Abstract
Background
Branched-chain amino acids (BCAA) have been previously linked to survival in colorectal cancer (CRC) patients. It is unclear whether BCAAs are prognostic biomarkers or surrogate markers for energy balance.
Objectives
We aimed to determine correlations of BCAAs with markers of energy balance over time and to investigate prognostic significance of BCAAs in CRC.
Methods
We used urinary samples from newly diagnosed CRC patients [n=163; (stage I – IV)] from the ColoCare study in Heidelberg, Germany, collected at surgery (n=163), 6 (n=83) and 12 months follow-up (n=54). Isoleucine, leucine, valine, (2Z)-3-methylglutaconic acid (3HM), 2-ethylhydracrylic acid (2EA), 2-methyl-3-hydroxybutyrate (2M3H) were detected using gas-chromatography mass-spectrometry and proton-nuclear-magnetic-resonance spectroscopy. Partial correlation coefficients between BCAAs with body mass index (BMI), physical activity (metabolic equivalent [MET]) and muscle area were computed and adjusted for sex and age at diagnosis. We used Cox proportional hazard models to investigate overall survival (OS) after 24 months of follow-up.
Results
We did not observe significant correlations between BCAAs and parameters of energy balance at all time points (correlation ranges: BMI: r= −0.13 to −0.01; METs: r=−0.14 to 0.02; dorsal muscle: r=−0.03 to 0.10). BCAAs were not associated with risk of death in stage I-III (e.g., valine: HRlog2=1.62, p=0.25) or in stage IV tumors. Elevated concentrations of 2EA and 2M3H were significantly associated with OS, independent of stage (2EA: stage I-III: HRlog2=0.42, p=0.04; stage IV: HRlog2=0.51, p=0.01).
Conclusion
Our study suggests that BCAAs in colorectal cancer patients do not reflect parameters of energy balance and may be independently associated with overall survival.
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer-related death worldwide. (Siegel et al. 2016) At the same time, the number of CRC survivors continues to increase. (Miller et al. 2016) Today, the most accurate means for predicting prognosis for CRC remains pathological stage, although substantial clinical heterogeneity in treatment response exists among patients with the same cancer stage. (Usher-Smith et al. 2016) There is an urgent need to identify more precise prognostic markers for CRC patients that can be translated to clinical settings.
CRC progression is associated with essential changes in amino acid metabolism due to the demands of the tumor and its interaction with the host. (Deberardinis et al. 2008; Kroemer and Pouyssegur 2008) The branched-chain amino acids (BCAAs) leucine, isoleucine and valine account for 35% of the essential amino acids in muscle proteins. (Uneyama et al. 2016) BCAAs are unique substrates for cell metabolism, differentiation and proliferation. (O’Connell 2013)
There is in vitro and in vivo evidence suggesting that BCAAs may slow cancer development and progression, (Baracos and Mackenzie 2006; O’Connell 2013; Nakano et al. 2013; Sugiyama et al. 1998; Ishizaki et al. 2013; Budhathoki et al. 2017). Prior studies have shown BCAA-induced apoptosis (Terakura et al. 2012; Shimizu et al. 2014) and inhibition of angiogenesis in cancer cells. (Murata and Moriyama 2007; Cha et al. 2013). Sugiyama and colleagues demonstrated that in vitro growth of human hepatocellular cancer (HCC) cells can be suppressed by enriched BCAA levels in medium.(Sugiyama et al. 1998) Long-term BCAA treatment in an obese-rats model of hepatocellular cancer induced cell cycle arrest and apoptosis and suppressed the development of pre-cancerous lesions. (Ishizaki et al. 2013)
Recently, high plasma concentrations of leucine, valine and total BCAA were inversely associated with risk of colorectal adenomas, an established precursor of CRC.(Budhathoki et al. 2017) This is in agreement with in vivo studies that have linked BCAAs to reduced relapse rates (Ishikawa et al. 2013) and improved survival rates in patients diagnosed with hepatocellular carcinoma. (Tada et al. 2015; Uneyama et al. 2016) Supplementation of BCAAs has also shown some clinical benefit for cancer patients suffering from cancer cachexia. (Imanaka et al. 2016)
This evidence, however, is in contrast to animal studies that have linked BCAAs to enhancement of pancreatic tumor growth (Liu et al. 2014) as well as breast cancer progression (Zhang and Han 2017; Thewes et al. 2017) via activation of the mammalian target of rapamycin (mTOR) pathway. There is currently no clear consensus on the role of BCAA metabolism in cancer development and progression.
To date, only a few, relatively small studies have investigated the role of BCAAs in colorectal cancer development. (Chen et al. 2012; Qiu et al. 2009; Gao et al. 2016) Valine and leucine measured in urine were significantly lower in advanced CRC cases (stage III and IV) compared to patients in the early stage group (stage I and stage II) [n=20 CRC cases; n=14 healthy controls] (Chen et al. 2012). Similarly, serum metabolite profiling in n=60 CRC cases and n=63 healthy controls showed significantly lower concentrations of valine and leucine in CRC patients compared to healthy controls. (Qiu et al. 2009) In addition, targeted amino acid analysis in tissue of CRC cases and advanced adenomas exhibited that valine and isoleucine can successfully differentiate CRC from advanced adenomas with an area under the receiver operating characteristic curve of 0.99 [n=22 CRC samples; n=10 advanced adenomas samples]. (Gao et al. 2016). BCAAs continue to emerge as biomarkers that can differentiate between colorectal cancer cases, colorectal adenomas and healthy controls as well as between early and late stage CRC.
BCAA metabolism can be altered by various mechanisms, including reduction of dietary intake, change in physical activity, or increased skeletal muscle catabolism (e.g., increased protein degradation and reduced protein synthesis). (Baracos and Mackenzie 2006; O’Connell 2013) To date, it is unclear whether BCAAs can be utilized as independent prognostic markers for CRC survival or whether they are surrogate markers for parameters of energy balance (e.g., body mass index (BMI), physical activity (PA) or muscle area), which have been independently associated with CRC survival. (Wang et al. 2017; Malietzis et al. 2015; Hardikar et al. 2015; Meyerhardt et al. 2009a; Meyerhardt et al. 2009b; Kocarnik et al. 2016) For example, elevated BMI is a predictor of recurrence and prognosis in CRC patients. (Wang et al. 2017; Kocarnik et al. 2016) Increased pre-diagnostic physical activity has been linked to improved survival (Hardikar et al. 2015) and reduced mortality rates (Schmid and Leitzmann 2014) in CRC survivors.
The aim of our present study is to evaluate associations between BCAAs and catabolites with parameters of energy balance (e.g., BMI, physical activity measured as metabolic equivalent (Gould et al.) hours/week and muscle area) over time to test the hypothesis, that urinary BCAAs may be solely surrogate measures for BMI, physical activity or muscle area. We have used urine samples of n=163 CRC patients collected at three time-points (at surgery, 6 and 12 months after surgery) from the well-established prospective ColoCare Study to investigate correlations of BCAAs and its catabolites in urine with parameters of energy balance at several time points. Further, we investigated the association of BCAAs and catabolites with 2 year overall survival in CRC patients.
Methods
The ColoCare Consortium is a multicenter international prospective cohort recruiting newly diagnosed colorectal cancer patients (stages I through IV; International Classification of Diseases, 10th edition, C18-C20) and aiming to investigate predictors of cancer recurrence, treatment toxicities, survival, and health-related quality of life (ClinicalTrials.gov: NCT02328677). (D. Liesenfeld et al. 2015; Skender et al. 2015; Ristau et al. 2014; D. B. Liesenfeld et al. 2015; Bohm et al. 2017) The present study was performed in n=163 patients recruited at the ColoCare site in Heidelberg, Germany between October 2010 and January 2013. After study approval by the Institutional Review Board of the Medical Faculty at the University of Heidelberg, written informed consent was obtained by all participants.
Demographic information as well as data on established risk factors were collected using standardized questionnaires at surgery, 6 and 12 months. BMI was measured and calculated as kg/m2 at all time points. Baseline examination includes questionnaires, anthropometric measurements, assessment of physical activity and biospecimen collection (e.g., blood and urine samples). Information on adjuvant chemotherapy, and medical history were abstracted from clinical records. Patients’ survival data were monitored both, by access to medical records and as part of the active patient follow-up within ColoCare. If patients’ died, the time point of death was collected.
Physical activity
We used the VITAL Physical Activity Questionnaire to assess participants’ physical activity. (Skender et al. 2015) This questionnaire assesses quantity (frequency and hours per week) and type of activity during the past four weeks. The VITAL questionnaire has been validated in the English and Spanish language. For this study the modified questionnaire was translated into German (Skender et al. 2015) and metabolic equivalent (MET; hours/week) were calculated for the baseline assessment and 6- and 12-months follow-up.
Quantification of skeletal muscle area
The area of the dorsal muscles, abdominal muscles and M. psoas major were quantified using abdominal computed tomography (CT)-scans as part of clinical care, predominantly before surgery. (Nattenmueller et al. 2016) CTs from pre- and post-surgery were similar and thus, combined for statistical analyses.28
Specific regions of interest (ROI) of muscle area (dorsal muscles, abdominal muscles and the M. psoas major) were manually determined and measured on two levels (vertebral body L3/4 and L4/5) by a volumetric tool (MMWP, Syngo Volume tool, Siemens Healthcare, Munich, Berlin, Germany). (Nattenmueller et al. 2016) The measurements limits for aforementioned selected ROIs were at a minimum attenuation of 40 Hounsfield Units (HU) to maximum 100 HU to avoid measuring errors including muscles’ lipid content and application of contrast media. (Goodpaster et al. 2000)
Biospecimens collection
Spot urinary samples were collected at the National Center of Tumor Diseases (NCT), Heidelberg at three time points [prior to surgery (n=126) and in some cases 1-8 days after surgery (n=37), 6 months (n=83) and 12 months (n=54) after surgery]. If patients underwent adjuvant chemotherapy, follow-up visits were scheduled at least 2 weeks after their last chemotherapy cycle had been completed. Urine samples were aliquoted immediately and stored at −80 °C until analysis. Self-reported food and fluid intake before sample collection was monitored during follow-up visits.
Laboratory analyses and quality control measurements
We used gas-chromatography mass-spectrometry (GC-MS) and proton-nuclear-magnetic resonance spectroscopy (1H-NMR) for the metabolomics profiling of urinary samples, as described in detail previously. (D. Liesenfeld et al. 2015)
Sample preparation and quality control: GC-MS
Preparation of urine samples for GC-MS was based on the method by Cheng and colleagues. (Cheng et al. 2012) Urine samples were thawed and vortexed briefly and aliquots of 50 μL were transferred into a reaction tube and 10 μL internal standard solution was added. The sample was transferred into an amber vial with micro inserts and subjected to GC-MS analysis. We used pooled quality control (QC) samples and Kovats retention mixtures (50 μg*mL-1) injected with every analytical batch. A LOESS function was used to correct drifts for each metabolite and relative standard deviations (%RSD) were used to estimate the analytical noise. In addition, we analyzed paired samples in the same analytical batches to account for time-dependent bias due to analytical drifts. Analyses were carried out on an Agilent 6890 GC/ 5973 MS single quadrupole system. Data acquisition was started after a solvent delay of 5 minutes. Spectra were acquired over a range of 50-500 m/z. (D. Liesenfeld et al. 2015)
Sample preparation and quality control: 1H-NMR
Sample preparation for 1H-NMR was performed according to the method by Xiao and colleagues: 540μL urine were spiked with 60μL K2HPO4/NaH2PO4 buffer in D2O (pH 6.5, 1.5M). (C. Xiao et al. 2009) QC samples were run at the beginning and end of each analytical batch. Citrate was measured in duplets during all data acquisitions and a 14.7% RSD in the raw 1H-NMR data already indicated an analytically robust method and a short term variance (across n=100 samples) of a median %RSD of 5%.
Data pre-processing
GC–MS: Raw files were converted to netCFD format, imported into MZMine 2.0 (Pluskal et al. 2010) and chromatograms crop filtered (5-32 minutes) and baseline corrected. Masses were detected with centroid algorithm and chromatogram building was carried out with minimum time span of 0.1 minutes, minimum height of 1E3 and mass accuracy 0.5 m/z. The retention time (Rt), mass spectrometric base peak used for quantification, confidence level in identification, matches in the Human Metabolome Database (HMDB) and PubChem are listed in Table 1.
Table 1.
List of all metabolites detected during the GC–MS urine metabolomics analysis or including data on retention time (Rt), mass spectrometric base peak used for quantification confidence level in identification and matches in the Human Metabolome Database, PubChem, and 1H-NMR spectroscopic bins for which resonances of urinary metabolites were reported, based on the Chenomx Library version 7.7 or the Human Metabolome Database
| Metabolite | Method | Rt (min) |
Basepeak (m/z) | Confidence | Match | HMDB1 | PubChem |
|---|---|---|---|---|---|---|---|
| 2-Ethylhydracrylic acid | GC–MS | 8.277 | 247 | Level 2 | 2-Ethylhydracrylic acid | 00396 | 188979 |
| (2Z)-3-Methylglutaconic acid | GC–MS | 11.532 | 198 | Level 1 | 3-Methylglutaconic acid | 00522 | 1551553 |
| Valine | GC–MS | 8.112 | 144 | Level 1 | L-Valine | 00883 | 6287 |
| 2-Methyl-3-hydroxybutyric acid | GC–MS | 7.862 | 191 | Level 2 | 2-Methyl-3-hydroxybutyric acid | 00354 | 160471 |
|
Bin high |
Bin low |
||||||
| Isoleucine | 1H-NMR | 1.0307 | 0.9906 | L-Isoleucine | 00172 | ||
| Leucine | 1H-NMR | 0.9753 | 0.9161 | L-Leucine | 00687 |
HMDB: Human Metabolome Database
1H NMR: Spectra were processed automatically using the Bruker TopSpin software with zero-filling (6-fold), the application of 0.3 Hz of line broadening, phase correction and referenced relative to TSP incorporated as an internal standard.(D. B. Liesenfeld et al. 2015) Processed data files where subsequently imported in batch mode into the dataChord spectrum miner software (OneMoon Scientific, New Jersey, USA). Each region was integrated and the total integral area of each bin divided by the area of the internal standard TSP creating the final data table for statistical analysis. The spectroscopic bins for which resonances of urinary metabolites were reported, based on the Chenomx Library version 7.7 / HMDB are listed in Table 1.
Statistical analysis
Data processing of urine metabolite measures from 1H-NMR and GC-MS were conducted separately using the commercially available Metaboanalyst software 3.0. (Xia et al. 2015) To account for heteroscedasticity data were normalized by sum, log-transformed and auto-scaled using the Metaboanalyst software. (Xia et al. 2015) Missing values have been replaced by half the minimum observed value for the respective metabolite.
Mean and standard deviations were calculated for continuous variables at baseline, 6 and 12 months follow-up. Data on BMI and METs were available for all 3 time points, while muscle area measurements were solely assessed at baseline. One-way ANOVA was applied for multiple group comparison. Pearson Chi-squared test was conducted for testing distribution differences by time points for categorical variables. Pearson’s partial correlation coefficients were calculated adjusting for sex and age. As we observed similar results from analyses adjusting in addition for tumor stage (data not shown), we only present data adjusted for age and sex. In sensitivity analyses we excluded patients diagnosed with stage IV cancer, as results may have been impacted by tumor cachexia (data not shown). Scatter plots were used to visualize the relationship between parameters of energy balance at baseline.
Cox proportional hazard models were used to investigate overall survival after 24 months of follow-up; adjusted for age, sex, stage and analytical batch. Time at risk was estimated from the date of recruitment to the date of death, loss to follow-up, or the end of follow-up, whichever occurred first. Patients that were still alive after 24 months of follow-up were censored. We adjusted for residual confounding based on the method from Willet et al. to account for the collinearity between the investigated biomarkers.(Willett et al. 1997)
To summarize risk associated with the investigated metabolites, a risk score was created based on tertiles of metabolite concentrations. Patients were assigned values based on tertiles of metabolite concentration for BCAAs: zero (1st tertile); one (2nd tertile) and two (3rd tertile). For catabolite concentrations, participants in the 3rd tertile were assigned a zero, a one for the 2nd tertile and a two for the 3rd tertile as prior analyses have shown an inverse association with survival. After assigning a score for each metabolite, the scores were summed to generate an individual metabolite risk score. The continuous score variable was then redefined in tertiles.
Statistical analyses and figure-plotting were performed using SAS for Windows, version 9.4. All tests were two-sided with significance level 0.05.
Results
Patient characteristics are presented in Table 2. We included a total of n=163 CRC patients at baseline with an average age of 64.1 years at enrollment. Patients were more likely to be male (67%) with an average BMI of 26.6 kg/m2 at baseline. About half of the patients were diagnosed with colon cancer and 53% of patients were diagnosed with advanced tumors (stage III and IV). After 24 months of clinical follow-up n=31 patients died.
Table 2.
Patient characteristics at baseline, 6 and 12 months follow-up.*
| Variables | Baseline (n=163) |
6 months (n=83) |
12 months (n=57) |
p-Value |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female | 53 (32%) | 23 (28%) | 18 (32%) | 0.74 |
| Male | 110 (68%) | 60 (72%) | 39 (68%) | 0.74 |
| Age (years), mean (±std) | 64 (12) | 62 (12) | 61 (10) | 0.16 |
| Cancer site, n (%) | ||||
| Colon | 78 (48%) | 37 (45%) | 22 (39%) | 0.48 |
| Rectum | 85 (52%) | 46 (55%) | 35 (61%) | 0.48 |
| Cancer stage, n (%) | ||||
| I/II | 76 (47%) | 36 (43%) | 32 (56%) | 0.31 |
| III/IV | 87 (53%) | 47 (57%) | 25 (44%) | 0.31 |
| Parameters of energy balance, mean (±std) | ||||
| BMI (kg/m2) | 26.6 (±4.0) | 25.3 (±3.9) | 26.5 (±3.9) | 0.26 |
| METs (hours/week) | 12.3 (±16.8) | 10.3 (±14.8) | 12.2 (±15.9) | 0.63 |
| Adipose tissue area (cm2), mean (±std) | ||||
| Dorsal muscles L3/L4 | 30.1 (±14.5) | 31.3 (±14.5) | 31.6 (±14.4) | 0.87 |
| Dorsal muscles L4/L5 | 23.7 (±10.4) | 26.0 (±11.7) | 25.0 (±11.8) | 0.58 |
| Psoas muscles L3/L4 | 16.3 (±6.9) | 17.9 (±6.9) | 17.3 (±5.8) | 0.42 |
| Psoas muscles L4/L5 | 19.1 (±7.7) | 22.1 (±13.6) | 21.8 (±13.9) | 0.31 |
| Abdominal muscles L3/L4s | 32.0 (±14.7) | 34.7 (±14.0) | 48.4 (±1.1) | 0.31 |
| Abdominal muscles L4/L5 | 26.3 (±9.2) | 29.0 (±8.2) | 32.3 (±2.3) | 0.55 |
Sample availability for the urinary metabolomics analysis of study population.
Follow-up data were available for n=83 patients at 6 months and n=57 patients at 12 months follow-up. Repeated measurements for all three time points were available for n=12 patients. For n=38 patients we had measurements at baseline and 6-months follow-up, for n=22 patients we had measurements at 6-months and 12-months follow-up, for n=8 patients we had measurements at baseline and 12-months follow-up. Single measurements were available for baseline (n=129), 6-months follow-up (n=17) and 12-months follow-up (n=20).
The BCAAs (isoleucine, leucine and valine) where highly and significantly correlated at each time point (e.g., baseline: >0.75, 6 months follow-up correlation: >0.88, 12 months follow-up correlations: >0.91; Supplementary Table 1). For the three catabolites 2EA, 2M3HA and 3HM correlations with each other and over time were modest ranging between 0.41 and 0.56 (Supplementary Table 1).
The correlations with the main BCAAs and the three catabolites were significant but comparably low, ranging from −0.03 – 0.14 (baseline); −0.02 – 0.18 (6 months follow-up) and -0.07 – 0.19 (12 months follow-up; Supplementary Table 1).
In Table 3 and Table 4 we have summarized Pearson’s partial correlations (adjusted for age and sex) for BCAAs and the catabolites with parameters of energy balance at each time point. We did not observe significant correlations for any of the investigated metabolites with parameters of energy balance. At all three time points, the correlations of isoleucine, leucine and valine with the parameters of energy balance were small and not statistically significant: e.g., BMI: leucine: range of correlations: −0.12 to 0.03 (p>0.35); isoleucine: range of correlations: −0.13 to −0.03 (p>0.35); valine: range of correlations: −0.11 to 0.05 (p>0.43).
Table 3.
Pearson correlation between isoleucine, leucine and valine with BMI, physical activity (METs) and muscle area over time (adjusted for age and sex).
| Variable | Time point | Isoleucine | Leucine | Valine | |
|---|---|---|---|---|---|
| BMI | Baseline | n=163 | −0.03 (0.69) | −0.01 (0.86) | −0.01 (0.95) |
| 6 months | n=32 | −0.06 (0.75) | −0.04 (0.84) | 0.05 (0.78) | |
| 12 months | n=55 | −0.13 (0.35) | −0.12 (0.40) | −0.11 (0.43) | |
|
| |||||
| METs | Baseline | n=156 | 0.02 (0.81) | −0.03 (0.72) | −0.02 (0.77) |
| 6 months | n=82 | −0.01 (0.94) | 0.00 (0.99) | −0.03 (0.80) | |
| 12 months | n=55 | −0.11 (0.45) | −0.14 (0.33) | −0.10 (0.47) | |
|
| |||||
| Dorsal muscles 4/5 | Baseline | n=65 | 0.00 (0.99) | −0.03 (0.83) | 0.00 (1.00) |
| 6 months | n=41 | 0.04 (0.82) | 0.02 (0.92) | 0.02 (0.89) | |
| 12 months | n=36 | 0.10 (0.57) | 0.06 (0.75) | 0.10 (0.57) | |
|
| |||||
| Psoas muscles 4/5 | Baseline | n=66 | −0.25 (0.05) | −0.18 (0.16) | −0.26 (0.04) |
| 6 months | n=43 | 0.21 (0.18) | 0.19 (0.23) | 0.20 (0.21) | |
| 12 months | n=40 | 0.14 (0.42) | 0.10 (0.54) | 0.09 (0.58) | |
Table 4.
Pearson correlation between 2EA, 2M3HA, 3HM with BMI, physical activity (METs) and muscle area over time (adjusted for age and sex).
| Variable | Time point | 2EA | 2M3HA | 3HM | |
|---|---|---|---|---|---|
| BMI | Baseline | n=163 | −0.11 (0.17) | −0.14 (0.07) | −0.13 (0.11) |
| 6 months | n=32 | 0.16 (0.38) | 0.06 (0.74) | −0.07 (0.73) | |
| 12 months | n=55 | 0.11 (0.42) | −0.10 (0.47) | −0.03 (0.81) | |
|
| |||||
| METs | Baseline | n=156 | 0.00 (0.97) | 0.04 (0.60) | 0.04 (0.60) |
| 6 months | n=82 | 0.27 (0.02) | 0.19 (0.10) | 0.18 (0.12) | |
| 12 months | n=55 | 0.04 (0.80) | 0.00 (0.98) | −0.04 (0.76) | |
|
| |||||
| Dorsal muscles 4/5 | Baseline | n=65 | 0.11 (0.39) | 0.11 (0.38) | 0.25 (0.05) |
| 6 months | n=41 | 0.24 (0.14) | 0.35 (0.03) | 0.12 (0.46) | |
| 12 months | n=36 | 0.06 (0.73) | 0.07 (0.70) | −0.14 (0.43) | |
|
| |||||
| Psoas muscles 4/5 | Baseline | n=66 | 0.00 (1.00) | 0.01 (0.92) | −0.04 (0.73) |
| 6 months | n=43 | 0.02 (0.88) | −0.01 (0.94) | 0.16 (0.32) | |
| 12 months | n=40 | 0.04 (0.84) | 0.07 (0.66) | 0.02 (0.91) | |
We observed only one nominally significant inverse correlation with muscle area and valine at baseline (r=−0.26, p=0.04) and with 2M3HA at 6 months follow-up (r=0.35, p=0.03). Based on the number of hypotheses tested, we would have expected at least 2 significant correlations at p<0.05 by chance. The correlations presented in Table 3 and Table 4 were similar if we adjusted for cancer stage in addition (data not shown).
In sensitivity analyses excluding patients diagnosed with stage IV cancer led to similar results (data not shown). To visualize the independence of the biomarkers with parameters of energy balance at baseline, we used scatter plots for isoleucine with BMI, METs, and muscle area (shown in Figure 1).
Figure 1.
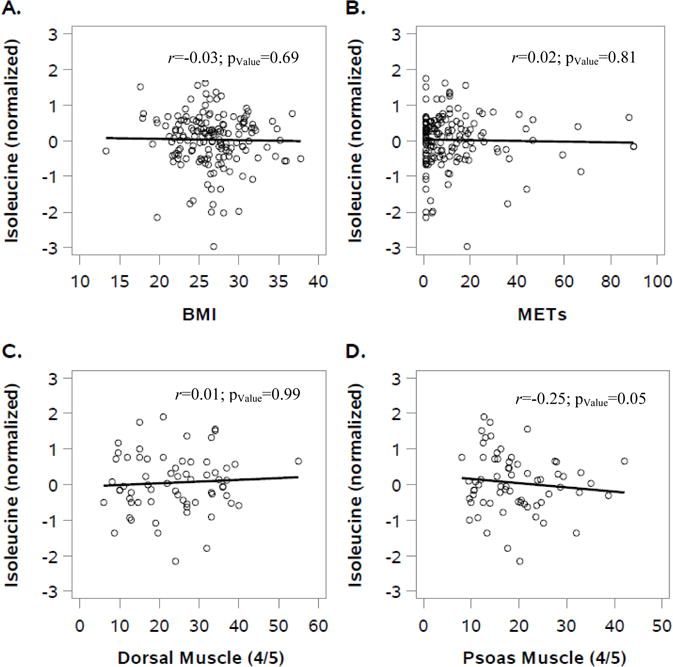
Scatter plot of the correlation of isoleucine with parameters of energy balance at baseline: BMI (A), METs (B) dorsal muscle area (L4/L5) (C) and psoas muscle area (L4/L5) (D) with isoleucine at baseline. This figure illustrates that there are no meaningful correlations between branched-chain amino acids (e.g., isoleucine) and markers of energy balance in colorectal cancer patients.
The results of the overall survival analyses are presented in Figure 2. We conducted separated analyses for (i) stage I, II and III and (ii) stage IV; adjusted for age, sex and analytical batch. Valine and isoleucine were not associated with risk of death in patients diagnosed with stage I through III CRC (valine: HRlog2=1.62, 95% CI: 0.72-3.61; isoleucine: HRlog2=1.28, 95% CI: 0.56-2.90). There was no association between valine and isoleucine in patients diagnosed with stage IV tumors (valine: HRlog2=1.04, 95% CI: 0.57-1.90; isoleucine: HRlog2=1.09, 95% CI: 0.56-2.12). A significant inverse association was observed for 2EA and 2M3H with overall survival, independent of cancer stage at diagnosis (e.g., 2EA: stage I-III: HRlog2=0.42, 95% CI: 0.19-0.94; stage IV: HRlog2=0.51, 95% CI: 0.30-0.88). In analysis adjusting for residual confounding, we observed comparable HR compared to those adjusted for age, sex and batch only (e.g., 2EA:stage I - III: not adjusted for residual confounding: HRlog2=0.42, 95% CI: 0.19-0.94; adjusted for residual confounding: HRlog2=0.12, 95% CI: 0.02-0.89; Supplementary Table 2). We further have used data on BCAA and catabolite concentrations to develop a summary risk score. Patients with a summary score between 8-12 had a 4-fold increase in risk of death compared to patient with a summary score between 1-4 (Summary Score 8-12: HRlog2=3.96, 95% CI: 1.11-14.0; Table 5).
Figure 2.
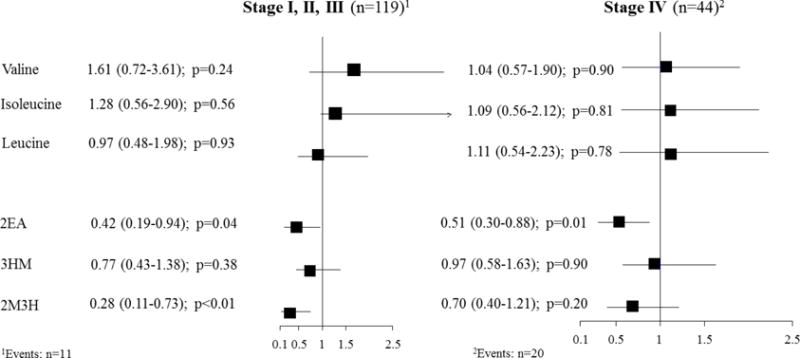
Forest plot of the associations of urinary measurements of valine, isoleucine, leucine, 2EA, 3HM, 2M3H with overall 2 year survival in colorectal cancer patients, stratified by stage at diagnosis.
Table 5.
Summary risk score for associations of metabolite concentrations with overall survival in colorectal cancer patients.*
| Summary score | HR | 95% CI | pValue |
|---|---|---|---|
| 1–4 | Ref | ||
| 5–7 | 2.19 | 0.63–7.54 | 0.22 |
| 8–12 | 3.96 | 1.11–14.07 | 0.03 |
adjusted for age, sex, batch and stage
Discussion
In the present study urinary concentrations of BCAAs and their catabolites were not correlated with parameters of energy balance in colorectal cancer patients at different time points after diagnosis (e.g., surgery, 6 and 12 months after surgery). Survival analyses identified BCAAs and their catabolites as potential biomarkers for colorectal cancer survival.
The absence of correlations between BCAAs and markers of energy balance is in contrast to numerous studies in healthy individuals.(Moore et al. 2014; Newgard et al. 2009; Felig et al. 1969; Q. Xiao et al. 2016; Lustgarten et al. 2014) Serum-based concentrations of BCAAs have been consistently linked to (i) BMI in healthy individuals, (Moore et al. 2014; Newgard et al. 2009; Felig et al. 1969) (ii) physical activity in a cohort of healthy Chinese adults (Q. Xiao et al. 2016) or (iii) thigh muscle cross-sectional area in functionally limited, but otherwise healthy, older adults. (Lustgarten et al. 2014) To the best of our knowledge, there is no prior study that has investigated correlations of urinary BCAAs with parameters of energy balance in colorectal cancer patients. The role of BCAAs in cancer metabolisms are just starting to be explored. Thus, there may also be alternative explanations for the observed lack of correlations of BCAAs with parameters of energy balance in cancer patients. BCAAs have been consistently linked to cachexia – the involuntarily loss of muscle area. (Porporato 2016) Prior studies have shown decreased BCAA concentrations in patients diagnosed with cachexia. (Norton et al. 1985; Beck and Tisdale 1989; Anthony et al. 2000) This is in agreement with the present data, showing that elevated concentrations of BCAA in patients diagnosed with stage IV cancer were not related to overall survival. Another reason may be a potential difference between BCAA concentrations measured in serum/plasma compared to concentrations measured in urine in cancer patients. To date, there is limited data on the comparison of amino acid profiles in serum and urine of prostate cancer patients (n=49) and controls (n=40) showing reasonable correlations between BCAAs in urine and serum with comparable predictive accuracies. (Derezinski et al. 2017) In addition, the authors observed comparable sensitivity and specificity to distinguish between cases and controls for both types of biospecimens. (Derezinski et al. 2017)
A plausible explanation for the absence of correlations in our study may be that changes in BCAAs in cancer patients reflect truly tumor metabolism, (Ananieva 2015; Commisso et al. 2013) rather than muscle area, BMI or physical activity. There is evidence regarding BCAA-induced apoptosis in cancer cells (Terakura et al. 2012; Shimizu et al. 2014) and inhibition of angiogenesis. (Murata and Moriyama 2007; Cha et al. 2013) Using a summary risk score we observed that patients in the top tertile had a significant 4-fold increase of risk of death compared to patients in the bottom tertile. The numbers for this analyses were not stratified by stage due to the limited sample size. However, we adjusted for stage in these analyses. While we did not observe an increased risk evaluating metabolites separately, the score identified patients at increased risk of death.
In vitro and in vivo studies indicate that BCAAs may impact cancer development and progression (Baracos and Mackenzie 2006; O’Connell 2013; Nakano et al. 2013) possibly through the mTOR signaling pathway.(Nakano et al. 2013; Anthony et al. 2000) The mTOR pathway is a promising therapeutic target for various cancer types, (Leelawat et al. 2007; Alvarez et al. 2007) including CRC (Weijenberg et al. 2013) as it controls protein translation, cell growth, proliferation, and autophagy. (Ananieva 2015) There is no direct evidence for a link between BCAAs and the mTOR pathway in colorectal cancer. Recent in vitro data, however, revealed that increased BCAA metabolism drives cancer progression in myeloid leukaemia (Hattori et al. 2017) and breast cancer. (Zhang and Han 2017; Thewes et al. 2017) A potential underlying mechanism may be the elevated expression of branched-chain amino acid transaminase 1 (BCAT1) with subsequent activation of mTOR signaling. (Zhang and Han 2017)
In survival analyses we observed differences in the associations between BCAAs and their catabolites with overall survival, with BCAAs being related to increased risk of overall death while catabolites such as 2-ethylhydracrylic acid (2EA) have been associated with a significant reduction in risk of death. Increased urinary concentrations of 2EA are an indicator for deficiencies in enzymes of the (S) pathway of isoleucine metabolism,(Ryan 2015) such as BCAT1. BCAT1 has just recently been shown to enhance intracellular production of BCAAs and has been linked to progression of myeloid leukemia (Hattori et al. 2017) and growth of breast cancer cells in vivo. (Zhang and Han 2017)
This study has strengths as well as limitations. This is the first study to date comprehensively investigating correlations of BCAAs with parameters of energy balance in cancer patients using GC-MS and 1H-NMR. Prior studies predominantly investigated BCAAs using serum- or plasma-based samples, instead of urine samples. A particular strength of this study is that urinary biomarkers can be assessed using a non-invasive approach for continuous disease monitoring. (Altobelli et al. 2016)
We used state-of the-art metabolomics for the measurement of urinary BCAAs. Isoleucine and leucine were measured by 1H-NMR, a quantitative method that does not require extra steps for sample preparation and determines very accurate measurement of compounds. Limited sensitivity and missing chromatographic separation of metabolites may complicate compound identification. However, prior research has shown the quality of 1H-NMR to measure and identify isoleucine and leucine in urine. (Bouatra et al. 2013) Valine and the catabolites were measured by GC-MS. This is an excellent approach that offers combined sensitivity and selectivity platforms for metabolomics research. However, in contrast to 1H-NMR where nearly all detectable peaks are identifiable, metabolite coverage by GC-MS tends to be relatively incomplete. However, for the metabolites we have strong confidence levels for compound identification (level 1 and level 2, see Table 1).
We tested a comprehensive set of parameters of energy balance (e.g., BMI, physical activity, muscle area) from a well-described prospective cohort of CRC patients. Determination of skeletal muscle area by CT is a reliable and noninvasive method to provide information on muscle area and composition. (Goodpaster et al. 2000) However, muscle area was only assessed at one single time point as part of patient’s clinical care. The sample size was reasonable to investigate correlations at baseline and 6 months follow-up, it was limited for analyses at 12 months follow-up. We had a limited number of events available (n=31) to investigate overall survival.
Conclusions
We observed no significant association between urinary BCAAs and their catabolites with physical activity, BMI and skeletal muscle area at baseline or at follow-up after 6 and 12 months in a well-described cohort study of prospectively followed CRC patients.
Elevated concentrations of valine and isoleucine were associated with a non-significant increase in risk of death in stage I-III patients with, while catabolites of BCAA metabolisms such as 2EA significantly reduced overall mortality, independent of cancer stage at diagnosis. These results imply that BCCAs measured in urine are not functioning as a biomarker for parameters of energy balance and should be investigated further as independent prognostic markers for Colorectal Cancer survival. Most important, urine is a biospecimen that is collected non-invasively, which makes it a prime candidate for the development of clinically useful biomarkers.
Supplementary Material
Acknowledgments
The authors thank all ColoCare study participants, the entire ColoCare study team who assisted in recruitment for the PA assessment.
The ColoCare study protocols, questionnaires, and procedures were developed in collaboration with the ColoCare Fred Hutchinson Cancer Research Center investigators. We thank our collaborators on the ColoCare study, particularly Hermann Brenner, Jenny Chang-Claude, and Michael Hoffmeister. We are grateful to all the study staff who have made this study possible, especially Torsten Kölsch, Susanne Jakob, Clare Abbenhardt, Stefanie Zschäbitz, Werner Diehl, Rifraz Farook, Anett Brendel, Marita Wenzel, Judith Kammer, Stephanie Skender, Verena Widmer, Manja Ghajar Rahimi, and Renate Skatula.
We thank the National Center for Tumor Diseases Tissue and Liquid Biobank for sample storage and handling.
Funding
This study was funded by the Lackas Foundation, the Division of Preventive Oncology (Dr. Ulrich) and the German Consortium of Translational Cancer Research (DKTK).
Cornelia M. Ulrich, Jürgen T. Böhm and Jennifer Ose received funding from the Huntsman Cancer Foundation.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number U01CA206110. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of Abbreviations
- 2EA
2-Ethylhydracrylic Acid
- 3HM
(2Z)-3-Methylglucaconic Acid
- 2M3H
2-Methyl-3-Hydroxybutyrate
- BCAAs
Branched-Chain Amino Acids
- BCAT1
Branched-Chain Amino Acid Transaminase 1
- BMI
Body Mass Index
- CRC
Colorectal Cancer
- CT
Computed Tomography
- GC-MS
Gas-Chromatography-Mass Spectrometry
- HMDB
Human Metabolome Database
- 1H-NMR
Protonnuclear-Magnetic Resonance Spectroscopy
- HU
Huntsfield Units
- MET
Metabolic Equivalent
- mTOR
mammalian Target of Rapamycin
- PA
Physical Activity
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Compliance with ethical requirements
The ColoCare study has been approved by the ethics committee of the medical faculty at the University of Heidelberg and study participants provided their written informed consent.
References
- Altobelli E, Angeletti PM, Latella G. Role of Urinary Biomarkers in the Diagnosis of Adenoma and Colorectal Cancer: A Systematic Review and Meta-Analysis. J Cancer. 2016;7(14):1984–2004. doi: 10.7150/jca.16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M, Roman E, Santos ES, Raez LE. New targets for non-small-cell lung cancer therapy. Expert Rev Anticancer Ther. 2007;7(10):1423–1437. doi: 10.1586/14737140.7.10.1423. [DOI] [PubMed] [Google Scholar]
- Ananieva E. Targeting amino acid metabolism in cancer growth and anti-tumor immune response. World J Biol Chem. 2015;6(4):281–289. doi: 10.4331/wjbc.v6.i4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130(10):2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Baracos VE, Mackenzie ML. Investigations of branched-chain amino acids and their metabolites in animal models of cancer. J Nutr. 2006;136(1 Suppl):237s–242s. doi: 10.1093/jn/136.1.237S. [DOI] [PubMed] [Google Scholar]
- Beck SA, Tisdale MJ. Nitrogen excretion in cancer cachexia and its modification by a high fat diet in mice. Cancer Res. 1989;49(14):3800–3804. [PubMed] [Google Scholar]
- Bohm J, Pianka F, Stuttgen N, Rho J, Gigic B, Zhang Y, et al. Discovery of novel plasma proteins as biomarkers for the development of incisional hernias after midline incision in patients with colorectal cancer: The ColoCare study. Surgery. 2017;161(3):808–817. doi: 10.1016/j.surg.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, et al. The human urine metabolome. PLoS One. 2013;8(9):e73076. doi: 10.1371/journal.pone.0073076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhathoki S, Iwasaki M, Yamaji T, Yamamoto H, Kato Y, Tsugane S. Association of plasma concentrations of branched-chain amino acids with risk of colorectal adenoma in a large Japanese population. Ann Oncol. 2017;28(4):818–823. doi: 10.1093/annonc/mdw680. [DOI] [PubMed] [Google Scholar]
- Cha JH, Bae SH, Kim HL, Park NR, Choi ES, Jung ES, et al. Branched-chain amino acids ameliorate fibrosis and suppress tumor growth in a rat model of hepatocellular carcinoma with liver cirrhosis. PLoS One. 2013;8(11):e77899. doi: 10.1371/journal.pone.0077899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Fan J, Yan LS, Guo HQ, Xiong JJ, Ren Y, et al. Urine Metabolite Profiling of Human Colorectal Cancer by Capillary Electrophoresis Mass Spectrometry Based on MRB. Gastroenterol Res Pract. 2012;2012:125890. doi: 10.1155/2012/125890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Xie G, Chen T, Qiu Y, Zou X, Zheng M, et al. Distinct urinary metabolic profile of human colorectal cancer. J Proteome Res. 2012;11(2):1354–1363. doi: 10.1021/pr201001a. [DOI] [PubMed] [Google Scholar]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497(7451):633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18(1):54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derezinski P, Klupczynska A, Sawicki W, Palka JA, Kokot ZJ. Amino Acid Profiles of Serum and Urine in Search for Prostate Cancer Biomarkers: a Pilot Study. Int J Med Sci. 2017;14(1):1–12. doi: 10.7150/ijms.15783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281(15):811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- Gao P, Zhou C, Zhao L, Zhang G, Zhang Y. Tissue amino acid profile could be used to differentiate advanced adenoma from colorectal cancer. J Pharm Biomed Anal. 2016;118:349–355. doi: 10.1016/j.jpba.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- Gould DW, Lahart I, Carmichael AR, Koutedakis Y, Metsios GS. Cancer cachexia prevention via physical exercise: molecular mechanisms. J Cachexia Sarcopenia Muscle. 2013;4(2):111–124. doi: 10.1007/s13539-012-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardikar S, Newcomb PA, Campbell PT, Win AK, Lindor NM, Buchanan DD, et al. Prediagnostic Physical Activity and Colorectal Cancer Survival: Overall and Stratified by Tumor Characteristics. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1130–1137. doi: 10.1158/1055-9965.EPI-15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A, Tsunoda M, Konuma T, Kobayashi M, Nagy T, Glushka J, et al. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature. 2017;545(7655):500–504. doi: 10.1038/nature22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka K, Ohkawa K, Tatsumi T, Katayama K, Inoue A, Imai Y, et al. Impact of branched-chain amino acid supplementation on survival in patients with advanced hepatocellular carcinoma treated with sorafenib: A multicenter retrospective cohort study. Hepatol Res. 2016;46(10):1002–1010. doi: 10.1111/hepr.12640. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kubota T, Horigome R, Kimura N, Honda H, Iwanaga A, et al. Branched-chain amino acids to tyrosine ratio (BTR) predicts intrahepatic distant recurrence and survival for early hepatocellular carcinoma. Hepatogastroenterology. 2013;60(128):2055–2059. doi: 10.5754/hge13488. [DOI] [PubMed] [Google Scholar]
- Ishizaki S, Nishiyama M, Hagiwara A. Long-term Branched Chain Amino Acid Supplementation Ameliorates Diethylnitrosamine-induced Liver Glutathione S-transferase-p Positivity in Zucker Fatty Rats. J Clin Exp Hepatol. 2013;3(3):192–197. doi: 10.1016/j.jceh.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocarnik JM, Chan AT, Slattery ML, Potter JD, Meyerhardt J, Phipps A, et al. Relationship of prediagnostic body mass index with survival after colorectal cancer: Stage-specific associations. Int J Cancer. 2016;139(5):1065–1072. doi: 10.1002/ijc.30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13(6):472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Leelawat K, Leelawat S, Narong S, Hongeng S. Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2007;13(10):1561–1568. doi: 10.3748/wjg.v13.i10.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld D, Habermann N, Toth R, Owen R, Frei E, Böhm J, et al. Changes in urinary metabolic profiles of colorectal cancer patients enrolled in a prospective cohort study (ColoCare) Metabolomics. 2015;11(4):998–1012. doi: 10.1007/s11306-014-0758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld DB, Grapov D, Fahrmann JF, Salou M, Scherer D, Toth R, et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: the ColoCare study. Am J Clin Nutr. 2015;102(2):433–443. doi: 10.3945/ajcn.114.103804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KA, Lashinger LM, Rasmussen AJ, Hursting SD. Leucine supplementation differentially enhances pancreatic cancer growth in lean and overweight mice. Cancer Metab. 2014;2(1):6. doi: 10.1186/2049-3002-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustgarten MS, Price LL, Chale A, Phillips EM, Fielding RA. Branched chain amino acids are associated with muscle mass in functionally limited older adults. J Gerontol A Biol Sci Med Sci. 2014;69(6):717–724. doi: 10.1093/gerona/glt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malietzis G, Aziz O, Bagnall NM, Johns N, Fearon KC, Jenkins JT. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol. 2015;41(2):186–196. doi: 10.1016/j.ejso.2014.10.056. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009a;169(22):2102–2108. doi: 10.1001/archinternmed.2009.412. [pii] 1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt JA, Ogino S, Kirkner GJ, Chan AT, Wolpin B, Ng K, et al. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009b;15(18):5931–5936. doi: 10.1158/1078-0432.CCR-09-0496. doi: 1078-0432.CCR-09-0496 [pii] 10.1158/1078-0432.CCR-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- Moore SC, Matthews CE, Sampson JN, Stolzenberg-Solomon RZ, Zheng W, Cai Q, et al. Human metabolic correlates of body mass index. Metabolomics. 2014;10(2):259–269. doi: 10.1007/s11306-013-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Moriyama M. Isoleucine, an essential amino acid, prevents liver metastases of colon cancer by antiangiogenesis. Cancer Res. 2007;67(7):3263–3268. doi: 10.1158/0008-5472.CAN-06-3739. [DOI] [PubMed] [Google Scholar]
- Nakano M, Nakashima A, Nagano T, Ishikawa S, Kikkawa U, Kamada S. Branched-chain amino acids enhance premature senescence through mammalian target of rapamycin complex I-mediated upregulation of p21 protein. PLoS One. 2013;8(11):e80411. doi: 10.1371/journal.pone.0080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattenmueller J, Hoegenauer H, Boehm J, Scherer D, Paskow M, Gigic B, et al. CT-based compartmental quantification of adipose tissue versus body metrics in colorectal cancer patients. Eur Radiol. 2016;26(11):4131–4140. doi: 10.1007/s00330-016-4231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JA, Gorschboth CM, Wesley RA, Burt ME, Brennan MF. Fasting plasma amino acid levels in cancer patients. Cancer. 1985;56(5):1181–1186. doi: 10.1002/1097-0142(19850901)56:5<1181::aid-cncr2820560535>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- O’Connell TM. The complex role of branched chain amino acids in diabetes and cancer. Metabolites. 2013;3(4):931–945. doi: 10.3390/metabo3040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. doi: 10.1038/oncsis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Cai G, Su M, Chen T, Zheng X, Xu Y, et al. Serum metabolite profiling of human colorectal cancer using GC-TOFMS and UPLC-QTOFMS. J Proteome Res. 2009;8(10):4844–4850. doi: 10.1021/pr9004162. [DOI] [PubMed] [Google Scholar]
- Ristau J, Staffa J, Schrotz-King P, Gigic B, Makar KW, Hoffmeister M, et al. Suitability of circulating miRNAs as potential prognostic markers in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2632–2637. doi: 10.1158/1055-9965.epi-14-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RO. Metabolic annotation of 2-ethylhydracrylic acid. Clin Chim Acta. 2015;448:91–97. doi: 10.1016/j.cca.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25(7):1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Shirakami Y, Hanai T, Imai K, Suetsugu A, Takai K, et al. Pharmaceutical and nutraceutical approaches for preventing liver carcinogenesis: chemoprevention of hepatocellular carcinoma using acyclic retinoid and branched-chain amino acids. Mol Nutr Food Res. 2014;58(1):124–135. doi: 10.1002/mnfr.201300538. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Skender S, Schrotz-King P, Bohm J, Abbenhardt C, Gigic B, Chang-Claude J, et al. Repeat physical activity measurement by accelerometry among colorectal cancer patients–feasibility and minimal number of days of monitoring. BMC Res Notes. 2015;8:222. doi: 10.1186/s13104-015-1168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama K, Yu L, Nagasue N. Direct effect of branched-chain amino acids on the growth and metabolism of cultured human hepatocellular carcinoma cells. Nutr Cancer. 1998;31(1):62–68. doi: 10.1080/01635589809514679. [DOI] [PubMed] [Google Scholar]
- Tada T, Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, et al. Impact of the branched-chain amino acid to tyrosine ratio and branched-chain amino acid granule therapy in patients with hepatocellular carcinoma: A propensity score analysis. J Gastroenterol Hepatol. 2015;30(9):1412–1419. doi: 10.1111/jgh.12954. [DOI] [PubMed] [Google Scholar]
- Terakura D, Shimizu M, Iwasa J, Baba A, Kochi T, Ohno T, et al. Preventive effects of branched-chain amino acid supplementation on the spontaneous development of hepatic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Carcinogenesis. 2012;33(12):2499–2506. doi: 10.1093/carcin/bgs303. [DOI] [PubMed] [Google Scholar]
- Thewes V, Simon R, Hlevnjak M, Schlotter M, Schroeter P, Schmidt K, et al. The branched-chain amino acid transaminase 1 sustains growth of antiestrogen-resistant and ERalpha-negative breast cancer. Oncogene. 2017 doi: 10.1038/onc.2017.32. [DOI] [PubMed] [Google Scholar]
- Uneyama H, Kobayashi H, Tonouchi N. New Functions and Potential Applications of Amino Acids. Adv Biochem Eng Biotechnol. 2016 doi: 10.1007/10_2016_35. [DOI] [PubMed] [Google Scholar]
- Usher-Smith JA, Walter FM, Emery JD, Win AK, Griffin SJ. Risk Prediction Models for Colorectal Cancer: A Systematic Review. Cancer Prev Res (Phila) 2016;9(1):13–26. doi: 10.1158/1940-6207.capr-15-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Khankari NK, Cai H, Li HL, Yang G, Gao YT, et al. Prediagnosis body mass index and waist-hip circumference ratio in association with colorectal cancer survival. Int J Cancer. 2017;140(2):292–301. doi: 10.1002/ijc.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijenberg MP, Hughes LA, Bours MJ, Simons CC, van Engeland M, van den Brandt PA. The mTOR Pathway and the Role of Energy Balance Throughout Life in Colorectal Cancer Etiology and Prognosis: Unravelling Mechanisms Through a Multidimensional Molecular Epidemiologic Approach. Curr Nutr Rep. 2013;2(1):19–26. doi: 10.1007/s13668-012-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res. 2015;43(W1):W251–257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Hao F, Qin X, Wang Y, Tang H. An optimized buffer system for NMR-based urinary metabonomics with effective pH control, chemical shift consistency and dilution minimization. Analyst. 2009;134(5):916–925. doi: 10.1039/b818802e. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Moore SC, Keadle SK, Xiang YB, Zheng W, Peters TM, et al. Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. Int J Epidemiol. 2016;45(5):1433–1444. doi: 10.1093/ije/dyw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Han J. Branched-chain amino acid transaminase 1 (BCAT1) promotes the growth of breast cancer cells through improving mTOR-mediated mitochondrial biogenesis and function. Biochem Biophys Res Commun. 2017;486(2):224–231. doi: 10.1016/j.bbrc.2017.02.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


