are recorded at the highest dose of epinephrine. Phentolamine pretreatment (P) is protective in all parameters. Mean values are plotted with error bars indicating SEM; # indicates difference following epinephrine alone from baseline value; + indicates difference in the phentolamine pretreatment group from their baseline value; * indicates difference between the two groups. Symbol number indicates significance: one symbol, P less than 0.05; two symbols, P less than 0.01; three symbols, P less than 0.001, four symbols, P less than 0.0001.
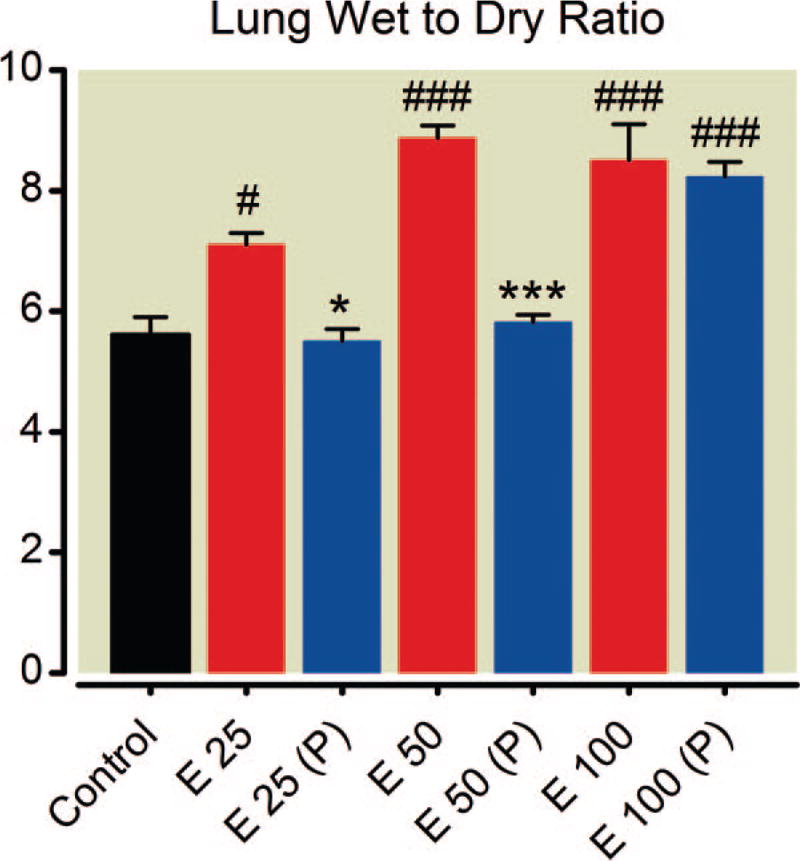
Fig. 9.
Dose-dependent effects of epinephrine on lung wet-to-dry ratio. Epinephrine causes increased lung water at all doses. Phentolamine pretreatment (P) is protective at the lower two doses. Mean values are plotted with error bars indicating SEM; # indicates difference following epinephrine alone from baseline value; + indicates difference in the phentolamine pretreatment group from their baseline value; * indicates difference between the two groups. Symbol number indicates significance: one symbol, P less than 0.05; three symbols, P less than 0.001.
Isolated Lung Experiments
Capillary Pressures
Infusion of epinephrine (100 µM) resulted in increased Ppc with modest, parallel increases in precapillary and postcapillary pressures (a typical experiment is shown in fig. 10). Median values for Ppc (95% confidence limits) were 7.0 (5.95–7.55) and 8.5 (6.73–9.77) cm H20 for baseline and epinephrine-added Ppc, respectively; P = 0.014 by paired, two-tailed Student t test. However, lung weight did not change with epinephrine treatment alone (P = 0.36).
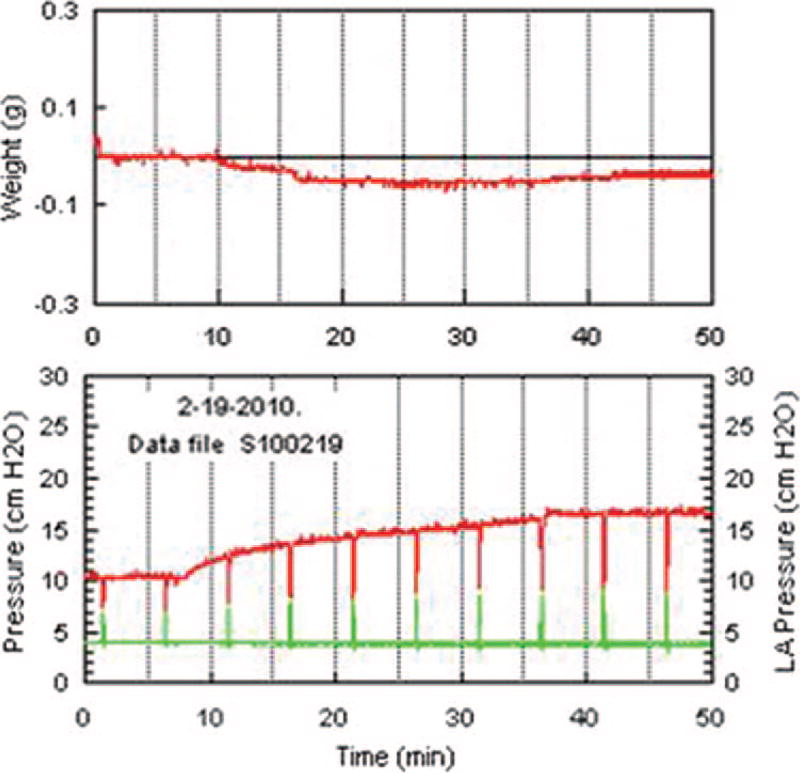
Fig. 10.
Epinephrine infusion and double occlusion pressure. This is a representative experiment where epinephrine infusion (100 µM) was begun 7 min after start of lung perfusion (zero time). The lower chart shows pulmonary artery pressure in red and pulmonary venous pressure in green. Periodic vertical excursions in pulmonary artery pressure and pulmonary venous pressure indicate points of double occlusion. The pulmonary capillary pressure (double occlusion pressure) is taken as the midpoint of the spikes. LAPressure = left atrial pressure.
Effects of Increasing Inflow
Doubling the pulmonary artery inflow rate caused the Ppc to increase dramatically (a representative experiment is shown in fig. 11). The baseline Ppc for all four lungs was 7.0 cm H2O and the median during epinephrine treatment with increased flow was 12.0 (10.9–14.1, 95% CI) cm H2O (P = 0.0016 for the change from baseline value). Furthermore, lung weight increased in the setting of simulated elevation in cardiac output; median δ = 0.080g (0.009 – 0.131, 95% CI), P = 0.035.
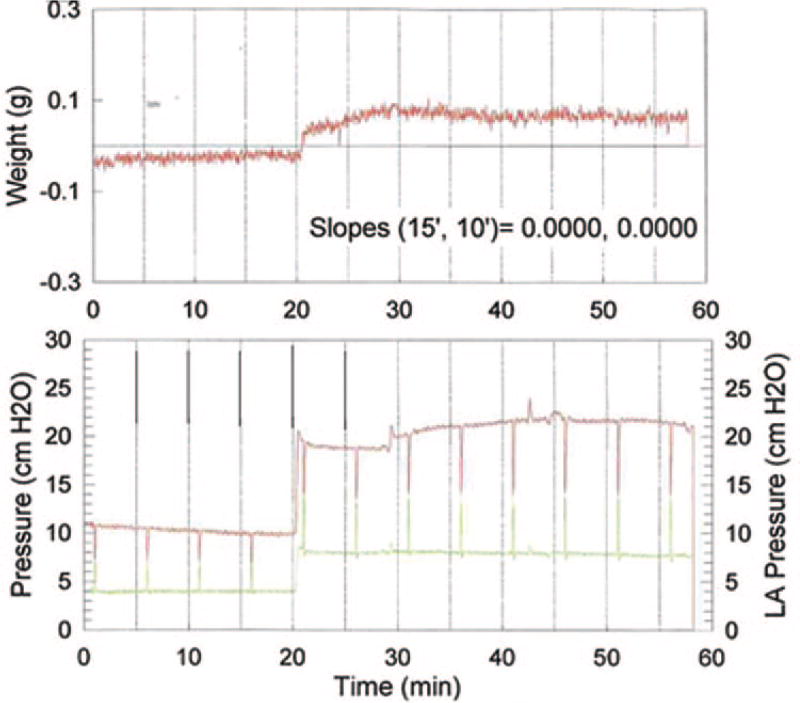
Fig. 11.
Epinephrine infusion and increased pulmonary flow. This is atypical experiment with epinephrine infusion coupled to an increase in flow rate from 2 ml/min to 4 ml/min. Flow rate increase was commenced 21 min after start of perfusion (zero time). Epinephrine infusion (100 µM) was begun at 30 min. Pulmonary artery pressure is in red and pulmonary venous pressure is in green. Vertical excursions in pulmonary artery pressure and pulmonary venous pressure resulted from the periodic double occlusions. LAPressure = left atrial pressure.
Discussion
We found that intravenous epinephrine had rapid, dose-dependent, adverse effects on pulmonary gas exchange, cardiac function, and metabolic parameters in healthy, anesthetized rats. The doses were modest compared with other experimental models and similar to those used in clinical situations after standard allometric scaling. The drop in arterial oxygen tension occurred within 1 min even at the lowest dose examined. Pretreatment with phentolamine attenuated the epinephrine-induced pulmonary effects at all but the highest dose examined (100 mcg/kg). Experiments in isolated lung indicated that epinephrine caused pulmonary edema only when pulmonary flow rates were increased and pulmonary capillary pressures exceeded a threshold of 12 cm H2O. These effects might have implications for the routine use of epinephrine during resuscitation, particularly in the anesthetized patient.
Pulmonary Effects
Epinephrine administration to healthy rats produced rapid deterioration in pulmonary gas exchange, leading to increased alveolar-arterial oxygen gradient, arterial hypoxemia, and hypercapnia. Experimental accounts of adrenergic-induced pulmonary edema and hypoxemia are not new but have generally followed prolonged (~hours) infusion of epinephrine.6,7 However, we found that alveolar-arterial oxygen gradients increased and arterial hypoxemia occurred within a minute of epinephrine injection—the rapidity of this effect is a novel observation. The effects on oxygenation were dose-and time- dependent and, except at the highest dose of epinephrine, were blunted (50 mcg/kg epinephrine) or even prevented entirely (25 mcg/kg epinephrine) by pretreatment with phentolamine. Phentolamine did not protect against changes in carbon dioxide exchange.
The effects on arterial oxygenation were ascribed to pulmonary edema, as fluid collected in the expiratory limb of the breathing circuit and wet-to-dry lung ratios increased. This is surprising in light of several studies showing that β-adrenergic stimulation augments clearance of lung interstitial water.8–10 Previous experimental observations of epinephrine-induced pulmonary edema occurred in the setting of resuscitation from local anesthetic overdose, a low output state where increases in peripheral vascular resistance might further impair cardiac output and induce pulmonary edema. This phenomenon has also been reported in clinical scenarios where epinephrine was used in resuscitating patients from toxic cardiomyopathy.11–13 These observations beg the question of an underlying mechanism.
We used a mouse isolated lung model to discriminate direct effects of epinephrine on lung water and pulmonary capillary pressures. Although epinephrine alone induced modest, parallel increases in precapillary and postcapillary pressures and increased Ppc (typically 1–2 cm H2O), these were not associated with a change in lung weight (P = 0.036) at normal flow rates. Therefore epinephrine itself did not directly contribute to pulmonary edema. However, lung weight increased when epinephrine was coupled with a flow rate that was doubled to mimic the effect of epinephrine on cardiac output. This combination increased the Ppc (mean δ, 5.5 cm H2O) and lung water. In every case the increase in lung weight appeared at a Ppc of approximately 12 cm H20. This observation agrees with the recent finding by Dull et al.14 of a nonlinear relationship between Ppc and capillary filtration coefficient. In their experiments, the filtration coefficient virtually doubled above a double occlusion pressure of 12 cm H2O. This argues that in our experiments hydrostatic pulmonary edema occurred when increased pulmonary flow coupled to epinephrine administration resulted in a Ppc above a threshold level of approximately 12 cm H2O.
Berk et al.15 and others16,17 theorized that shunt during prolonged epinephrine infusion was initially due to pulmonary arterial flow redistribution with secondary ventilation-perfusion inequalities and that only after several hours was it caused by interstitial and alveolar edema. They ascribed these effects to β-adrenergic stimulation as they were reversed by propranolol whereas phenoxybenzamine pretreatment had only minor protective effects. We found that phentolamine pretreatment reduced the wet-to-dry ratio and protected against the drop in arterial hypoxemia—a finding that implies a key role for α-adrenergic stimulation. This could theoretically reflect a salutary effect of phentolamine on pulmonary ventilation-perfusion matching; further studies would be required to test this hypothesis. The lack of a dramatic increase in isolated lung postcapillary pressures after epinephrine treatment alone favors the edemagenic role of peripheral vasoconstriction with secondary increases in left atrial and pulmonary vascular pressures over a direct effect on pulmonary postcapillary resistance. Therefore, it is likely that the potent, combined effects of epinephrine on both pulmonary artery flow (β stimulation) and pulmonary capillary pressure (peripheral α stimulation) caused gross transudation of water into the pulmonary interstitium and alveolae. We surmise that the major benefit of phentolamine treatment was countering epinephrine activation of peripheral α-adrenergic receptors. The appearance of erythrocytes in the pulmonary edema fluid suggests that capillary endothelial disruption might also be involved. This mechanism is supported by the observations that both α and β receptor-mediated increases in pulmonary capillary pressure can damage the endothelium, disrupt the basement membrane, and cause interstitial and alveolar extravasation.16,17
Metabolic Effects
The epinephrine-induced changes in pH and lactate formation were reduced by phentolamine pretreatment. Increased lactate is associated with adverse outcomes in the critically ill, and lactic acid clearance correlates with patient survival.18,19 Although shock states produce hyperlactatemia through both tissue hypoxemia and adrenergic stimulation (endogenous and exogenous pathways), it is unclear which mechanism predominates. Epinephrine exerts direct metabolic effects that increase blood lactate concentrations without reducing tissue perfusion.20,21 We found a strong positive correlation between serum lactate concentrations at 10 min and the bolus dose of epinephrine administered. The highest doses of epinephrine reliably produced the worst metabolic profiles. This conforms to the notion that increased blood lactate correlates with poor patient outcomes.22,23
Circulatory Effects
Cardiovascular function was depressed by 10 min after a dose of 100 mcg/kg epinephrine. Unlike pulmonary function, phentolamine pretreatment was protective at all epinephrine doses tested. The correlation of epinephrine administration with adverse cardiovascular outcomes is not novel. Epinephrine infusion has been known for more than a century to induce shock.24 It is arrhythmogenic, increases myocardial oxygen demand, reduces subendocardial perfusion, and reduces myocardial function after resuscitation.25 Clinical and laboratory data suggest that higher versus standard doses of epinephrine can worsen recovery in various shock states. It is arguable whether patients in such clinical studies received more adrenaline support because of their poor baseline physiologic status or if the persistent adrenergic stimulation contributed to their decline. However, this question is addressed in the current study, because the test rats were healthy before epinephrine treatment and baseline values for key hemodynamic and metabolic parameters were not different among the groups. Our findings confirm that epinephrine alone reliably induces shock in experimental animals.24,26 This, along with a lack of proven benefit for epinephrine, weighs against its routine use during cardiac arrest. Clinical evidence does not support the use of epinephrine for treating the pulseless patient For instance, Olasveengen et al.2 showed that use of epinephrine in out-of-hospital cardiac arrest provided no improvement in survival to hospital discharge or long-term survival. Apparently, the benefit of epinephrine administration is short-lived and illusory at best.
Limitations
Our study is limited by the relatively short experimental duration (10 min). Longer-term survival was not determined because the primary objective was to assess only acute effects of epinephrine on cardiopulmonary physiology. Interspecies differences in pulmonary physiology are also important confounders. Murine models may display large metabolic and circulatory buffer systems due to their high metabolic activity.25 Nevertheless, major adverse metabolic responses to epinephrine were observed.
Extrapolating doses of epinephrine used in these experiments to the clinical setting is not straightforward, given the difficulties of allometric scaling between species. However, standard conversions (for rat to human = 6) suggest the highest dose in these experiments (100 mcg/kg) is equivalent to 17 mcg/kg in humans, a typical dose for cardiopulmonary resuscitation. The doses used in these experiments are typical for animal studies of pressor therapy in resuscitation. It can also be argued that volatile anesthesia is an important confounder in this model. However, given the requirement for anesthesia due to ethical concerns, we preferred agents that would potentially blunt the hypertensive reaction to the planned dose of epinephrine that previous studies indicated could cause pulmonary hemorrhage. Despite this, the model may be relevant to patients under general anesthesia and could guide modifications of resuscitation in this clinical context. Furthermore, we did not evaluate the contribution of other mechanisms beyond α-1 receptor stimulation. Finally, one can question the applicability of a healthy animal model to studying epinephrine effects because this drug is only given to patients in extremis. However, our approach avoids the pathophysiologic confounders of cardiac arrest and chest compressions that unavoidably alter cardiopulmonary and metabolic measures.
Conclusion
The central finding of our study is that epinephrine caused rapid cardiopulmonary deterioration in previously healthy, anesthetized rats. Hypoxemia occurred within 1 min and was caused by hydrostatic pulmonary edema. Isolated lung experiments indicated the increased Ppc resulted from the combination of epinephrine and increased pulmonary blood flow. Pulmonary edema was seen when Ppc exceeded a threshold of approximately 12 cm H2O. We believe that the pulmonary edema, decreased arterial oxygen tension, poor RPP, and hyperlactatemia could call into question the use of standard epinephrine doses during cardiopulmonary resuscitation, particularly for the anesthetized patient. Further study is needed to confirm the rationale for using traditional doses of epinephrine in the pulseless patient.
What We Already Know about This Topic
Previous studies have demonstrated that epinephrine adversely affects arterial oxygenation when administered in a rat model of local anesthetic overdose
The current study investigated whether epinephrine might exert acute, deleterious effects on pulmonary oxygen exchange in anesthetized, intact rats
What This Article Tells Us That Is New
Epinephrine caused rapid cardiopulmonary deterioration in previously healthy, anesthetized rats. Effects were blunted by a-adrenergic receptor blockade
Acknowledgments
Support was provided by the United States Veterans’ Administration Merit Grant (to Dr. Weinberg), Central Office Research and Development, Washington, D.C.
Footnotes
Abstract presented at the Annual Meeting of the American Society of Anesthesiologists, San Diego, California, October 18, 2010 (Dr. Hiller)
References
- 1.Gueugniaud PY, David JS, Chanzy E, Hubert H, Dubien PY, Mauriaucourt P, Bragana C, Billres X, Clotteau-Lambert MP, Fuster P, Thiercelin D, Debaty G, Ricard-Hibon A, Roux P, Espesson C, Querellou E, Ducros L, Ecollan P, Halbout L, Savary D, Guillaume F, Maupoint R, Capelle P, Bracq C, Dreyfus P, Nouguier P, Gache A, Meurisse C, Boulanger B, Lae C, Metzger J, Raphael V, Beruben A, Wenzel V, Guinhouya C, Vilhelm C, Marret E. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2008;359:21–30. doi: 10.1056/NEJMoa0706873. [DOI] [PubMed] [Google Scholar]
- 2.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: A randomized trial. JAMA. 2009;302:2222–9. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 3.McCaul CL, McNamara PJ, Engelberts D, Wilson GJ, Romaschin A, Redington AN, Kavanagh BP. Epinephrine increases mortality after brief asphyxial cardiac arrest in an in vivo rat model. Anesth Analg. 2006;102:542–8. doi: 10.1213/01.ane.0000195231.81076.88. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg GL, Di Gregorio G, Ripper R, Kelly K, Massad M, Edelman L, Schwartz D, Shah N, Zheng S, Feinstein DL. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108:907–13. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- 5.Hiller DB, Gregorio GD, Ripper R, Kelly K, Massad M, Edelman L, Edelman G, Feinstein DL, Weinberg GL. Epinephrine impairs lipid resuscitation from bupivacaine overdose: A threshold effect. Anesthesiology. 2009;111:498–505. doi: 10.1097/ALN.0b013e3181afde0a. [DOI] [PubMed] [Google Scholar]
- 6.Berk JL, Hagen JF, Koo R, Beyer W, Dochat GR, Rupright M, Nomoto S. Pulmonary insufficiency caused by epinephrine. Ann Surg. 1973;178:423–35. doi: 10.1097/00000658-197310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berk JL, Hagen JF, Tong RK, Levy ML, Martin PJ. The role of adrenergic stimulation in the pathogenesis of pulmonary insufficiency. Surgery. 1977;82:366–72. [PubMed] [Google Scholar]
- 8.Berthiaume Y, Staub NC, Matthay MA. β-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J Clin Invest. 1987;79:335–43. doi: 10.1172/JCI112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank JA, Wang Y, Osorio O, Matthay MA. Beta-adrenergic agonist therapy accelerates the resolution of hydrostatic pulmonary edema in sheep and rats. J Appl Physiol. 2000;89:1255–65. doi: 10.1152/jappl.2000.89.4.1255. [DOI] [PubMed] [Google Scholar]
- 10.Tibayan FA, Chesnutt AN, Folkesson HG, Eandi J, Matthay MA. Dobutamine increases alveolar liquid clearance in ventilated rats by beta-2 receptor stimulation. Am J Respir Crit Care Med. 1997;156:438–44. doi: 10.1164/ajrccm.156.2.9609141. [DOI] [PubMed] [Google Scholar]
- 11.Levsky ME, Miller MA. Cardiovascular collapse from low dose bupivacaine. Can J Clin Pharmacol. 2005;12:e240–5. [PubMed] [Google Scholar]
- 12.Reinikainen M, Hedman A, Pelkonen O, Ruokonen E. Cardiac arrest after interscalene brachial plexus block with ropivacaine and lidocaine. Acta Anaesthesiol Scand. 2003;47:904–6. doi: 10.1034/j.1399-6576.2003.00188.x. [DOI] [PubMed] [Google Scholar]
- 13.Sirianni AJ, Osterhoudt KC, Calello DP, Muller AA, Water-house MR, Goodkin MB, Weinberg GL, Henretig FM. Use of lipid emulsion in the resuscitation of a patient with prolonged cardiovascular collapse after overdose of bupropion and lamotrigine. Ann Emerg Med. 2008;51:412–5. doi: 10.1016/j.annemergmed.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Dull RO, Cluff M, Kingston J, Hill D, Chen H, Hoehne S, Malleske DT, Kaur R. Lung heparan sulfates modulate K(fc) during increased vascular pressure: Evidence for glycocalyx-mediated mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2012;302:L816–28. doi: 10.1152/ajplung.00080.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berk JL, Hagen JF, Koo R. Effect of alpha and beta adrenergic blockade on epinephrine induced pulmonary insufficiency. Ann Surg. 1976;183:369–76. doi: 10.1097/00000658-197604000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dnser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: Adverse effects of adrenergic stress. J Intensive Care Med. 2009;24:293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 17.Kreienberg PB, Vincent PA, Bell DR, Saba TM, Minnear FL. Isoproterenol decreases protein permeability in edematous isolated rabbit lungs: Estimation of PS and sigma. J Appl Physiol. 1994;77:325–31. doi: 10.1152/jappl.1994.77.1.325. [DOI] [PubMed] [Google Scholar]
- 18.Arnold RC, Shapiro NI, Jones AE, Schorr C, Pope J, Casner E, Parrillo JE, Dellinger RP, Trzeciak S Emergency Medicine Shock Research Network (EMShockNet) Investigators. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock. 2009;32:35–9. doi: 10.1097/shk.0b013e3181971d47. [DOI] [PubMed] [Google Scholar]
- 19.Donnino MW, Miller J, Goyal N, Loomba M, Sankey SS, Dolcourt B, Sherwin R, Otero R, Wira C. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation. 2007;75:229–34. doi: 10.1016/j.resuscitation.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 20.McCarter FD, James JH, Luchette FA, Wang L, Friend LA, King JK, Evans JM, George MA, Fischer JE. Adrenergic blockade reduces skeletal muscle glycolysis and Na(+), K(+)-ATPase activity during hemorrhage. J Surg Res. 2001;99:235–44. doi: 10.1006/jsre.2001.6175. [DOI] [PubMed] [Google Scholar]
- 21.McCarter FD, Nierman SR, James JH, Wang L, King JK, Friend LA, Fischer JE. Role of skeletal muscle Na+-K+ ATPase activity in increased lactate production in sub-acute sepsis. Life Sci. 2002;70:1875–88. doi: 10.1016/s0024-3205(02)01475-3. [DOI] [PubMed] [Google Scholar]
- 22.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–7. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 23.Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, Davies A, Stachowski E, Reade MC, Bailey M, Cooper DJ. Relative hyperlactatemia and hospital mortality in critically ill patients: A retrospective multi-centre study. Crit Care. 2010;14:R25. doi: 10.1186/cc8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlanger J, Gasser H. Studies in secondary traumatic shock. Circulatory failure due to adrenalin. Am J Physiol. 1919;49:345–76. [Google Scholar]
- 25.Tang W, Weil MH, Sun S, Noc M, Yang L, Gazmuri RJ. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation. 1995;92:3089–93. doi: 10.1161/01.cir.92.10.3089. [DOI] [PubMed] [Google Scholar]
- 26.Freeman N, Freedman H, Miller C. The production of shock by the prolonged continuous injection of adrenalin in un-anesthetized dogs. Am J Physiol. 1941;131:545–53. [Google Scholar]