Abstract
Background
Human studies investigating the link between postnatal polyunsaturated fatty acids and preterm brain growth are limited, despite emerging evidence of potential effects on outcomes.
Methods
Sixty preterm neonates <32 weeks gestational age with MRI scanning at near-birth and near-term age were assessed for brain tissue volumes including cortical grey matter, white matter, deep grey matter, cerebellum, brainstem and ventricular cerebrospinal fluid. Red blood cell fatty acid content was evaluated within 1 week of each MRI scan. Neurodevelopmental outcome at 30-36 months corrected age was assessed.
Results
Adjusting for potential confounders, higher near-birth docosahexaenoic acid levels are associated with larger cortical grey matter, deep grey matter, and brainstem volumes, and higher near-term levels with larger deep grey matter, cerebellar, and brainstem volumes at near-term age; lower near-birth linoleic acid levels are correlated with larger white matter volume at near-term age. By 30-36 months corrected age, larger cortical and deep grey matter, cerebellar, and brainstem volumes by term age are associated with improved language scores, and larger cerebellar and brainstem volumes with improved motor scores.
Conclusion
Specific polyunsaturated fatty acid levels have differential and time-dependent associations with brain region growth. Larger brain volumes are associated with improved outcomes at preschool age.
INTRODUCTION
Preterm birth accounts for 11% of all live births worldwide (1). In addition to neurodevelopmental difficulties, preterm birth is associated with reduced total and regional brain volumes, including white and grey matter, cerebellum, hippocampus, caudate and corpus callosum (2,3). Considering increasing survival rates and a consequential increased risk of adverse neurodevelopmental outcomes (4), it is pertinent to determine modifiable risk factors to improve the health and welfare of preterm babies.
Nutrition, including polyunsaturated fatty acids (PUFAs), plays a central role in brain development (5, 6). Most brain PUFAs, are accumulated between the last trimester of gestation and 2 years of life, during the phase of rapid brain growth (7–9). Preterm birth interrupts the transfer of placental PUFAs to the fetus, limiting preterm neonatal PUFA stores, and interfering with brain development (6–8).
MRI studies investigating the effects of nutrition, including PUFAs, on brain volumes of people born preterm are limited and results are variable (5,10–12). Limitations of prior studies include variability in nutritional interventions and lack of postnatal MRI scanning. To our knowledge, no studies have explored the effects of PUFAs on brain volumes of preterm neonates using postnatal MRI scans. Further, of the studies that have utilized near-term MRI scans to examine the relationship between brain volumes and neurodevelopmental outcome in the preterm population, all demonstrate that smaller brain volumes – total and regional – predict worse developmental outcomes during childhood (13–16).
We recently reported that during the postnatal period, higher ω-3 and lower ω-6 fatty acid levels are associated with improved microstructural brain development and improved developmental outcomes at preschool age in preterm infants (17). Here, we will expand upon our previous findings (17) by assessing the association between postnatal PUFA levels and preterm macrostructural brain development – brain tissue volumes acquired using serial postnatal MRI scans – and also investigate the relationship between brain tissue volumes and developmental outcomes at preschool age in infants born preterm. All things considered (13–17), it is hypothesized that during the postnatal period, higher ω-3 and lower ω-6 fatty acid levels will be associated with larger brain tissue volumes, which will predict improved developmental outcomes at 30-36 months corrected gestational age.
METHODS
Study population
Sixty preterm infants (<32 weeks gestational age) born between March 2010 and November 2011 admitted to the NICU at either UCSF Benioff Children’s Hospital at the University of California San Francisco or University of British Columbia affiliated BC Women’s Hospital were studied. Infants were excluded if they had a congenital malformation or syndrome, congenital infection, or were too unstable to transport to the MRI scanner. Clinical history was obtained from patient charts. This study was approved by both institutions’ research ethics boards. Parental consent was obtained for each participating infant. Patient demographics and detailed feeding practices at both study sites were previously described (17).
MRI studies
MRI scans were performed soon after birth as clinically stable and again at near-term age, using 1.5-T MRI scanners (General Electric Sigma, GE Medical Systems, Milwaukee, WI, or Siemens Avanto, Siemens Medical Solutions, Malvern, PA). Conventional MRI sequences were acquired at both study sites (18). Blinded to patient medical history, one neuroradiologist at each study site (AJB, KJP) reviewed and graded all scans for severity of intraventricular haemorrhage (IVH) and white matter injury. The highest severity of injury score from both scans was used to delineate the patient’s severity of injury (17).
Brain tissue volumes
Each subject’s T1-weighted MRI scan was automatically segmented into tissue classes using a spatio-temporal atlas driven expectation maximization algorithm (19). This incorporated a bias estimation step and used a 4D atlas built from a database of 32 manually delineated MRI scans. This approach uses patch-based priors to augment the age specific atlas to better account for abnormal anatomy not captured by the atlas training data. The system has been carefully evaluated using a similar cohort of T1-weighted scans with manually marked tissues as a reference. The Dice Similarity Coefficient was used to quantify the accuracy of the automatic segmentation with an average of 0.883 across tissue classes (19). Tissue classes included: cortical grey matter, white matter, deep grey matter, cerebellum, brainstem and ventricular cerebrospinal fluid (CSF; Figure 1). After labelling all MRI voxels, the volume of each tissue class was calculated for each subject scan.
Figure 1. Brain tissue volumes at near-birth and near-term age.
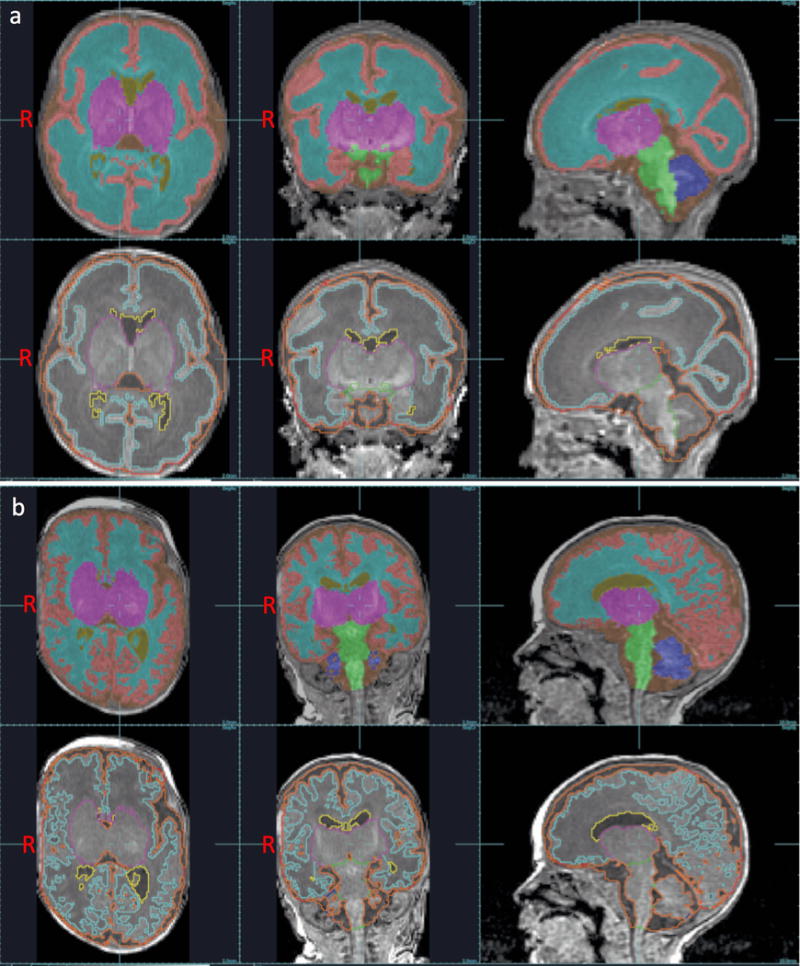
Segmentations of cortical grey matter (peach), white matter (light blue), deep grey matter (fuchsia), cerebellum (dark blue), brainstem (green), and ventricular CSF (yellow) superimposed on 3D T1-weighted MRI images obtained near-birth (a), and near-term (b) age. Images are shown in axial, coronal, and sagittal planes.
Blood samples
For each patient, within 7 days of MRI, a 1 ml blood sample was collected at both near-birth and near-term MRI scans, for a total of 2 blood samples. Red blood cell membrane PUFA levels (percent total fatty acids), including ω-3 fatty acids docosahexaenoic acid (DHA; 22:6ω3) and eicosapentaenoic acid (EPA; 20:5ω3) and ω-6 fatty acids arachidonic acid (ARA; 20:4ω6) and linoleic acid (LA; 18:2ω6), were determined by gas chromatography-flame ionization detector (20).
Neurodevelopmental outcome
At 30-36 months corrected age, infants were assessed with the Bayley Scales of Infant and Toddler Development, 3rd Edition (Bayley-3). Blinded to the infant’s neonatal course, a developmental psychologist or physiotherapist determined the infant’s language, motor, and cognitive composite scores according to the infant’s corrected age.
Statistical analysis
Statistical analyses were performed using R version 3.1.1 (The R Foundation for Statistical Computing 2014, Vienna, Austria). A cube-root transformation for brain tissue volumes was used in the analyses. Mixed regression models were used to assess the relationship between fatty acid levels from the near-birth blood sample and brain tissue volumes from both scans to account for repeated measures. Linear regression analysis was used to assess the relationship between fatty acid levels and brain tissue volumes acquired at near-term age. Results for the mixed regression analyses and the linear regression analyses were first only adjusted for age at MRI scan (known clinical risk factor for brain injury), and then further adjusted for measures of brain injury, including IVH severity, white matter injury severity, and cerebellar haemorrhage (for cerebellar volumes only), and for known clinical risk factors for brain injury, including duration of intubation, sepsis, and patent ductus arteriosus. All analyses were also adjusted for study site to account for site-specific variations. Linear regression analysis was used to assess the relationship between near-term brain tissue volumes and Bayley-3 composite scores at 30-36 months corrected age. Results were adjusted for age at near-term MRI scan. The above analyses were then repeated using the same study cohort, but excluding subjects with IVH. Associations were considered significant at P ≤ 0.050.
RESULTS
Study cohort
The study cohort comprises 60 preterm neonates <32 weeks gestational age enrolled at two study sites. All subjects had an MRI acquired as soon after birth as clinically stable (mean 31.54±2.26 week GA) and associated blood sampling within 7 days. Of 60, 44 (73%) subjects also had an MRI at near-term age (mean 37.71±2.98 week GA), along with associated blood sampling (Table 1). Details regarding patient demographics and fatty acid composition of blood samples were previously reported (17).
Table 1.
Patient cohort characteristics at near-birth and near-term age.
| Near-Birth Age | Near-Term Age | |
|---|---|---|
|
| ||
| PUFAsa | ||
| DHA | 4.358 ± 0.963 % | 4.420 ± 1.268 % |
| EPA | 0.417 ± 0.495 % | 0.398 ± 0.181 % |
| ARA | 16.547 ± 1.917 % | 15.807 ± 2.925 % |
| LA | 9.610 ± 1.981 % | 10.461 ± 1.588 % |
|
| ||
| Brain Tissue Classesb | ||
| Cortical grey matter | 52.019 ± 15.911 cm3 | 107.579 ± 40.180 cm3 |
| White matter | 100.192 ± 18.666 cm3 | 150.982 ± 20.097 cm3 |
| Deep grey matter | 11.425 ± 2.461 cm3 | 17.625 ± 3.836 cm3 |
| Cerebellum | 7.592 ± 2.281 cm3 | 16.711 ± 5.283 cm3 |
| Brainstem | 3.971 ± 0.684 cm3 | 5.460 ± 0.813 cm3 |
Abbreviations: ARA = arachidonic acid; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; LA = linoleic acid; PUFA = polyunsaturated fatty acid
Fatty acid levels (percent total fatty acid) expressed as mean ± SD.
Brain tissue volumes expressed as mean ± SD.
Means represent subjects from both study sites.
Near-birth PUFA levels and brain tissue volumes
Of 60, 59 (98%) near-birth MRI scans had adequate scan quality to undergo automatic segmentation of tissue classes. Of 44, 39 (89%) near-term MRI scans were of adequate quality to undergo automatic segmentation. Near-birth PUFA levels were correlated with brain tissue volumes from both near-birth and near-term MRI scans. Adjusting for age at each MRI scan, mixed effects analysis revealed positive associations for DHA with cortical grey matter (β = 3.285 cm3/1% PUFA, P = 0.008), deep grey matter (β = 0.729 cm3/1% PUFA, P = 0.003), cerebellar (β = 0.650 cm3/1% PUFA, P = 0.002), and brainstem (β = 0.189 cm3/1% PUFA, P = 0.005) volumes, and for ARA with deep grey matter (β = 0.263 cm3/1% PUFA, P = 0.040) and cerebellar (β = 0.226 cm3/1% PUFA, P = 0.045) volumes, and negative associations for EPA with cortical grey matter (β = −16.704 cm3/1% PUFA, P = 0.034), white matter (β = −25.922 cm3/1% PUFA, P = 0.021), deep grey matter (β = −3.874 cm3/1% PUFA, P = 0.012), and cerebellar (β = −3.283 cm3/1% PUFA, P = 0.018) volumes, and for LA with cortical grey matter (β = −1.404 cm3/1% PUFA, P = 0.020), white matter (β = −2.488 cm3/1% PUFA, P = 0.003), deep grey matter (β = −0.384 cm3/1% PUFA, P = 0.001), cerebellar (β = −0.246 cm3/1% PUFA, P = 0.022), and brainstem (β = −0.074 cm3/1% PUFA, P = 0.029) volumes (Table 2, Model 1). After further adjusting for IVH severity, white matter injury severity, duration of intubation, sepsis, patent ductus arteriosus, and cerebellar haemorrhage (for cerebellar volumes only), only DHA remained positively associated with cortical grey matter (β = 3.366 cm3/1% PUFA, P = 0.022), deep grey matter (β = 0.721 cm3/1% PUFA, P = 0.007), and brainstem (β = 0.168 cm3/1% PUFA, P = 0.042) volumes, and only LA remained negatively associated with white matter (β = −1.964 cm3/1% PUFA, P = 0.050) volume (Table 2, Model 2). One percent increase in DHA was associated with a 3.366 cm3 increase in cortical grey matter volume, 0.721 cm3 increase in deep grey matter volume, and 0.168 cm3 increase in brainstem volume at near-term age. Further, a 1% increase in LA was associated with a 1.964 cm3 decrease in white matter volume at near-term age. No associations were found for any studied PUFA levels and ventricular CSF volumes (P > 0.100).
Table 2.
Mixed effects analyses comparing red blood cell fatty acid levels (percent total fatty acid) from first blood sample and brain tissue volumes (cm3) from two MRI scans acquired as soon after birth as clinically stable and at near-term age.
| Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Δc volume (cm3) | SE | P-value | Δc volume (cm3) | SE | P-value | |
|
| ||||||
| DHA | ||||||
| Cortical grey matter | 3.285 | 1.230 | 0.008 | 3.366 | 1.468 | 0.022 |
| White matter | 1.750 | 1.874 | 0.350 | 0.410 | 2.188 | 0.851 |
| Deep grey matter | 0.729 | 0.243 | 0.003 | 0.721 | 0.267 | 0.007 |
| Cerebellum | 0.650 | 0.211 | 0.002 | 0.407 | 0.253 | 0.108 |
| Brainstem | 0.189 | 0.068 | 0.005 | 0.168 | 0.083 | 0.042 |
| Ventricular CSF | −0.336 | 0.223 | 0.132 | −0.255 | 0.273 | 0.350 |
|
| ||||||
| EPA | ||||||
| Cortical grey matter | −16.704 | 7.878 | 0.034 | 3.683 | 10.057 | 0.714 |
| White matter | −25.922 | 11.265 | 0.021 | −6.735 | 13.843 | 0.627 |
| Deep grey matter | −3.874 | 1.548 | 0.012 | 0.630 | 1.882 | 0.738 |
| Cerebellum | −3.283 | 1.386 | 0.018 | 0.304 | 1.701 | 0.858 |
| Brainstem | −0.606 | 0.447 | 0.176 | 0.395 | 0.556 | 0.477 |
| Ventricular CSF | −0.415 | 1.426 | 0.771 | −1.354 | 1.739 | 0.436 |
|
| ||||||
| ARA | ||||||
| Cortical grey matter | 0.908 | 0.645 | 0.159 | −0.024 | 0.770 | 0.975 |
| White matter | 1.392 | 0.928 | 0.134 | −0.137 | 1.074 | 0.898 |
| Deep grey matter | 0.263 | 0.128 | 0.040 | 0.002 | 0.144 | 0.991 |
| Cerebellum | 0.226 | 0.113 | 0.045 | 0.004 | 0.130 | 0.974 |
| Brainstem | 0.036 | 0.036 | 0.314 | −0.031 | 0.043 | 0.470 |
| Ventricular CSF | −0.112 | 0.117 | 0.340 | −0.026 | 0.146 | 0.856 |
|
| ||||||
| LA | ||||||
| Cortical grey matter | −1.404 | 0.603 | 0.020 | −0.845 | 0.750 | 0.260 |
| White matter | −2.488 | 0.850 | 0.003 | −1.964 | 1.002 | 0.050 |
| Deep grey matter | −0.384 | 0.117 | 0.001 | −0.220 | 0.138 | 0.111 |
| Cerebellum | −0.246 | 0.107 | 0.022 | −0.078 | 0.128 | 0.544 |
| Brainstem | −0.074 | 0.034 | 0.029 | −0.043 | 0.042 | 0.306 |
| Ventricular CSF | −0.107 | 0.115 | 0.351 | −0.191 | 0.132 | 0.146 |
Abbreviations: ARA = arachidonic acid; CSF = cerebrospinal fluid; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; LA = linoleic acid
Model 1: Adjusted for age at each MRI scan.
Model 2: Adjusted for potential confounders, including age at each MRI scan, IVH severity, white matter injury severity, duration of intubation, sepsis, patent ductus arteriosus, and cerebellar haemorrhage (cerebellar volumes only).
Change values represent change in volume measures per 1% increase in red blood cell fatty acid level.
Considering previously reported results in the same cohort of an association between IVH and DHA and the potential impact of IVH on brain growth (17), subanalysis was performed excluding infants with IVH. Adjusting for age at each MRI scan, white matter injury severity, duration of intubation, sepsis, patent ductus arteriosus, and cerebellar haemorrhage (for cerebellar volumes only), mixed effects analysis revealed positive associations for only DHA with cortical grey matter (β = 3.598 cm3/1% PUFA, P = 0.028), and deep grey matter (β = 0.975 cm3/1% PUFA, P = 0.002) volumes. After excluding infants with IVH, DHA was no longer associated with brainstem (β = 0.155 cm3/1% PUFA, P = 0.131) volume, and LA was no longer associated with white matter (β = −0.143 cm3/1% PUFA, P = 0.916) volume. As in prior analyses, no associations were found for EPA (P > 0.200) or ARA (P > 0.400) with any studied brain tissue volumes.
Near-term PUFA levels and near-term brain tissue volumes
Near-term PUFA levels were correlated with brain tissue volumes from near-term MRI scans. Adjusting for age at near-term scan, linear regression analysis revealed positive associations for DHA with deep grey matter (β = 0.689 cm3/1% PUFA, P = 0.046), cerebellar (β = 0.908 cm3/1% PUFA, P = 0.021), and brainstem (β = 0.226 cm3/1% PUFA, P = 0.013) volumes, and for ARA with deep grey matter (β = 0.283 cm3/1% PUFA, P = 0.039) volume (Table 3, Model 1). After further adjusting for IVH severity, white matter injury severity, duration of intubation, sepsis, patent ductus arteriosus, and cerebellar haemorrhage (for cerebellar volumes only), only DHA remained positively associated with deep grey matter (β = 0.822 cm3/1% PUFA, P = 0.032), cerebellar (β = 0.964 cm3/1% PUFA, P = 0.028), and brainstem (β = 0.245 cm3/1% PUFA, P = 0.021) volumes (Table 3, Model 2). No associations were found for EPA (P > 0.100) or LA (P > 0.100) with any studied brain tissue volumes, or for any studied PUFA levels and white matter (P > 0.100) or ventricular CSF (P > 0.100) volumes. One percent increase in DHA was associated with a 0.822 cm3 larger deep grey matter volume, 0.964 cm3 larger cerebellar volume, and 0.245 cm3 larger brainstem volume at near-term age.
Table 3.
Linear regression analyses comparing red blood cell fatty acid levels (percent total fatty acid) from second blood sample and brain tissue volumes (cm3) from MRI scan acquired at near-term age.
| Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Δc volume (cm3) | SE | P-value | Δc volume (cm3) | SE | P-value | |
|
| ||||||
| DHA | ||||||
| Cortical grey matter | 3.386 | 2.117 | 0.119 | 4.472 | 2.542 | 0.092 |
| White matter | 2.746 | 2.882 | 0.347 | 2.322 | 3.416 | 0.504 |
| Deep grey matter | 0.689 | 0.334 | 0.046 | 0.822 | 0.359 | 0.032 |
| Cerebellum | 0.908 | 0.375 | 0.021 | 0.964 | 0.410 | 0.028 |
| Brainstem | 0.226 | 0.086 | 0.013 | 0.245 | 0.098 | 0.021 |
| Ventricular CSF | −0.356 | 0.317 | 0.269 | −0.414 | 0.370 | 0.276 |
|
| ||||||
| EPA | ||||||
| Cortical grey matter | 14.188 | 16.137 | 0.385 | 29.217 | 19.487 | 0.148 |
| White matter | −21.236 | 21.397 | 0.328 | −18.071 | 26.558 | 0.504 |
| Deep grey matter | 1.401 | 2.617 | 0.596 | 3.363 | 2.924 | 0.262 |
| Cerebellum | 0.747 | 2.880 | 0.797 | 1.470 | 3.475 | 0.676 |
| Brainstem | 0.905 | 0.682 | 0.194 | 1.315 | 0.817 | 0.122 |
| Ventricular CSF | −2.197 | 2.383 | 0.363 | −3.918 | 2.763 | 0.171 |
|
| ||||||
| ARA | ||||||
| Cortical grey matter | 1.288 | 0.844 | 0.136 | 1.043 | 0.926 | 0.272 |
| White matter | 1.297 | 1.121 | 0.255 | 2.036 | 1.198 | 0.104 |
| Deep grey matter | 0.283 | 0.132 | 0.039 | 0.248 | 0.130 | 0.069 |
| Cerebellum | 0.223 | 0.154 | 0.158 | 0.288 | 0.158 | 0.082 |
| Brainstem | 0.050 | 0.036 | 0.168 | 0.054 | 0.040 | 0.187 |
| Ventricular CSF | −0.015 | 0.129 | 0.908 | 0.027 | 0.134 | 0.840 |
|
| ||||||
| LA | ||||||
| Cortical grey matter | 0.466 | 1.461 | 0.752 | 1.018 | 1.719 | 0.560 |
| White matter | −1.527 | 1.863 | 0.418 | 0.377 | 2.192 | 0.865 |
| Deep grey matter | −0.277 | 0.231 | 0.238 | −0.117 | 0.254 | 0.649 |
| Cerebellum | −0.431 | 0.248 | 0.091 | −0.267 | 0.290 | 0.367 |
| Brainstem | −0.056 | 0.060 | 0.352 | −0.020 | 0.071 | 0.781 |
| Ventricular CSF | −0.134 | 0.219 | 0.543 | −0.156 | 0.244 | 0.530 |
Abbreviations: ARA = arachidonic acid; CSF = cerebrospinal fluid; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; LA = linoleic acid
Model 1: Adjusted for age at near-term MRI scan.
Model 2: Adjusted for potential confounders, including age at near-term MRI scan, IVH severity, white matter injury severity, duration of intubation, sepsis, patent ductus arteriosus, and cerebellar haemorrhage (cerebellar volumes only).
Change values represent change in volume measures per 1% increase in red blood cell fatty acid level.
Excluding infants with IVH and adjusting for measures of brain injury and known clinical risk factors for brain injury did not meaningfully change the positive associations found for DHA with deep grey matter (β = 0.852 cm3/1% PUFA, P = 0.032), cerebellar (β = 0.768 cm3/1% PUFA, P = 0.033), and brainstem (β = 0.257 cm3/1% PUFA, P = 0.007) volumes. However, after excluding infants with IVH, LA became positively associated with cortical grey matter (β = 3.472 cm3/1% PUFA, P = 0.037) volume. As in prior analyses, no associations were found for EPA (P > 0.070) or ARA (P > 0.090) with any studied brain tissue volumes.
Near-term brain tissue volumes and developmental outcome
Of 60 subjects, 45 (75%) underwent developmental assessments at a mean corrected age of 32.8 ± 3.6 months. Means for Bayley-3 composite scores were 101.0 ± 17.8 for language, 98.1 ± 14.3 for motor, and 103.6 ± 14.4 for cognitive. Of the 39 subjects who had near-term MRI scans with adequate quality to undergo automatic segmentation of tissue classes, 31 (79%) underwent developmental assessments at 30-36 months corrected age. Near-term brain tissue volumes were correlated with Bayley-3 composite scores at 30-36 months corrected age. Adjusting for age at near-term scan, linear regression analysis revealed positive associations for cortical grey matter (β = 0.546 pt/1cm3, P < 0.001), deep grey matter (β = 3.379 pt/1cm3, P = 0.004), cerebellar (β = 3.480 pt/1cm3, P = 0.001), and brainstem (β = 10.853 pt/1cm3, P = 0.033) volumes with language scores, and for cerebellar (β = 2.073 pt/1cm3, P = 0.016) and brainstem (β = 8.444 pt/1cm3, P = 0.030) volumes with motor scores at 30-36 months corrected age (Table 4). No associations were found for ventricular CSF volume and Bayley-3 composite score in any domain (P > 0.400), or for any studied brain tissue volume with cognitive scores (P > 0.080) at 30-36 months corrected age. A 1 cm3 larger cortical grey matter volume at near-term age was associated with 0.5 point higher language scores at 30-36 months corrected age. A 1 cm3 larger near-term deep grey matter volume was associated with 3.4 point higher language scores. Further, a 1 cm3 larger cerebellar volume at near-term age was associated with 3.5 point higher language scores and 2.1 point higher motor scores. Lastly, a 1 cm3 larger near-term brainstem volume was associated with 10.9 point higher language scores and 8.4 point higher motor scores.
Table 4.
Linear regression analysis comparing brain tissue volumes (cm3) from MRI scan acquired at near-term age and Bayley-3 language, motor, and cognitive scores at 30-36 months corrected age.
| Changea | SE | P-value | |
|---|---|---|---|
|
| |||
| Cortical Grey Matter | |||
| Language | 0.546 | 0.127 | <0.001 |
| Motor | 0.147 | 0.123 | 0.242 |
| Cognitive | 0.095 | 0.127 | 0.460 |
|
| |||
| White Matter | |||
| Language | 0.141 | 0.166 | 0.401 |
| Motor | 0.247 | 0.122 | 0.052 |
| Cognitive | 0.223 | 0.125 | 0.085 |
|
| |||
| Deep Grey Matter | |||
| Language | 3.379 | 1.090 | 0.004 |
| Motor | 1.685 | 0.917 | 0.077 |
| Cognitive | 1.349 | 0.958 | 0.170 |
|
| |||
| Cerebellum | |||
| Language | 3.480 | 0.964 | 0.001 |
| Motor | 2.073 | 0.805 | 0.016 |
| Cognitive | 0.999 | 0.896 | 0.274 |
|
| |||
| Brainstem | |||
| Language | 10.853 | 4.828 | 0.033 |
| Motor | 8.444 | 3.694 | 0.030 |
| Cognitive | 4.345 | 4.032 | 0.290 |
|
| |||
| Ventricular CSF | |||
| Language | 1.149 | 1.453 | 0.436 |
| Motor | 0.589 | 1.129 | 0.606 |
| Cognitive | 0.547 | 1.148 | 0.638 |
Abbreviations: Bayley-3 = Bayley Scales of Infant Development, 3rd Edition; CSF = cerebrospinal fluid
Change values represent change in Bayley-3 composite score per 1 cm3 increase in brain tissue volume.
Results are adjusted for age at near-term MRI scan
Excluding infants with IVH and adjusting for age at each MRI scan did not meaningfully change the positive associations found for cortical grey matter (β = 0.430 pt/1cm3, P = 0.004), deep grey matter (β = 2.414 pt/1cm3, P = 0.049), and cerebellar (β = 3.053 pt/1cm3, P = 0.011) volumes with language scores. However, brainstem (β = 7.453 pt/1cm3, P = 0.199) volume was no longer associated with language scores, and cerebellar (β = 1.097 pt/1cm3, P = 0.184), and brainstem (β = 3.966 pt/1cm3, P = 0.298) volumes were no longer associated with motor scores. As in prior analyses, no associations were found for any studied brain tissue volume with cognitive scores (P > 0.300) at 30-36 months corrected age.
DISCUSSION
Utilizing serial blood samples, neuroimaging, and developmental assessments in preterm neonates, we demonstrate opposing associations with respect to ω-3 and ω-6 fatty acid levels and their effect on brain tissue volumes during the neonatal period, showing larger neonatal brain tissue volumes associated with improved developmental outcomes at preschool age. Higher near-birth DHA levels are associated with larger cortical and deep grey matter, and brainstem volumes, and higher near-term DHA levels are associated with larger deep grey matter, cerebellar, and brainstem volumes at near-term age. Moreover, we demonstrate an association between higher near-birth LA levels and decreased near-term white matter volumes. Larger cerebellar and brainstem volumes at near-term age predict improved Bayley-3 language and motor scores at 30-36 months corrected age. Further, larger near-term cortical and deep grey matter volumes predict improved Bayley-3 language scores at 30-36 months corrected age.
Considering that DHA accretion relative to brain weight is highest during fetal development and early infancy (21), and its preferential placental transfer to the fetus during the third trimester, it is not surprising that, as the most abundant ω-3 fatty acid in the mammalian brain, DHA is essential to brain development (5,7,21,22). This study finds that for only DHA levels, both near-birth and near-term blood samples are associated with increased brain tissue volumes at near-term age, albeit differences in affected brain regions. Increased DHA levels at both near-birth and near-term time points are associated with larger deep grey matter and brainstem volumes, suggesting a larger sensitive time window for DHA to positively affect these regions. Higher DHA levels only at the near-birth time point are associated with larger cortical grey matter volumes, suggesting a shorter and earlier time period for DHA’s influence, and providing evidence that near-birth DHA levels predict near-term cortical grey matter volumes. Further, greater DHA levels only at the near-term time point are correlated with larger cerebellar volume, suggesting a shorter window for DHA to impact the cerebellum. The mechanisms – neuroprotection or growth enhancement – by which DHA is affecting individual brain tissue regions have yet to be elucidated. Although DHA is present throughout the brain, the cortex has the highest percentage of DHA, whereas the medulla has the lowest (23). DHA is also present in high quantities in the phospholipids of brain grey matter (9,24,25). Of note, whole blood DHA levels are lower in preterm than in full-term newborns (22). However, no studies have previously reported the effects of DHA on volumes of specific brain regions.
Initially, it may seem as though the current DHA results conflict with previously reported DTI results in the same cohort, which showed that higher DHA was associated with DTI measures in regions of interest located in white matter tracts (17), whereas the current study found no association between DHA and white matter volumes. However, since prior DTI results showed higher DHA associated with decreased mean diffusivity and no association with fractional anisotropy, it was suggested that improved microstructural development was independent of myelination (17). This supports the current findings that DHA levels were not associated with white matter volumes.
In contrast to DHA, levels of the ω-6 fatty acid LA remain relatively stable during late gestation (26), are lower in fetal than maternal plasma and erythrocyte phospholipids (27,28), and LA transfer to the fetus via the placenta during pregnancy is restricted (29). This study determines that LA levels only at the near-birth time point are associated with brain tissue volumes. Higher near-birth LA levels are correlated with decreased near-term white matter volumes, suggesting an earlier and shorter sensitive window for LA to negatively affect white matter, and providing evidence that near-birth LA levels predict near-term white matter volumes. Previous findings suggest that low placental transfer of LA is a favourable biological mechanism that ultimately facilitates DHA accretion in the fetus (29). LA and α-linoleic acid compete for the same desaturation enzymes that ultimately convert these essential fatty acids to ARA and DHA, respectively. In the developing brain, although α-linoleic acid is the preferred substrate for these desaturases, elevated levels of LA can result in inhibition of DHA production, potentially posing developmental problems during the perinatal period and into childhood (30). This supports our finding that DHA and LA have opposing associations with brain tissue volumes, which is strengthened by previous findings that revealed that higher DHA and lower LA levels were associated with improved microstructural development (17). Future studies need to decipher details behind the negative association between near-birth LA levels and near-term white matter volume. Animal studies have shown that during the neonatal period the difference in LA concentration across brain regions is not very apparent (23). Studies have also shown that LA levels in preterm newborns are altered when compared to full-term infants (22). No previous investigations have described a relationship between LA and regional brain volumes.
We previously determined that higher DHA and lower LA levels during the postnatal period were associated with improved developmental outcomes in preterm newborns (17). Higher near-birth DHA levels were associated with higher language and motor scores, and lower near-birth LA levels were associated with higher motor and cognitive scores on the Bayley-3 (17). This study reveals that improved macrostructural brain development – larger brain tissue volumes – at near-term age is also associated with improved developmental outcomes at 30-36 months corrected age, supporting and expanding upon previous findings. Larger cortical and deep grey matter, cerebellar, and brainstem volumes at near-term age are associated with improved language scores, suggesting language development in the preterm population may be dependent on postnatal volumes of multiple brain regions, and disruption of typical regional brain growth by preterm birth may lead to delayed and/or impaired language development detected at preschool age. Further, larger cerebellar and brainstem volumes are also associated with improved motor scores, suggesting preterm motor development at preschool age can be predicted by postnatal cerebellar and brainstem volumes, and that the cerebellum and brainstem have a more global impact on the development of preterm born children. Of the few studies that employed near-term MRI scans to investigate the relationship between brain volumes and neurodevelopmental outcome in preterm newborns, all illustrate that smaller global and regional brain tissue volumes–cerebrum, frontal lobes, cortical grey matter, basal ganglia, thalami, cerebellum—at near-term age predict worse neurodevelopmental outcomes at preschool age (13,15,16), supporting our findings.
The impact of neonatal feeding practices on PUFA levels for this study cohort was previously reported (17). Reported results suggest that feeding regimens currently employed in the NICU have no significant effect on postnatal DHA levels for the study cohort, and thus should not impact significant associations of DHA with brain tissue volumes reported here. However, higher near-birth LA levels were found to be associated with increased duration of intravenous nutrition (β = 1.96, P = 0.001) and lipid supplementation (β = 1.88, P = 0.001). This finding can be expected since the intravenous fat emulsion contains LA, and suggests that the measures of estimated nutritional exposures should be able to identify differences in feeding.
Since the sickest newborns were harder to feed, it could be argued that measures of poor brain growth – smaller brain tissue volumes – are related to disease rather than PUFA levels. This is unlikely to be the case for findings presented here because the analyses were controlled for measures of brain injury, including IVH severity, white matter injury severity, and cerebellar haemorrhage (for cerebellar volumes only), and for known clinical risk factors for brain injury, including age at MRI scan, duration of intubation, sepsis, and patent ductus arteriosus. Additionally, since previously reported results in the same cohort illustrated an association between IVH and DHA and the potential impact of IVH on brain growth (17), analyses were repeated excluding infants with IVH. In general, after adjusting for potential confounders, the associations between the studied PUFAs (i.e., DHA, EPA, ARA, and LA) and brain tissue volumes did not meaningfully change. This strengthens the likelihood that the associations found between brain tissue volumes and PUFA levels are real. However, the influence of disease on the brain tissue volumes cannot be ignored entirely, as there may be residual confounding.
Of note, red blood cell membrane PUFA level is a cell marker depicting a cell membrane synthesized weeks earlier (6), thus near-birth PUFA levels presented here may not only represent early postnatal levels, but may also reflect intrauterine levels. Since this study finds that DHA levels from near-birth blood samples are significantly associated with larger brain regions, DHA may be important for growth and/or neuroprotection of certain brain regions throughout the perinatal period as opposed to only the postnatal period. This evidence along with other studies that have determined that women with higher plasma DHA during pregnancy give birth to newborns with higher DHA (24,25), provide support for therapeutic intervention with DHA to help improve region-specific brain growth in at-risk pregnant women.
Although the size of study cohort is limited, adjustments for analyses were made for potential confounders selected a priori based on previous studies (17) and the number of potential confounders controlled for were limited due to sample size. However, many associations between postnatal PUFA levels and brain tissue volumes, and between brain tissue volumes and developmental outcomes at 30-36 months corrected age remained across multiple analyses, providing evidence that these study findings are genuine.
This study demonstrates that the associations between PUFAs and the brain during development are not homogenous. We find that ω-3 and ω-6 fatty acids have opposing associations with brain tissue volumes, with higher ω-3 and lower ω-6 fatty acids levels correlated with larger volumes. Further, the correlations between PUFAs and brain growth were found to be time-dependent and region-specific. The associations between DHA and brain tissue volumes are variable and occur throughout the perinatal period, whereas only near-birth LA levels predict only white matter volumes. In accordance with previous literature (13,15–17), albeit differences in affected brain regions and associated outcomes, this study provides further evidence that improved macrostructural brain development during the neonatal period can predict improved developmental outcomes during the preschool period. Future studies need to investigate therapeutic windows and thresholds for individual PUFAs and their associations with specific brain regions during development, and associations with long-term developmental outcomes. Our current findings suggest it may prove beneficial for therapeutic interventions to target both at-risk expectant mothers and preterm neonates.
Acknowledgments
The authors thank Sheila M. Innis, RD, PhD, for her assistance with analysing blood samples for fatty acid levels. The authors also thank Kenneth J. Poskitt, MD, FRCPC, for his assistance reviewing MRI scans at the University of British Columbia study site.
STATEMENT OF FINANCIAL SUPPORT
The study was supported by the Gerber Foundation (Fremont, MI) [EWYT,DMF], Canadian Institutes for Health Research (CIHR) CHI 151135 [SPM], National Institutes of Health (NIH) NIH/NINDS R01 NS 061957 [CS], NIH/NINDS R01 NS 055064 [CS], NIH R01 NS346432 [AJB], NIH P01NS082330 [DMF], and NeuroDevNet (Vancouver, Canada) [SPM]. The funders had no role in study design, data collection, analysis or interpretation, decision to publish, or preparation of the manuscript.
Footnotes
DISCLOSURE
The authors have no financial or other conflicts of interest to declare.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.de Kieviet JF, Zoetebier L, van Elburg RM, et al. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 2012;54:313–23. doi: 10.1111/j.1469-8749.2011.04216.x. [DOI] [PubMed] [Google Scholar]
- 3.Soria-Pastor S, Padilla N, Zubiaurre-Elorza L, et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009;124:e1161–70. doi: 10.1542/peds.2009-0244. [DOI] [PubMed] [Google Scholar]
- 4.Petrou S, Henderson J, Bracewell M, et al. Pushing the boundaries of viability: The economic impact of extreme preterm birth. Early Hum Dev. 2006;82:77–84. doi: 10.1016/j.earlhumdev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Almaas AN, Tamnes CK, Nakstad B, et al. Long-chain polyunsaturated fatty acids and cognition in VLBW infants at 8 years: an RCT. Pediatrics. 2015;135:972–80. doi: 10.1542/peds.2014-4094. [DOI] [PubMed] [Google Scholar]
- 6.Fleith M, Clandinin MT. Dietray PUFA for preterm and term infants: review of clinical studies. Crit Rev Food Sci Nutr. 2005;45:205–29. doi: 10.1080/10408690590956378. [DOI] [PubMed] [Google Scholar]
- 7.Lapillonne A. Enteral and parental lipid requirements of preterm infants. World Rev Nutr Diet. 2014;110:82–98. doi: 10.1159/000358460. [DOI] [PubMed] [Google Scholar]
- 8.Lauterbach R. A supplementation of DHA and AA to human milk-fed VLBW infants has no significant cognitive improvement or measurable neuroanatomical effects when evaluated at 8 years of age. Evid Based Med. 2015;20:177. doi: 10.1136/ebmed-2015-110242. [DOI] [PubMed] [Google Scholar]
- 9.Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–9. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- 10.Isaacs EB, Gadian DG, Sabatini S, et al. The effect of early human diet on caudate volumes and IQ. Pediatr Res. 2008;63:308–14. doi: 10.1203/PDR.0b013e318163a271. [DOI] [PubMed] [Google Scholar]
- 11.Isaacs EB, Fischl BR, Quinn BT, et al. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res. 2010;67:357–62. doi: 10.1203/PDR.0b013e3181d026da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan M, Abernethy L, Cooke R. Improving head growth in preterm infants—a randomized controlled trial II: MRI and developmental outcomes in the first year. Arch Dis Child Fetal Neonatal Ed. 2008;93:342–46. doi: 10.1136/adc.2007.124255. [DOI] [PubMed] [Google Scholar]
- 13.Lind A, Parkkola R, Lehtonen L, et al. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr Radiol. 2011;41:953–61. doi: 10.1007/s00247-011-2071-x. [DOI] [PubMed] [Google Scholar]
- 14.Monson BB, Anderson PJ, Matthews LG, et al. Examination of the pattern of growth of cerebral tissue volumes from hospital discharge to early childhood in very preterm infants. JAMA Pediatr. 2016;170:772–9. doi: 10.1001/jamapediatrics.2016.0781. [DOI] [PubMed] [Google Scholar]
- 15.Skiöld B, Alexandrou G, Padilla N, et al. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr. 2014;164:1012–18. doi: 10.1016/j.jpeds.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Van Kooij BJ, Benders MJ, Anbeek P, et al. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev Med Child Neurol. 2012;54:260–6. doi: 10.1111/j.1469-8749.2011.04168.x. [DOI] [PubMed] [Google Scholar]
- 17.Tam EW, Chau V, Barkovich AJ, et al. Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr Res. 2016;79:723–30. doi: 10.1038/pr.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam EW, Miller SP, Studholme C, et al. Differential effects of intraventricular haemorrhage and white matter injury on preterm cerebellar growth. J Pediatr. 2011;158:366–71. doi: 10.1016/j.jpeds.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Kitsch A, Miller SP, et al. Patch-based augmentation of Expectation-Maximization for brain MRI tissue segmentation at arbitrary age after premature birth. Neuroimage. 2016;127:387–408. doi: 10.1016/j.neuroimage.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innis SM, Nelson CM, Rioux MF, et al. Development of visual acuity in relation to plasma erythrocyte omega-6 and omega-3 fatty acids in healthy term gestation infants. Am J Clin Nutr. 1994;60:347–52. doi: 10.1093/ajcn/60.3.347. [DOI] [PubMed] [Google Scholar]
- 21.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–59. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- 22.Martin CR, DaSilva DA, Cluette-Brown JE, et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr. 2011;159:743–49. doi: 10.1016/j.jpeds.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Y, Huang Y, Chen ZY. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in the rat brain. Br J Nutr. 2005;94:544–50. doi: 10.1079/bjn20051539. [DOI] [PubMed] [Google Scholar]
- 24.Innis SM. Fatty acids and early human development. Early Hum Dev. 2007;83:761–6. doi: 10.1016/j.earlhumdev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr. 2003;143:S1–8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- 26.Birch EE, Birch DG, Hoffman DR, et al. Dietary essential fatty acid supply and visual acuity development. Invest Ophthalmol Vis Sci. 1992;33:3242–53. [PubMed] [Google Scholar]
- 27.Elias SL, Innis SM. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal fatty acids, length of gestation, and birth weight and length. Am J Clin Nutr. 2001;73:807–14. doi: 10.1093/ajcn/73.4.807. [DOI] [PubMed] [Google Scholar]
- 28.Vlaardingerbroek H, Hornstra G. Essential fatty acids in erythrocyte phospholipids during pregnancy and at delivery in mothers and their neonates: comparison with plasma phospholipids. Prostaglandins Leukot Essent Fatty Acids. 2004;71:363–74. doi: 10.1016/j.plefa.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Novak EM, Ling DJ, Innis SM. Low linoleic acid may facilitate Δ6 desaturase activity and docosahexaenoic acid accretion in human fetal development. Prostaglandins Leukot Essent Fatty Acids. 2012;86:93–8. doi: 10.1016/j.plefa.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Salvati S, Attorri L, Avellino C, et al. Diet, lipids and brain development. Dev Neurosci. 2000;22:481–7. doi: 10.1159/000017479. [DOI] [PubMed] [Google Scholar]


