Abstract
Metastatic pheochromocytoma and paraganglioma (mPHEO/PGL) are frequently associated with succinate dehydrogenase B (SDHB) mutations. Cyclophosphamide-dacarbazine-vincristine (CVD) regimen is recommended as standard chemotherapy for advanced mPHEO/PGL. There is limited evidence to support the role of metronomic schemes (MS) of chemotherapy in mPHEO/PGL treatment. We report 2 patients with SDHB-related mPGL who received a regimen consisting of MS temozolomide (TMZ) and high-dose lanreotide after progression on both CVD chemotherapy and high-dose lanreotide. Molecular profiling of the tumor tissue from both patients revealed hypermethylation of the O6-methylguanine-DNA-methyltransferase (MGMT) promoter. In one patient, progression-free survival was 13 months and the second patient remained under treatment after 27 months of stabilization of metabolic response of his disease. Treatment was well tolerated, and adverse effects were virtually absent. A modification in the scheme of TMZ from standard schemes to MS is safe and feasible and can be considered in patients with progressive mPHEO/PGL refractory to dacarbazine in standard doses.
Keywords: Paraganglioma, SDHB, metastatic, temozolomide, metronomic
Background
Pheochromocytomas (PHEOs) and paragangliomas (PGLs) are rare, frequently heritable and highly vascularized neuroendocrine tumors (NETs) that originate from chromaffin cells of the adrenal medulla or paraganglia located outside of the adrenal gland, respectively. Although they are usually benign, PHEO/PGL can exhibit malignant behavior. This rate of malignancy has long been cited as 10%,1 although some authors point at a higher percentage of 26%,2 which would be specially higher in patients with secretive PGLs.3 Patients with mPHEO/PGLs, defined by the presence of metastases, have a 5-year overall survival rate of 60%.4 Approximately 40% of PGLs are ascribed to germline mutations in 1 of more than 12 well-identified genes including succinate dehydrogenase (SDH) type A, B, C, or D; SDH complex assembly factor 2 (SDHAF2); fumarate hydratase (FH); von Hippel-Lindau (VHL); malate dehydrogenase (MDH) type 2; endothelial pas domain protein 1/hypoxia-inducible factor type 2A (EPAS1/HIF2A); prolyl hydroxylase (PHD) type 1 and 2; rearranged during transfection (RET); neurofibromatosis type 1 (NF1); transmembrane protein 127 (TMEM127); and MYC-associated factor X (MAX).5–7 Mutations in the SDHB gene are associated with mPHEO/PGL in up to 83% of cases.8,9 SDH-related tumorigenesis is characterized by a pseudo(hypoxic) pathway signature and stabilization of the hypoxia-inducible factor,10,11 which results in activation of target genes involved in angiogenesis, proliferation, invasiveness, and metastasis.12,13 More specifically, SDHB-mutated PGLs exhibit a pronounced hypermethylator phenotype14 resulting in decreased expression of target genes involved in neuroendocrine differentiation.15 This mechanism leads to activation of the epithelial-to-mesenchymal transition pathway and explains the invasive phenotype of these tumors.14,15 In patients with progressive mPHEO/PGL, the treatment goals are to manage hormone-related symptoms, control tumor growth, and prolong the overall survival (OS) of patients. Treatment options are limited to debulking surgery, localized radiotherapy, and tumor-specific systemic therapies. Combination treatment with cyclophosphamide-dacarbazine-vincristine (CVD; cyclophosphamide 750 mg/m2 vincristine 1.4 mg/m2, and dacarbazine 600 mg/m2 on day 1 and dacarbazine 600 mg/m2 on day 2) is considered the standard first-line chemotherapy regimen in patients with inoperable and progressive mPHEO/PGL based on 6 retrospective studies.16–22 However, the results may be overestimated and have not been confirmed by randomized controlled trials. Molecular targeted chemotherapies with mammalian target of rapamycin (mTOR) inhibitors (NCT01152827) and tyrosine kinase inhibitors with strong antiangiogenic activity such as pazopanib (NCT01340794) and axitinib (NCT01967576) have not shown any clear benefit in mPHEO/PGL in phase 2 clinical trials. Ongoing clinical trials are testing the efficacy of vascular endothelial growth factor (VEGF) pathway inhibitors, such as sunitinib (NCT01371201) and lenvatinib (NCT03008369); VEGF/MET (VEGF/hepatocyte growth factor receptor) pathway inhibitors, such as cabozantinib (NCT02302833); and the immune-modulating antibodies against the PD-1/PDL-1 pathway, such as pembrolizumab (NCT02721732).
Temozolomide (TMZ) is an alkylating agent and an oral chemotherapy alternative to intravenous dacarbazine, which historically has been used both as monotherapy and in combination with other antitumoral agents. In a recent study, TMZ, when administered at a mean dose of 172 mg/m2/d for 5 days in 28-day cycles, resulted in clinical benefit in 67% of the enrolled patients with progressive mPHEO/PGL.23 Interestingly, 80% of these responders exhibited low tumor levels of O6-methylguanine-DNA methyltransferase (MGMT).23 A similar pattern has been previously reported in patients with glioblastomas and gastroenteropancreatic NETs.24,25 Furthermore, this study reported a correlation between SDHB-mutated tumors and hypermethylation of the MGMT promoter region.23
In this report, we detail the outcomes for 2 patients with SDHB-related PGL progressive metastatic disease, who had been already treated and progressed on both intravenous dacarbazine-based chemotherapy and high doses of lanreotide (Table 1). At the time of progression (TTP), standard scheme (SS) of chemotherapy was rotated to metronomic schemes (MS) with TMZ 75 mg/m2 (21/28-day regimen).26 Both patients responded well. The administration of MS in our 2 patients was based on further genetic profiling of metastatic tumor tissue in both patients who revealed methylation of the MGMT promoter. This epigenetic silencing pattern23 and the theoretical possibility to overcome chemotherapy resistance to conventional SS were considered compelling molecular findings to recommend rechallenging with TMZ and to explore MS dosing of the same.
Table 1.
Clinical characteristics of patients treated with metronomic TMZ and high-dose lanreotide.
| Patient no. | Sex | Age at diagnosis Primary |
Ki 67 index, % | Age at mPGL diagnosis | Site of metastases | SDHB mutation | MGMT methylation | Treatment cycles, n | Biochemical response | Therapies | PFS to prior therapies, mo | Best response to prior therapies | PERCIST 1.0 | PFS, mo | Survival status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 58 | 20 | 60 | Bone, soft tissue | Yes | Yes | 27 | PRa | Surgery | 24 | PMRb | NR | Alive | |
| Thermoablation | |||||||||||||||
| Sunitinib | 2 | PMD | |||||||||||||
| 2 monthly lanreotide + CVD | 6 | PMR | |||||||||||||
| 2 | F | 46 | 5 | 46 | Bone, soft tissue | Yes | Yes | 13 | CRa | CVD | 8 | PMR | PMRb | 13 | Alive |
| High-dose lanreotide | 15 | PMR | |||||||||||||
| Surgery | |||||||||||||||
| Thermoablation |
Abbreviations: CR, complete response; CVD, cyclophosphamide, vincristine, and dacarbazine; F, female; M, male; MGMT, O6-methylguanine-DNA methyltransferase; mPGL, malignant pheochromocytoma and paraganglioma; PERCIST, positron emission tomography response criteria in solid tumors; PFS, progression-free survival; PMD, progressive metabolic disease; PMR, partial metabolic response; PR, partial response; SDHB, succinate dehydrogenase subunit B; TMZ, temozolomide; NR, not reached.
Biochemical response in patient 1 was based on urinary normetanephrine levels obtained at baseline and after 4 and 11 cycles of chemotherapy. Urinary normetanephrine levels were used to monitor disease progression because the biochemical phenotype of the tumor was noradrenergic (mainly producing norepinephrine and normetanephrine). In patient 2, biochemical response was based on plasma chromogranin A levels. Other biochemical markers were not available at regular time intervals.
Both patients demonstrated PMR as their best response to this treatment regimen on PERCIST 1.0 criteria. While patient 1 continued to demonstrate a stabilized PMR after 21 cycles, patient 2 demonstrated progressive metabolic disease (PMD) after 13 cycles with dissociated response.
In general, the administration of optimal MS chemotherapy can result in an improved OS compared with SS27,28 with relatively low toxicity in various solid malignancies,28–30 even in heavily pretreated patients.28,29 As a consequence, chemotherapy paradigms are shifting to focus on MS.31–33 Metronomic scheme targets the proliferating tumor endothelial cells34 and theoretically can result in a significant antiangiogenic effect31–34 and may implicate immunologic host effects32,35 as treatment outcome seems to rely on the immune environment.33 However, the antiproliferative pathways of MS are not fully elucidated.
Experimental Methods and Data Analysis
After pathological review, formalin-fixed paraffin-embedded (FFPE) metastatic tissues were macrodissected and DNA was extracted using the Qiagen DNA FFPE Tissue Kit (Qiagen, Valencia, CA, USA) and measured using the Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). To identify somatic alterations, an AmpliSeq custom panel (OncoDNA, Gosselies, Belgium) was designed to amplify by next-generation sequencing (NGS) 207 amplicons covering hotspot mutations of 65 genes (OncoDEEP). Briefly, the targeted sequencing libraries were generated using the Ion AmpliSeq Library Kit 2.0 according to the manufacturer’s instructions (Thermo Fisher Scientific). The starting material consisted of 10 ng DNA from FFPE. The primers used for amplification were partially digested by the Pfu enzyme and the product of digestion was then ligated with corresponding barcoded adapters and purified using Ampure Beads (Agilent Genomics Inc., Santa Clara, CA, USA). The product of purification was amplified for 5 more cycles and subsequently purified using Ampure Beads. The quality of the libraries was assessed using the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific). About 10 pM of each library was loaded into the Ion Chef System (Thermo Fisher Scientific) for the emulsion polymerase chain reaction. An average coverage of 1000× was targeted to be able to detect variants down to 5%. Ion PGM (Personal Genome Machine) System was used for primary processing of NGS data and identification of putative somatic mutations. The data generated were aligned to the human reference sequence and annotated using the consensus coding DNA sequences, RefSeq, and Ensembl databases. The NGS data were first analyzed using the Torrent Suite Software (Thermo Fisher Scientific).
Then, somatic mutations were identified using the Variant Caller 4.0 software using the somatic high-stringency parameters (Thermo Fisher Scientific). Mutations were separated into those associated with a described biological impact on the function of the proteins and those common germline mutations found in the NCBI dbSNP human variation sets in VCF (variant call format) version 138 (https://www.ncbi.nlm.nih.gov/variation/docs/human_variation_vcf/). For immunohistochemistry (IHC), each IHC was analyzed by microscopy in a double-blind fashion. A score was calculated based on a predefined ISO-accredited scoring method (which is IHC dependent). MGMT promoter methylation was determined using pyrosequencing. To assess clinical relevance of the analyses, a literature search was performed to identify published official guidelines, retrospective, and prospective clinical studies, pertaining to genomic alterations of each gene and their association with outcomes in patients with cancer, and to remove variants known to be benign or likely to be benign. The same analysis was performed for the results of the IHC and methylation test. The treatments recommended for each assay fell into 3 different categories: associated with “Potential clinical benefit,” “Lack of potential clinical benefit,” and “Unknown.” In both cases, the methylation of the MGMT promoter was revealed as the main target for personalized treatment (Table 2).
Table 2.
Results of exhaustive genomic profiling, next-generation sequencing (NGS) of 65 genes (Ion Torrent technology [Life Technologies, Carlsbad, CA, USA]), methylation profiling, and immunohistochemistry (IHC) of the tumor tissue (OncoDEEP) from both patients.
| Patient 1 | Patient 2 | |||
|---|---|---|---|---|
| NGS | ||||
| Variants | N | |||
| Variants of uncertain significance (VUS) | 1 | KDR p.R961Q | ||
| Probably polymorphism | 1 | PIK3CA p.I391M | 1 | DPYD p.S534N |
| IHC | ||||
| Protein/biomarker | Expression | Clinical impact | ||
| P16 | Positive | Potential lack of clinical benefits of CDK4/6 inhibitors | Negative | Potential lack of clinical benefits of CDK4/6 inhibitors |
| CDK4 | Negative | Potential lack of clinical benefits of CDK4 inhibitors | Moderate | Potential clinical benefits of CDK4 inhibitors |
| Phospho-Rb | Negative | Potential lack of clinical benefits of CDK4/6 inhibitors | Positive | Potential clinical benefits of CDK4/6 inhibitors |
| Fusion panel (ALK/ROS1/RET) | Negative | Potential lack of clinical benefits of Crizotinib | Negative | Potential lack of clinical benefits of Crizotinib |
| MGMT methylation | Positive | Potential clinical benefits of temozolomide | Positive | Potential clinical benefits of temozolomide |
| CD8 | Negative | Potential lack of clinical benefits of PD-1/PD-L1 inhibitors | Negative | Potential lack of clinical benefits of PD-1/PD-L1 inhibitors |
| PD-L1 | Low | Potential lack of clinical benefits of PD-1/PD-L1 inhibitors | Low | Potential lack of clinical benefits of PD-1/PD-L1 inhibitors |
| p4EBP1 | Low | Potential lack of clinical benefits of mTOR inhibitors | Low | Potential lack of clinical benefits of mTOR inhibitors |
| PTEN | Positive | Potential lack of clinical benefits of PIK3CA and/or mTOR inhibitors | Positive | Potential lack of clinical benefits of PIK3CA and/or mTOR inhibitors |
| VEGF | Positive | Treatment based on angiogenesis inhibitors associated with undetermined clinical benefit in paraganglioma | Negative | Potential lack of clinical benefits of angiogenesis inhibitors |
| VEGFR2 | Low | Potential lack of clinical benefits of VEGFR2 inhibitors | ||
| EGFR | Negative | Potential lack of clinical benefits of EGFR inhibitors | Negative | Potential lack of clinical benefits of EGFR inhibitors |
Case Reports
Patient 1
In January 2017, a 63-year-old man with a history of SDHB (c.637dupA)-related mPGL presented to the National Institutes of Health. In November 2012, he presented with a 5-year history of profuse sweating at night, hypertension, and diffuse abdominal pain. Imaging studies revealed a 7.5 cm × 5 cm paraaortic mass, which was resected and was confirmed to be PGL. Immunohistochemistry demonstrated positivity for chromogranin (CgA), synaptophysin, S-100, and Ki-67 index of 20%. After a disease-free survival period of 2 years, the patient developed severe cervical pain and was found to have multifocal bone lesions on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT). First-line systemic treatment with sunitinib 25 mg daily was initiated, increasing the dose to 37.5 mg after 1 week. Treatment was continued for 62 days with poor tolerability and progression of the disease. Thus, during the treatment, the patient developed profuse sweating due to worsening hypertension, severe bone pain, and myalgia with increased analgesic requirement, asthenia grade 2, and lost 44 lbs with Eastern Cooperative Oncology Group performance status (ECOG PS) of 4. Restaging studies in January 2015, after 69 days of treatment, showed progression of metabolic disease (PMD) based on positron emission tomography response criteria in solid tumors (PERCIST) 1.0 criteria. Hypertension and pain and control were achieved with a combination of olmesartan 20 mg and hydrochlorothiazide 12.5 mg daily and high-dose transdermal fentanyl patches (200 µg every 3 days), reaching an ECOG PS of 2.
In February 2015, the patient started second-line systemic treatment with a combination regimen of extended-release lanreotide (Somatuline Autogel) at a dose of 120 mg every 14 days, zoledronic acid 4 mg every 28 days, and SS chemotherapy with CVD (cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2, and dacarbazine 600 mg/m2 on day 1 and dacarbazine 600 mg/m2 on day 2) every 21 days. Although a partial metabolic response (PMR) was noted after 4 cycles, the 18F-FDG PET CT after 6 cycles showed PMD with clinical worsening (ECOG PS 3). The CVD chemotherapy was discontinued, and the patient remained on the same doses of lanreotide and zoledronic acid.
In December 2015, genomic profiling of the tumor tissue revealed methylation of the MGMT promoter. Based on this epigenetic silencing pattern, MS TMZ was added to lanreotide and zoledronic acid at a dose of 75 mg/m2/d with a schedule of 3 weeks on treatment followed by 1 week off treatment (21/28-day regimen).26 After 17 cycles, treatment was discontinued in February 2017 due to grade 3 lymphopenia according to Common Terminology Criteria for Adverse Events v3.0 (CTCAE) and restarted after 2 weeks, in March 2017, at the same dose but with prolongation of the courses to 5 weeks (3 weeks on treatment followed by 2 weeks off treatment).26 Currently, the patient remains under treatment after 27 cycles.
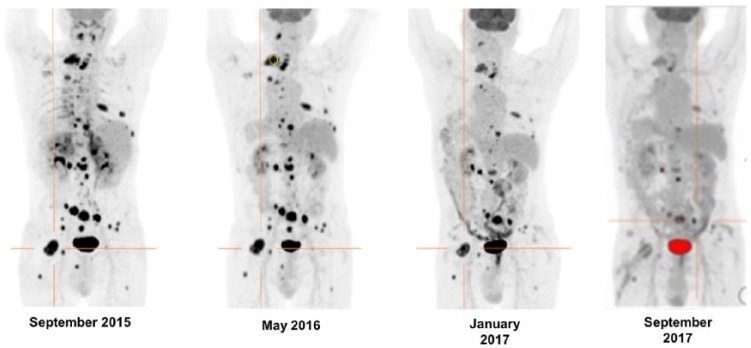
The treatment regimen was being well tolerated with improvement in ECOG PS 0-1 with significant improvement in pain control as noted by progressive strength reductions in fentanyl analgesia. In June 2016, the patient achieved complete control pain without analgesia. Continuous daily TMZ can cause lymphopenia, potentially increasing the risk of opportunistic infections. Expected hematological toxicity with grade 1-2 lymphopenia from the fourth cycle of therapy onward required addition of oral prophylactic TMP/SMX (trimethoprim/sulfamethoxazole) to prevent Pneumocystis carinii pneumonia.36 Patient’s blood pressure and heart rate remain in the normal range without need for antihypertensive medications. Biochemical response was noted after 14 cycles, evaluated with both CgA and urinary normetanephrine (NMN) levels, at 47% and 63% from baseline, respectively. Restaging 18F-FDG PET CTs performed in May 2016, after 5 cycles, and in March 2017, after 15 cycles, demonstrated a PMR and a stabilized PMR, respectively (Figure 1). On his latest 18FDG-PET/CT of September 2017 after 22 cycles, the patient continued to demonstrate prolonged stabilized PMR and currently he continues under 28th course of treatment (Figure 1).
Figure 1.
This image demonstrates the metabolic changes observed on the 18F-FDG PET/CT at baseline (prior to initiating MS with TMZ in September 2015), restaging studies performed after 5 cycles, in May 2016, and 15 cycles, in March 2017, of the chemotherapy regimen consisting of MS with TMZ and high-dose lanreotide. In September 2015, maximum standardized uptake value (SUVmax) normalized to lean body mass (SULmax) of the most active lesion (marked in orange) was 23.12. In May 2016, a PMR was noted (SULmax) of the target lesion = 12.49, which is 45.9% reduction). In March and September 2017, there was stabilization of the PMR and no new lesions were detected on either of the studies. 18F-FDG PET/CT: 18F-fluorodeoxyglucose positron emission tomography/computed tomography; PMR: partial metabolic response; TMZ: temozolomide.
Patient 2
In May 2016, a 49-year-old woman with an SDHB (del ex 6-8)-related mPGL presented to the National Institutes of Health. She was originally diagnosed at age 46, when she reported a 3-month history of debilitating hip pain, flushing profuse sweating as well as a 3-year history of palpitations, hypertension, and progressive deterioration of ECOG to status 3. The patient endorsed a family history of neck PGL, 18F-FDG PET CT showed a 6.8 cm × 7.5 cm × 8.5 cm hypermetabolic mass subjacent to the area of the third portion of the duodenum and multifocal lytic bone lesions. Biochemical testing showed elevated urinary norepinephrine, as well as plasma NMN and CgA levels, with a high degree of suspicion for a diagnosis of mPGL. Systemic treatment with CVD was prioritized and commenced chemotherapy in May 2013. An initially deferred biopsy of a right iliac bone lesion performed after 2 courses confirmed the diagnosis of mPGL. Restaging following 3 cycles of therapy showed no evidence of clinical response, with stable disease based on response evaluation criteria in solid tumors (RECIST) 1.1 and PMR based on PERCIST 1.0 criteria.
Following a fifth course of therapy, the patient underwent somatostatin receptor scintigraphy evaluation with 111In-pentetreotide ([111In-DTPA0]-octreo-tide), which demonstrated a positive uptake. Based on this finding, extended-release lanreotide (Somatuline Autogel) at a dose of 120 mg was administered alongside 6 cycles of CVD chemotherapy, starting September 2013. This regimen was discontinued in February 2014 (after 4 cycles of CVD monotherapy and 4 cycles of combination therapy) due to worsening symptoms and biochemical markers; lanreotide dosing intervals were shortened fortnightly to 2 weeks. Following 6 cycles of lanreotide monotherapy, the patient demonstrated a pronounced clinical response, with improvement in self-care activities and symptoms to ECOG PS 0, and a biochemical response with normalization of plasma CgA levels. Restaging 18F-FDG PET CT in May 2014 revealed a PMR based on PERCIST 1.0 criteria. A maximal cytoreductive debulking surgery with complete resection of the primary retroperitoneal PGL was performed in October 2014. Lanreotide was continued at the same dose until July 2015.
A year and half later, in May 2015, the patient presented with symptomatic progression of a painful soft tissue mass located at the level of T9-T10 vertebrae, which was treated with external beam radiation (30 cGy daily for 10 days, total dose of 300 Gy). At the same time, a 6.5-cm femoral lesion, extending into the diaphysis and with noted endosteal resorption, was identified consistent with disease progression. Second line of SS chemotherapy following Strosberg et al (capecitabine 750 mg/m2 twice a day, on days 1-14 and TMZ 200 mg/m2 daily, on days 1-5 in 28-day cycles)37 and monthly denosumab 120 mg, was initiated. Lanreotide was continued at the same dose. However, in July 2015, patient underwent thermoablation and cementoplasty to stabilize the femur that was complicated by a fourth-degree skin burn with full thickness ulceration. As a result, chemotherapy was discontinued due to the associated risk of infection. During this period, the patient’s biochemical markers continued to worsen. In addition, genetic and molecular profiling of the tumor tissue revealed methylation of the MGMT promoter. Following consideration of the NGS findings, in September 2015, the chemotherapy regimen was rotated to MS TMZ 75 mg/m2 (21/28-day regimen),26 denosumab 120 mg, and lanreotide 120 mg every 14 days with excellent tolerability.
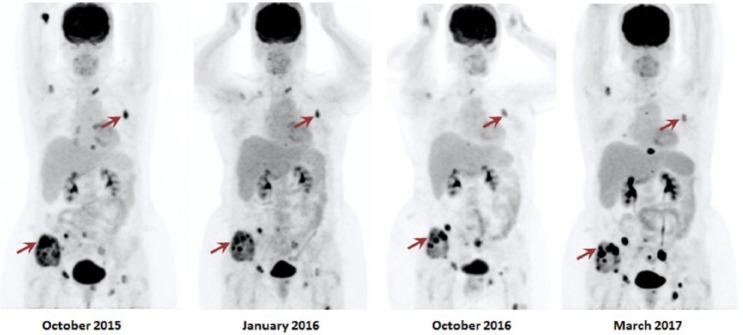
Following 6 courses of treatment, the patient achieved a complete biochemical response with normalization of CgA levels. Restaging 18F-FDG PET CT after 4 cycles demonstrated PMR of >40% in target metastatic bone lesions. The patient did not develop any adverse effects apart from grade 2 lymphopenia, which was managed with prophylactic antibiotics. 18F-FDG PET CT in October 2016 demonstrated a dissociated metabolic response and no new lesions were detected (Figure 2). Despite this, the regimen was continued due to limited available treatment options while other therapies were being considered. Patient completed 17 cycles of this chemotherapy regimen in March 2017. 18F-FDG PET CT in March 2017 revealed PMD and this treatment regimen was discontinued (Figure 2). The patient was started on peptide receptor radionuclide therapy with Lutetium-177 (177Lu)-DOTA0-Tyr3-octreotate (DOTATATE) in April 2017.
Figure 2.
This image demonstrates the metabolic changes observed on the 18F-FDG PET CT 1 month after initiating TMZ in September 2015, restaging studies performed after 4 cycles, in January 2016, after 13 cycles, in October 2016, and after 17 cycles, in March 2017, of the chemotherapy regimen consisting of MS TMZ, denosumab, and high-dose lanreotide. In October 2015, maximum standardized uptake values (SUVmax) of the most active lesions located at the right iliac crest and the left third rib (marked in red) were 16.0 and 13.6, respectively. In January 2016, a PMR was noted of the right iliac crest lesion and of the left third rib lesion (SUVmax of 9.2 and of 7.9), showing a 42.5% and 41.9% of SUVmax reduction, respectively. In October 2016, there was a dissociated metabolic response. While the SUVmax of the right iliac lesion increased to 21.62, the SUVmax of the left third rib lesion decreased by 19.8% to 6.33. No new lesions were noted in either restaging studies. New lesions and increased metabolic activity were noted in the 18F-FDG PET CT performed in March 2017, indicating PMD. 18F-FDG PET/CT indicates 18F-fluorodeoxyglucose positron emission tomography/computed tomography; PMD, progressive metabolic disease; PMR, partial metabolic response; TMZ, temozolomide.
Discussion
Temozolomide is a DNA alkylating agent, which primarily exerts its cytotoxic effect by causing methylation of the O6 position of guanine, resulting in DNA adduction. These DNA adducts represent 9% of the total DNA methylation events caused by TMZ,38–40 inducing DNA mismatch repair. The resulting double-strand breaks ultimately drive the cell to undergo apoptosis.41,42 The only cellular mechanism capable of repairing these adducts is the MGMT enzyme, which is irreversibly inactivated in this process, such that new MGMT protein synthesis is required.43,44 The cellular levels of MGMT affect the cytotoxicity of TMZ and play an important role in response to this chemotherapy agent. MGMT expression in tumor cells is regulated by epigenetic silencing of the gene, via hypermethylation of its promoter region. Both succinate45 and d-2-hydroxyglutarate46 are considered oncometabolites. Increased levels of succinate or d-2-hydroxyglutarate derive in epigenetic remodeling.47 As a consequence, the hypermethylator phenotypes of SDH-related PGL14 and IDH-related glioblastoma (GBM)46 result in overlapping. In both, hypermethylation of the promoter of the MGMT gene is frequently observed. However, MGMT is also depleted in normal cells, particularly hematopoietic stem cells, resulting in hematologic toxicity. Thus, the therapeutic window is largest in tumor cells with hypermethylated MGMT promoter, which effectively silences the gene.48,49 The benefit of MGMT gene silencing in patients with glioma undergoing chemotherapy with TMZ is well elucidated.26
Efforts to deplete MGMT activity to increase the cytotoxic potential of alkylating agents led to exploration of metronomic TMZ schedules in patients with glioma26,50,51 with the goal of improving antitumor activity and overcoming resistance.52–55 Several regimens consisting of MS TMZ in both newly diagnosed and recurrent gliomas as well as in melanoma have been investigated.52–56 In patients with GBM, retreatment with MS was shown to be safe and active at various doses including the 7/14-day regimen,51 the 21/28-day regimen,26 and the daily TMZ at 50 mg/m2/d.50 Among these, Brandes et al26 were the first to try to show correlation between MGMT promoter methylation status and treatment outcome. Unexpectedly, no significant difference was found between MGMT promoter methylation status and median progression-free survival (PFS) outcomes at 6 months in this trial (15.6 weeks and 20% for MGMT promoter methylation and unmethylation vs 11.9 weeks and 21.4%, respectively). In addition, the authors suggested that MGMT depletion achieved with extended TMZ increased the sensitivity of the unmethylated tumor. Subsequently, Wick et al52 obtained similar results and speculated that the negative findings were due to the effect of MS TMZ in the tumor microenvironment rather than the cytotoxic effect of TMZ. More recently, MS TMZ schedules showed also to improve survival in newly diagnosed GBMs56 were, again, no additional benefit has been observed in patients with methylated MGMT. However, these observations were based on a small number of patients26 and there were no functional investigations conducted in any of these 3 studies.
The increase in MS TMZ efficacy has been effectively clarified in other reports and can be explained by the activation of several mechanisms. These would include the inhibition of angiogenesis,57 MGMT activity depletion,58 or downregulation of nuclear factor κB (NF-κB)/p65 binding activity in epidermal growth factor receptor (EGFR)-overexpressing GBMs, independently of MGMT methylation status.59 This mechanism is even more evident in GBMs with phosphatase and tensin homolog (PTEN) loss59 as PTEN represents a major inhibitor of the EGFR/phosphoinositide 3-kinase/protein kinase B (EGFR/PI3K/Akt) pathway.
Regarding MS efficacy in mPHEO/PGL, one prior publication reported 2 patients who responded to metronomic doses of cyclophosphamide.60 Of these, only 1 patient was screened and was found positive for a germline VHL mutation, although no additional translational information was reported.60
To our knowledge, these are the first case reports showing the efficacy of metronomic TMZ in patients with mPGL who have been previously pretreated with SS dacarbazine-based chemotherapy and had progressed on it. Both patients responded well to therapy, with notable increases in PFS for both patients. In addition to the clinical benefit with complete resolution of symptoms, a continuous metabolic response after at least 12 months of treatment was noted. One patient remains under treatment with a 4 times durable response compared with previous conventional CVD. Whether the concomitant high doses of lanreotide played a synergistic role remains unclear. The combination proved to be safe and no adverse effects attributed to the addition of the second agent were noted. In our opinion, this regimen has promising activity and offers a judicious possibility of retreatment with TMZ in patients progressing on CVD chemotherapy or conventional TMZ.
This report highlights the efficacy of this regimen, even in patients with poor performance status, and opens new treatment scenarios for patients with mPHEO/PGL. Whether patients without MGMT methylation could also benefit from metronomic TMZ remains unelucidated and should be evaluated considering the results for patients with GBM. Therefore, we think that metronomic TMZ should be explored in MGMT promoter–unmethylated patients in prospective studies with patients stratified according to methylation status.
As mentioned above, MS TMZ efficacy relies also in the inhibition of angiogenesis57 and the downregulation of NF-κB/p65 binding activity in EGFR-overexpressing GBMs independently of MGMT methylation status.59 It is worthy to say that in our 2 patients, EGFR expression was negative (Figure 1). However, if patients with unmethylated MGMT mPHEO/PGL could present with EGFR-overexpressing tumors and could respond to MS TMZ remains something worthy to explore.
Metronomic treatment has some advantages over other salvage chemotherapies for progressive mPHEO/PGL as it offers better compliance than other intravenous treatments. In addition, treatment-related toxicities in our report were only related to manageable lymphopenia.
Conclusions
Few cases of mPHEO/PGL treated with MS have been described so far. This report suggests that a modification in the scheme of TMZ to MS is feasible and safe in patients with mPHEO/PGL refractory to conventional regimens with intravenous dacarbazine or oral TMZ.
We postulate that MS TMZ should be considered as a second-line regimen in patients with methylated MGMT mPHEO/PGL at the TTP to standard CVD chemotherapy or conventional TMZ schedules.
Whether patients without MGMT methylation could also benefit from metronomic TMZ remains unclear and should be explored in well-designed clinical trials.
Supplemental Material
Supplemental material, Supplementary_data for Successful Second-Line Metronomic Temozolomide in Metastatic Paraganglioma: Case Reports and Review of the Literature by Isabel Tena, Garima Gupta, Marcos Tajahuerce, Marta Benavent, Manuel Cifrián, Alejandro Falcon, María Fonfria, Maribel del Olmo, Rosa Reboll, Antonio Conde, Francisca Moreno, Julia Balaguer, Adela Cañete, Rosana Palasí, Pilar Bello, Alfredo Marco, José Luis Ponce, Juan Francisco Merino, Antonio Llombart, Alfredo Sanchez and Karel Pacak in Clinical Medicine Insights: Oncology
Acknowledgments
The authors would like to thank the PHEiPAS Alliance and the NIH for their support with patient care. They thank BioSequence SL, Valencia, Spain, for using the OncoDEEP solution (OncoDNA SA, Gosselies, Belgium) for performing genomic profiling of the paraffin-embedded metastatic tumor tissue from the 2 patients.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Spanish Society of Medical Oncology (SEOM grant for 2 years in a foreign reference center, 2015) and the PHEiPAS patient Alliance (2016).
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors Contributions: IT and GG analyzed the data, wrote the first draft of the manuscript, and jointly developed the structure and arguments for the paper. IT, GG, and KP contributed to the writing of the manuscript. IT, GG, MT, MB, MC, AF, MF, MdO, RR, AC, FM, JB, AC, RP, AM, JP, JFM, AS, AL, and KP agree with manuscript results and conclusions and made critical revisions and approved final version. All authors reviewed and approved the final manuscript.
References
- 1. Eisenhofer G, Bornstein SR, Brouwers FM, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423–436. [DOI] [PubMed] [Google Scholar]
- 2. Gimenez-Roqueplo AP, Favier J, Rustin P, et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615–5621. [PubMed] [Google Scholar]
- 3. Ayala-Ramirez M, Feng L, Johnson MM, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96:717–725. [DOI] [PubMed] [Google Scholar]
- 4. Jimenez C, Rohren E, Habra MA, et al. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep. 2013;15:356–371. [DOI] [PubMed] [Google Scholar]
- 5. Burnichon N, Buffet A, Gimenez-Roqueplo AP. Pheochromocytoma and paraganglioma: molecular testing and personalized medicine. Curr Opin Oncol. 2016;28:5–10. [DOI] [PubMed] [Google Scholar]
- 6. Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44:328–333. [DOI] [PubMed] [Google Scholar]
- 7. Mercado-Asis LB, Wolf KI, Jochmanova I, Taieb D. Pheochromocytoma: a genetic and diagnostic update. Endocr Pract. 2017;24:78–90. [DOI] [PubMed] [Google Scholar]
- 8. Amar L, Bertherat J, Baudin E, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005;23:8812–8818. [DOI] [PubMed] [Google Scholar]
- 9. Neumann HP, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–951. [DOI] [PubMed] [Google Scholar]
- 10. Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. [DOI] [PubMed] [Google Scholar]
- 11. Pollard PJ, Briere JJ, Alam NA, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. [DOI] [PubMed] [Google Scholar]
- 12. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. [DOI] [PubMed] [Google Scholar]
- 13. Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Letouze E, Martinelli C, Loriot C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–752. [DOI] [PubMed] [Google Scholar]
- 15. Loriot C, Burnichon N, Gadessaud N, et al. Epithelial to mesenchymal transition is activated in metastatic pheochromocytomas and paragangliomas caused by SDHB gene mutations. J Clin Endocrinol Metab. 2012;97:E954–E962. [DOI] [PubMed] [Google Scholar]
- 16. Tanabe A, Naruse M, Nomura K, Tsuiki M, Tsumagari A, Ichihara A. Combination chemotherapy with cyclophosphamide, vincristine, and dacarbazine in patients with malignant pheochromocytoma and paraganglioma. Horm Cancer. 2013;4:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel SR, Winchester DJ, Benjamin RS. A 15-year experience with chemotherapy of patients with paraganglioma. Cancer. 1995;76:1476–1480. [DOI] [PubMed] [Google Scholar]
- 18. Huang H, Abraham J, Hung E, et al. Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer. 2008;113:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deutschbein T, Fassnacht M, Weismann D, Reincke M, Mann K, Petersenn S. Treatment of malignant phaeochromocytoma with a combination of cyclophosphamide, vincristine and dacarbazine: own experience and overview of the contemporary literature. Clin Endocrinol (Oxf). 2015;82:84–90. [DOI] [PubMed] [Google Scholar]
- 20. Ayala-Ramirez M, Feng L, Habra MA, et al. Clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas or sympathetic extra-adrenal paragangliomas: insights from the largest single-institutional experience. Cancer. 2012;118:2804–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Averbuch SD, Steakley CS, Young RC, et al. Malignant pheochromocytoma: effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Ann Intern Med. 1988;109:267–273. [DOI] [PubMed] [Google Scholar]
- 22. Asai S, Katabami T, Tsuiki M, Tanaka Y, Naruse M. Controlling tumor progression with cyclophosphamide, vincristine, and dacarbazine treatment improves survival in patients with metastatic and unresectable malignant pheochromocytomas/paragangliomas. Horm Cancer. 2017;8:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hadoux J, Favier J, Scoazec JY, et al. SDHB mutations are associated with response to temozolomide in patients with metastatic pheochromocytoma or paraganglioma. Int J Cancer. 2014;135:2711–2720. [DOI] [PubMed] [Google Scholar]
- 24. Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. [DOI] [PubMed] [Google Scholar]
- 26. Brandes AA, Tosoni A, Cavallo G, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from gruppo italiano cooperativo di neuro-oncologia (GICNO). Br J Cancer. 2006;95:1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piedbois P, Rougier P, Buyse M, et al. ; Meta-analysis Group in Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16:301–308. [DOI] [PubMed] [Google Scholar]
- 28. Muthusamy P, Chary KV, Nalini GK. Metronomic chemotherapy: seems prowess to battle against cancer in current scenario. J Clin Diagn Res. 2016;10:FC09–FC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelley RK, Hwang J, Magbanua MJ, et al. A phase 1 trial of imatinib, bevacizumab, and metronomic cyclophosphamide in advanced colorectal cancer. Br J Cancer. 2013;109:1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jellvert A, Lissbrant IF, Edgren M, et al. Effective oral combination metronomic chemotherapy with low toxicity for the management of castration-resistant prostate cancer. Exp Ther Med. 2011;2:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klement G, Baruchel S, Rak J, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hao YB, Yi SY, Ruan J, Zhao L, Nan KJ. New insights into metronomic chemotherapy-induced immunoregulation. Cancer Lett. 2014;354:220–226. [DOI] [PubMed] [Google Scholar]
- 33. Bissell MJ, Hines WC. Why don’t we get more cancer? a proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nature reviews Cancer. 2005;5:516–525. [DOI] [PubMed] [Google Scholar]
- 35. Kaneno R, Shurin GV, Kaneno FM, Naiditch H, Luo J, Shurin MR. Chemotherapeutic agents in low noncytotoxic concentrations increase immunogenicity of human colon cancer cells. Cell Oncol (Dordr). 2011;34:97–106. [DOI] [PubMed] [Google Scholar]
- 36. Kovacs JA, Masur H. Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N Engl J Med. 2000;342:1416–1429. [DOI] [PubMed] [Google Scholar]
- 37. Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trivedi RN, Almeida KH, Fornsaglio JL, Schamus S, Sobol RW. The role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell death. Cancer Res. 2005;65:6394–6400. [DOI] [PubMed] [Google Scholar]
- 39. Tentori L, Graziani G. Pharmacological strategies to increase the antitumor activity of methylating agents. Curr Med Chem. 2002;9:1285–1301. [DOI] [PubMed] [Google Scholar]
- 40. Liu L, Taverna P, Whitacre CM, Chatterjee S, Gerson SL. Pharmacologic disruption of base excision repair sensitizes mismatch repair-deficient and -proficient colon cancer cells to methylating agents. Clin Cancer Res. 1999;5:2908–2917. [PubMed] [Google Scholar]
- 41. Roos WP, Batista LF, Naumann SC, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. [DOI] [PubMed] [Google Scholar]
- 42. Bignami M, O’Driscoll M, Aquilina G, Karran P. Unmasking a killer: DNA O(6)-methylguanine and the cytotoxicity of methylating agents. Mutat Res. 2000;462:71–82. [DOI] [PubMed] [Google Scholar]
- 43. Pegg AE, Dolan ME, Moschel RC. Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol. 1995;51:167–223. [DOI] [PubMed] [Google Scholar]
- 44. Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189–4199. [DOI] [PubMed] [Google Scholar]
- 45. Yang M, Pollard PJ. Succinate: a new epigenetic hacker. Cancer Cell. 2013;23:709–711. [DOI] [PubMed] [Google Scholar]
- 46. Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 48. Watts GS, Pieper RO, Costello JF, Peng YM, Dalton WS, Futscher BW. Methylation of discrete regions of the O6-methylguanine DNA methyltransferase (MGMT) CpG island is associated with heterochromatinization of the MGMT transcription start site and silencing of the gene. Mol Cell Biol. 1997;17:5612–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59:793–797. [PubMed] [Google Scholar]
- 50. Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28:2051–2057. [DOI] [PubMed] [Google Scholar]
- 51. Galldiks N, Berhorn T, Blau T, Dunkl V, Fink GR, Schroeter M. “One week on-one week off”: efficacy and side effects of dose-intensified temozolomide chemotherapy: experiences of a single center. J Neurooncol. 2013;112:209–215. [DOI] [PubMed] [Google Scholar]
- 52. Wick A, Felsberg J, Steinbach JP, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25:3357–3361. [DOI] [PubMed] [Google Scholar]
- 53. Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. [DOI] [PubMed] [Google Scholar]
- 54. Middleton MR, Lee SM, Arance A, Wood M, Thatcher N, Margison GP. O6-methylguanine formation, repair protein depletion and clinical outcome with a 4 hr schedule of temozolomide in the treatment of advanced melanoma: results of a phase II study. Int J Cancer. 2000;88:469–473. [PubMed] [Google Scholar]
- 55. Lee SM, Thatcher N, Crowther D, Margison GP. Inactivation of O6-alkylguanine-DNA alkyltransferase in human peripheral blood mononuclear cells by temozolomide. Br J Cancer. 1994;69:452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27:3861–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. [DOI] [PubMed] [Google Scholar]
- 58. Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88:1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cominelli M, Grisanti S, Mazzoleni S, et al. EGFR amplified and overexpressing glioblastomas and association with better response to adjuvant metronomic temozolomide. J Nat Cancer Inst. 2015;107:djv041. [DOI] [PubMed] [Google Scholar]
- 60. Gillon P, Godbert Y, Dupin C, Bubien V, Italiano A, Roubaud G. Long clinical benefit achieved in two patients with malignant paraganglioma treated by metronomic cyclophosphamide. Future Oncol. 2014;10:2121–2125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_data for Successful Second-Line Metronomic Temozolomide in Metastatic Paraganglioma: Case Reports and Review of the Literature by Isabel Tena, Garima Gupta, Marcos Tajahuerce, Marta Benavent, Manuel Cifrián, Alejandro Falcon, María Fonfria, Maribel del Olmo, Rosa Reboll, Antonio Conde, Francisca Moreno, Julia Balaguer, Adela Cañete, Rosana Palasí, Pilar Bello, Alfredo Marco, José Luis Ponce, Juan Francisco Merino, Antonio Llombart, Alfredo Sanchez and Karel Pacak in Clinical Medicine Insights: Oncology