Abstract
Ultraviolet-B (UV-B) radiation can have a negative impact on the growth and development of plants. Plants tolerant to UV-B alleviate these effects using UV-screening pigments that reduce the penetration of UV-B into mesophyll tissue. Little is known about the relative contribution of specific phenolic compounds to the screening capacity of leaves. The D1 and D2 proteins constituting the photosystem (PS) II reaction center heterodimer are targets of UV-B radiation and can be used as an in situ sensor for UV penetration into photosynthetic tissue. Degradation of these proteins occurs under very low fluences of UV-B, and is strongly accelerated in the presence of visible light. Using the D1-D2 degradation assay, we characterized UV-B sensitivity of Arabidopsis mutants (tt4, tt5, and fah1) that are genetically altered in their composition of phenolic compounds. We found that changes in phenol metabolism result in altered rates of PSII reaction center heterodimer degradation under mixtures of photosynthetically active radiation and UV-B. A comparison of D2 degradation kinetics showed increased UV sensitivity of the Landsberg (Landsberg erecta) tt5 mutant relative to the Landsberg tt4 mutant and the Landsberg wild type. Despite a lack of flavonoid accumulation, the tt4 mutant is not particularly UV sensitive. However, the tolerance of this mutant to UV-B may reflect the increased accumulation of sinapate esters that strongly absorb in the UV range, and may thus protect the plant against environmentally relevant UV-B radiation. This sinapate-mediated protection is less obvious for the tt4 mutant of Columbia ecotype, indicating that the relative contribution of particular phenolics to the total screening capacity varies with the genetic background. The role of sinapate esters in UV screening is further substantiated by the results with the fah1 mutant where absence of most of the sinapate esters results in a significantly accelerated degradation of D2 under mixed light conditions. Because the latter mutant is not expected to be deficient in flavonoids, the relative contribution of flavonoids as protectants of PSII reaction center heterodimer against UV-B damage in Arabidopsis needs to be re-evaluated vis-a-vis screening by simple phenolics like sinapate esters.
UV-B (280–320 nm) radiation is a minor component of the solar spectrum in the biosphere. Yet UV-B has the potential to disproportionately affect metabolic processes in the plant including growth, morphology, photosynthesis, flowering, pollination, and transpiration (Jordan, 1996; Jansen et al., 1998). Major molecular targets include DNA and RNA, proteins, lipids, and pigments (Jordan, 1996; Jansen et al., 1998). Understanding the mechanism(s) by which these targets are damaged, repaired, and/or protected is important for understanding the ecophysiological role of UV-B radiation. Detrimental effects of UV-B radiation on photosynthesis have been characterized in considerable detail. These effects include inactivation of photosystem II (PSII), reduced activity of Rubisco, decreased levels of chlorophylls and carotenoids, down-regulation of transcription of photosynthetic genes, and decreased thylakoid integrity and altered chloroplast ultrastructure (Friso et al., 1994; Strid et al., 1994; Teramura and Sullivan, 1994; Greenberg et al., 1996; Jansen et al., 1996a; Vass et al., 1996).
PSII is a protein-pigment complex, the reaction center core of which is formed by the D1 and D2 proteins (Mattoo et al., 1989, 1999; Barber et al., 1997). The D1 and D2 reaction center proteins are extremely UV sensitive, and degradation is driven by UV-B fluence rates as low as 1 μmol m−2 s−1 (Jansen et al., 1996a). UV-driven D1-D2 degradation is strongly accelerated in the presence of a background of visible radiation. The accelerated turnover of D2, as well as D1, under mixtures of UV-B radiation and photosynthetically active radiation (PAR), contrasts with the stability of the D2 protein under (excessive) flux densities of PAR alone (Jansen et al., 1996a; Babu et al., 1999). The UV-B-driven degradation of the D1-D2 proteins may be, but is not necessarily, accompanied by a loss of PSII functionality, i.e. a decrease in oxygen evolution or in variable chlorophyll fluorescence.
The environmental relevance of UV-B effects on photosynthesis is not clear. Many of the detrimental UV-B effects observed under laboratory conditions are not obvious under field conditions (Fiscus and Booker 1995; Rozema et al., 1997; Jansen et al., 1998). Plants respond to UV-B by balancing reactions that lead to damage, repair, and acclimation. A likely reason underlying the discrepancy between laboratory and field studies is a failure to take into consideration the naturally occurring tolerance mechanisms (Fiscus and Booker 1995; Rozema et al., 1997; González et al., 1998; Jansen et al., 1998). In a converse manner, the effects of UV-B on photosynthesis offer a convenient means to screen for repair and acclimation responses that can confer UV tolerance. We have assessed the role of UV-screening pigments in protecting chloroplast metabolism against UV-B radiation in the presence or absence of a background of PAR using the UV-sensitive D1-D2 protein degradation assay as a sensor for UV penetration. In comparison to the more common measurements of photosynthetic electron flow and/or efficiency of photosynthetic light utilization, this assay has several advantages: (a) It is only to a minor extent affected by non-physiological UV-C wavelengths (Greenberg et al., 1989; Jansen et al., 1996b); (b) in healthy plants, the response is triggered by a low threshold fluence (1 μmol m−2 s−1) of UV-B (Jansen et al., 1996a); (c) the degradation response is not diminished by a physiologically relevant background of PAR (Jansen et al., 1996b; Babu et al., 1999); and (d) the measured bonafide in vivo pulse-chase response directly reflects damage, i.e. not a steady-state balance comprised of damage and repair reactions.
UV-B attenuation is mainly attributed to flavonoids and related phenolic compounds that absorb UV-B radiation effectively while transmitting PAR to the chloroplasts (Caldwell et al., 1983; Li et al., 1993; Lois and Buchanan, 1994; Landry et al., 1995; Shirley et al., 1995; Reuber et al., 1996). Levels of these complex phenolic compounds vary considerably between plant species, with developmental stage, and with differing environmental conditions such as visible radiation levels, water, and nutrient supply (Caldwell, 1971; Murali and Teramura, 1985, 1986). In addition, exposure to UV-B radiation may increase the concentration of UV-B-absorbing compounds in the epidermis, rendering some plants less susceptible to photosynthetic damage due to UV-B exposure. Oilseed rape plants when pre-adapted to grow in light supplemented with UV-B, developed tolerance to UV-B (Wilson and Greenberg, 1993). These plants, which had elevated levels of epidermal flavonoids, were also observed to have an increased half-life of the UV-B-sensitive PSII D1 protein.
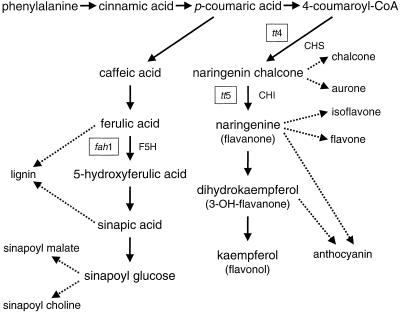
Arabidopsis mutants defective in the production of flavonoids have been successfully used in assessing the general effects of UV-B on plant growth, oxidative damage (Landry et al., 1995), and DNA repair (Landry et al., 1997). Although these studies have clearly demonstrated a general relationship between UV tolerance and flavonoid content, questions remain concerning (a) the extrapolation to different species, cultivars or ecotypes; (b) the protection of specific molecular targets; and (c) the relative contribution of specific (classes of) phenolics to the screening capacity. Using the D1-D2 degradation assay, we analyzed Arabidopsis mutants altered in the accumulation of aromatic secondary products. We tested the wild type along with mutants blocked in the function of either chalcone synthase (CHS; tt4), chalcone-flavanone isomerase (CHI; tt5) or ferulate-5-hydroxylase (F5H; fah1). The fah1 mutant and one tt4 mutant (tt4–2YY6) are derived from the Columbia ecotype, whereas the second tt4 mutant (tt4-W85) and the tt5 mutant are derived from the Landsberg (Landsberg erecta) ecotype. Exposure of some of these mutants to UV-B has previously been shown to result in macroscopic damage (Li et al., 1993; Landry et al., 1995), as well as general protein and lipid breakdown (Landry et al., 1995). Our study of a specific, well-defined UV-B target indicates that sinapate esters have a protective role against UV-B radiation damage and that the ecotype background is a key parameter that determines the quantitative effect of any knockout mutation.
RESULTS
The relationship between the flavonoid constituents in plants and the ability to protect against UV-B damage was tested in Arabidopsis mutants impaired in flavonoid biosynthesis. The PSII D1-D2 protein degradation assay was used as a sensor for UV-B penetration into photosynthetic tissues. We studied Arabidopsis mutants blocked in the expression of CHS (tt4), chalcone-flavanone isomerase (tt5) or F5H (fah1) genes (see Fig. 1 for the phenylpropanoid pathway and the indicated mutations). Two mutants (tt4–2YY6 and fah1) are derived from the Columbia ecotype. The other mutants (tt4-W85 and tt5) are derived from the Landsberg ecotype. The fah1 mutant fails to accumulate any of the sinapic acid esters due to the loss of activity of F5H, a cytochrome P450-dependent monooxygenase (Chapple et al., 1992). The tt4 and tt5 mutants are deficient in flavonoids, but the tt4 mutant has 40% higher levels of sinapate esters (Li et al., 1993). The tt4–2YY6 lesion is a point mutation in the non-coding region precluding the production of correctly spliced transcripts. The tt4-W85 has a point mutation in the coding region and the transcripts produced are of normal size and abundance. This could lead to leakiness of this mutant and some enzyme activity may be generated leading to flavonol accumulation below the level of detection. The tt5 mutant has reduced levels of sinapoylmalate in addition to flavonoid deficiency (Li et al., 1993; Table I).
Figure 1.
The biosynthetic pathway of plant phenolics: the intermediates and enzymes involved. Also shown are the blocked reactions in the Arabidopsis mutants used in the present study. See also Table I.
Table I.
Characteristics of the Arabidopsis mutants used in this study
| Ecotypes and Mutants | Gene Mutated | Deficiency |
|---|---|---|
| Columbia | ||
| Col (C) | None (WT) | |
| tt4-2YY6(Y) | CHS: A to G (non-coding region); splicing disrupted | CHS protein absent; increased sinapate esters; flavonoids absent |
| fah1 (F) | F5H | F5H enzyme absent; absence of sinapoylmalate, sinapoyl choline, and sinapate esters |
| Landsberg erecta | ||
| Ler (L) | None (WT) | |
| tt4-W85(W) | CHS: A to G (coding region) | CHS protein absent; flavonoids absent; increased sinapate esters |
| tt5 (T) | CHI: inversion | CHI protein absent; flavonoids absent; reduced sinapoylmalate |
The tt4-2YY6 allele contains a single nucleotide change (A to G transition) in a non-coding region that disrupts the splicing. There is no detectable CHS protein and flavonoids in these plants. The tt4-W85 allele contains a single nucleotide change in the coding region (A to G transition) that changes a Gly to a Ser residue. The levels of mRNA appear to be normal, but there are no detectable flavonoids in any tissue of these plants. The tt5 lesion consists of an inversion that results in reduced levels of mRNA and apparent loss of enzyme activity. There are no flavonoids in these plants. The fah1 mutant is modified on the F5H gene and lacks sinapate esters due to a deficiency in activity of F5H.
Changes in Phenolic Metabolism Alter UV-B-Driven Degradation of the D1 and D2 PSII Proteins
Exposure of Columbia or Landsberg leaves to UV-B radiation alone led to a rapid degradation of the D1 and D2 PSII proteins. Typical rate constants of D1 degradation were 0.17 and 0.25 h−1 under 0.62 and 1.24 μmol m−2 s−1 UV-B, respectively (Table II). This translates into half-life times of 6 and 4 h, respectively. D1 degradation is equally rapid in Columbia and Landsberg. In the UV-exposed mutants, D1 degradation appears slightly accelerated, but in most cases this effect is not significant (Table II).
Table II.
Rate constants of degradation of D1 and D2 PSII proteins in wild-type and mutant Arabidopsis plants exposed to low and high UV-B radiation
| Ecotype | Rate Constants of Protein Degradation
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Low UV-Ba
|
High UV-B
|
|||||||
| D1 | s.e.b | D2 | s.e. | D1 | s.e. | D2 | s.e. | |
| h−1 | ||||||||
| Columbia WT | 0.17 | 0.03 | 0.03 | 0.01 | 0.20 | 0.04 | 0.09 | 0.02 |
| Col. fah1 | 0.17 | 0.03 | 0.07 | 0.01 | 0.28 | 0.07 | 0.11 | 0.02 |
| Col. tt4-2YY6 | 0.21 | 0.03 | 0.17 | 0.05 | 0.25 | 0.05 | 0.23 | 0.08 |
| Landsberg erecta | 0.13 | 0.02 | 0.08 | 0.01 | 0.20 | 0.03 | 0.12 | 0.07 |
| Ler. tt4-W85 | 0.19 | 0.02 | 0.07 | 0.01 | 0.26 | 0.07 | 0.12 | 0.01 |
| Ler. tt5 | 0.16 | 0.04 | 0.12 | 0.01 | 0.32 | 0.10 | 0.24 | 0.08 |
Low UV-B represents 0.62 μmol m−2 s−1 and high UV-B represents 1.24 μmol m−2 s−1.
s.e., Standard error (n = 3–8).
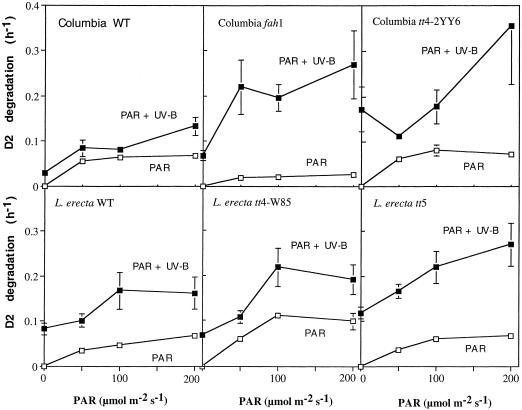
Degradation of the D2 protein is slower than that of the D1 protein in UV-exposed leaves. Rate constants of D2 degradation are in the range of 0.06 and 0.10 h−1 under 0.62 and 1.24 μmol m−2 s−1 UV-B, respectively, for Columbia and Landsberg leaves (Table II). This translates into half-life times of 17 and 10 h, respectively. D2 degradation is clearly more rapid in Landsberg than in Columbia. An additional increase in UV sensitivity is noted in the Landsberg tt5 mutant. It is interesting that the tt4 mutation from Landsberg ecotype has no effect, whereas it leads to a strong acceleration of D2 degradation in tt4 mutant from the Columbia ecotype. In comparison, the effects of the fah1 mutation in Columbia are minor.
Visible Light Has Similar Effects in Columbia and Landsberg Ecotypes
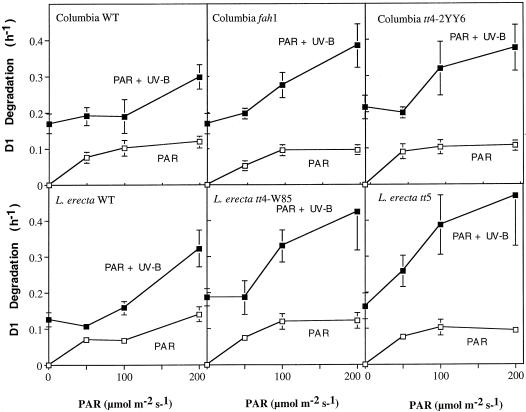
The effects of UV-B given alone may give a first indication about the UV sensitivity of the photosynthetic apparatus. However, from an environmental relevance point of view, the sensitivity of PSII to mixtures of UV-B and PAR is more relevant. To be able to analyze the contribution of the different wavelength regions to the mixed light response we first studied the effects of PAR alone. Rates of D1 degradation increased with increasing fluence of PAR (Fig. 2). D1 degradation was equally rapid in Columbia and Landsberg. Moreover, rates of D1 degradation did not significantly differ between the indicated mutants and their controls. D2 degradation also increased with increasing fluences of PAR and the rates were similar for the two wild-type Arabidopsis lines (Fig. 3). However, we note that visible light driven D2 degradation was significantly slowed down in the fah1 mutant.
Figure 2.
D1 protein degradation in Arabidopsis Columbia and Landsberg ecotypes, and in mutants fah1, tt4–2YY6, tt4-W85, and tt5. Leaves were pulse-labeled with [35S] Met for 3 h under 50 μmol m−2 s−1 PAR, rinsed, and chased for various periods of time at the PAR fluence indicated, in the presence (0.62 μmol m−2 s−1) or absence of a background of UV-B radiation. Following the exposure to radiation, plants were homogenized and the membrane proteins were isolated and fractionated by SDS/PAGE. Radiolabeled bands were detected by autoradiography and their degradation kinetics were determined as described (Greenberg et al., 1987). Values represent averages of data from several independent experiments (n = 4–8). se of the mean are shown.
Figure 3.
D2 protein degradation in Arabidopsis Columbia and Landsberg ecotypes, and in mutants fah1, tt4–2YY6, tt4-W85, and tt5. Leaves were pulse-labeled and chased as described in the legend to Figure 1. Leaves were exposed to the PAR fluence indicated, in the presence (0.62 μmol m−2 s−1) or absence of a background of UV-B radiation. Values represent averages of data from several independent experiments (n = 4–8). se of the mean are shown.
Alterations in Phenolic Metabolism Result in Accelerated PSII Reaction Center Heterodimer Degradation under Environmentally Relevant Mixtures of PAR and UV-B
It is important to determine effects of and mechanisms relevant to UV-B damage in terms of the plant's sensitivity to mixtures of UV-B and PAR. Leaves were, therefore, exposed to combinations of PAR and UV-B. Under these mixed light conditions, D1 degradation sharply accelerated with increasing levels of PAR (Fig. 2). In particular, at the highest level of PAR, D1 degradation is faster than would be predicted from the breakdown rates seen under PAR or UV-B alone. Such a synergistic stimulation of D1 degradation was found to be similar in the Columbia and Landsberg ecotypes. In the mutants, D1 degradation was even further accelerated relative to the controls. However, no significant differences were observed among the mutants.
Mixtures of UV-B and PAR also disproportionately boosted the degradation of the D2 protein (Fig. 3). Under mixed light conditions, D2 degradation appears slightly faster in Landsberg ecotype as compared with Columbia. Further acceleration occurs in the Landsberg tt5 mutant, particularly under the highest level of PAR. D2 degradation was not significantly increased under mixed light conditions in the Landsberg tt4 mutant relative to the Landsberg ecotype. These results resemble the UV-B-induced responses of the Landsberg lines measured in the absence of PAR (Table II). The relative lack of effect in the Landsberg tt4 knock out contrasts with the strong acceleration of D2 breakdown in the Columbia tt4 mutant under mixed light conditions. Results obtained under mixed light conditions resemble the response to UV-B alone. The D2 degradation response is also strongly accelerated in the fah1 mutant exposed to the mixed light conditions. However, in this case the acceleration of D2 degradation contrasts with the low rates of D2 degradation observed under either PAR or UV-B alone.
DISCUSSION
The D2 Degradation Reaction as a Sensor for UV-B Radiation
Despite functional and structural analogies between the D1 and D2 proteins of PSII, there are striking differences in the responses of these proteins to visible and/or UV-B radiation. The relative stability of the D2 protein under visible radiation contrasts with the rapid turnover of the D1 protein under these conditions. D2 degradation is strongly accelerated under UV-B radiation or mixtures of UV-B and PAR, such that its rate of degradation approaches that of the D1 protein. These observations have led to the hypothesis that the D2 protein, rather than D1, is a specific sensor for UV-B penetration. Here we do not see such an UV-specific acceleration of D2 degradation, although it is only at the level of D2 degradation that we were able to note the differences in UV sensitivity between Columbia and Landsberg ecotypes. Also, it is only at the level of D2 degradation that we observed altered UV sensitivity of tt4, tt5, or fah1 lines relative to their controls.
Fastest D2 degradation was observed under mixtures of UV-B and PAR. In particular, the combination of UV-B with the highest fluence of PAR triggered a rate of D2 degradation that is higher than would be predicted from the rates under PAR or UV-B alone (Figs. 2 and 3). Synergistic acceleration of D2 degradation was previously observed under a mixture of UV-B and a level of PAR that was high enough (1,000 μmol m−2 s−1) to saturate photosynthesis (Jansen et al., 1996a). It was concluded that the synergistic stimulation of UV-driven D2 (and D1) degradation under such background levels of PAR is potentially of environmental relevance, emphasizing the need for testing for UV sensitivity under mixed light conditions. A comparison of the magnitude of synergistically accelerated degradation, vis-a-vis rates under either UV-B or PAR, demonstrates this point, and is particularly underlined by our data on the fah1 mutant. Relatively low rates of UV-B-driven D1-D2 degradation in the fah1 mutant (Table II) might have led us to believe that the mutation has no effect on UV sensitivity. However, D1-D2 degradation is strikingly accelerated in this mutant under the mixed light conditions (Figs. 2 and 3).
Stimulation of D1-D2 Degradation under Mixed Light Conditions
The acceleration of D1-D2 degradation observed under mixtures of PAR and UV is particularly clear under the highest background of PAR used (Figs. 2 and 3). A synergistic acceleration of D2-degradation was previously noted to occur in Spirodela under a background fluence of 1,000 μmol m−2 s−1 PAR (Jansen et al., 1996a; Babu et al., 1999). A more moderate acceleration of D2 degradation was seen also in Spirodela under a background fluence of 50 μmol m−2 s−1 (Babu et al., 1999). UV-driven D1 degradation can be accelerated by a background fluence of merely 3 μmol m−2 s−1 red light (Jansen et al., 1993). The acceleration of the UV-driven D1-D2 degradation response at high levels of PAR contrasts sharply with many other UV effects that are, on the contrary, moderated in the presence of increasing background levels of PAR (Cen and Bornman, 1990). It is unclear what is the underlying mechanism of this unique response. The synergistic stimulation of D1-D2 degradation has been speculated to involve charged plastoquinones, tyrosines, or manganese ions, which are produced during photosynthetic electron flow (Babu et al., 1999). Thus the role of PAR in triggering accelerated degradation might be related to photosynthetic electron flow. Heat shock deactivation of the donor side of PSII nullifies the accelerated D1-D2 degradation that normally occurs under mixed light conditions (Babu et al., 1999). Singly reduced plastoquinones have long been suspected to be potential UV photosensitizers (Greenberg et al., 1989; Jansen et al., 1993). A very low rate of electron flow would be sufficient to yield a steady-state level of reduced quinone or oxidized Tyr. It is remarkable that our present data show that increasing fluences of PAR increasingly amplify D1-D2 degradation under mixed light conditions. Thus our data seem to question a possible role of singly reduced plastoquinone as the UV photosensitizer.
The Genetic Background Is a Key Factor That Governs the Effects of Mutations in the Phenylpropanoid Pathway
Exploiting the possibilities offered by the D1-D2 degradation assay, we have determined the effects of differences in genetic background and of blockages in the phenylpropanoid pathway on UV sensitivity of Arabidopsis. Despite the identical growth conditions, we have found that Columbia is more UV tolerant than Landsberg. Increased tolerance, i.e. reduced rates of D2 degradation, are noted under UV-B alone as well as under mixed light conditions. It is striking that the Columbia tt4 mutant is significantly more UV sensitive than the parental line. Yet the Landsberg tt4 mutant is only very slightly more UV sensitive than the parental line. Thus with regard to UV screening potential, it appears that either the CHS-gene product plays a more critical role in Columbia than in Landsberg or Columbia is better equipped to redirect the flow of phenolic intermediates in response to the genetic inactivation of CHS. These data demonstrate the potential importance of the genetic background when testing for UV sensitivity. An alternative explanation is that the different responses observed for the two tt4 mutants reflects the severity of their enzymatic defects (Li et al., 1993). The tt4–2YY6 lesion is a point mutation in the non-coding region and therefore correctly spliced transcript is not produced. The tt4-W85 lesion is a CHS point mutation in the coding region and the transcript produced is of normal size and abundance. This could be a leaky mutation that produces a low level of enzyme activity, leading to flavonol accumulation below the level of detection. This hypothesis could be verified if a known null tt4 mutation, such as a deletion allele, were available for testing.
A possible role for sinapate esters as UV screens, first postulated by Landry et al. (1995), is supported by the results with fah1 mutant where absence of most of the sinapate esters results in markedly accelerated degradation of D2 under mixed light conditions. This sinapate-mediated protection is not obvious, however, in the mutants from Columbia background. Comparison of the degradation rates of the D2 protein in the tt4 and tt5 mutants from Landsberg genetic background do support a protective role for sinapate esters against environmentally relevant UV-B radiation (i.e. under mixed light conditions), since both are deficient in flavonoids, but only tt4 accumulates sinapate esters. The difference in UV sensitivity between the Landsberg tt4 and tt5 mutants indicates the fluid character of the phenylpropanoid pathway. Thus two distinct genetic blockages in the major biochemical pathway leading to flavonoids result in different patterns of redistribution of the phenolic intermediates as demonstrated by the accumulation of sinapate esters in tt4 but not tt5. These secondary readjustments may well be responsible for the differential UV sensitivities of tt4 and tt5. Thus the physiological consequences of a simple genetic blockage may be hard to predict. A genetic lesion may simply result in a block in synthesis, with precursors suppressing early steps in the pathway through feed back inhibition.
In an alternative manner, redirection of the flow of phenolic precursors may lead to alteration in the composition of a population of phenolic compounds. The latter option does not necessarily affect UV screening as most phenolics screen well in the UV. It is striking that UV acclimation may involve alterations in the accumulation of several quite distinct types of phenolics. UV-B-induced accumulation of flavonoids in the epidermis has been extensively reported (Wilson and Greenberg, 1993). Levels of soluble ferulic acid (Liu et al., 1995) and total hydroxycinnamic acids (Tevini et al., 1991) also increase in response to UV. Lignin, which is mostly localized in the vascular system, increases in response to UV-B (Rozema et al., 1997). Thus different phenolic compounds may play distinct roles in UV acclimation. Our data showing UV sensitivity of the fah1 mutant, which is reportedly not deficient in flavonoids, suggest that the relative contribution of flavonoids in reducing UV penetration in Arabidopsis needs to be re-evaluated vis-a-vis screening by other phenolics. It is significant that the effect of the fah1 mutation is only significant under mixed light conditions. One has to reconcile these results with the possibility that plants use different mechanisms for protection against different light regimes (Bharti and Khurana, 1997).
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis wild-type (Landsberg [Landsberg erecta] and Columbia ecotypes) and mutant (tt4–2YY6, tt4-W85, and tt5) plants were a kind gift of Dr. B. Shirley (Virginia Polytechnic University, Blacksburg). The fah1 mutant was obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Arabidopsis plants were grown on a mixture of perlite:vermiculite:sphagnum (1:1:1) at 20°C, 85% relative humidity, and 80 μmol m−2 s−1 cool fluorescent light in a growth chamber as described (Somerville and Ogren, 1982).
In Vivo Pulse-Chase Experiments
To study D1 and D2 degradation rates, leaves from 1-month-old plants covering a 90-mm culture plate were pulse-labeled in 5 mL of one-half-strength Huntner's medium (Posner, 1967) for 3 h with 0.1 mCi/mL (1 Ci = 37 GBq) of [35S]Met under 50 μmol m−2 s−1 PAR and then rinsed. Radioactivity in proteins was chased for various periods of time in the same medium containing 1 mm non-radioactive Met (Greenberg et al., 1989) under different intensities of UV-B radiation with or without various background fluences of PAR. The higher the fluence rates applied to the plant, the shorter the irradiation times employed during the chase (Jansen et al., 1996a). After each chase period, the medium was drained and the leaves were frozen.
Radiation sources during the chase periods were as follows. In the PAR range, halogen lamps with heat absorbing glass and a 2.5-cm water filter were used. UV-B radiation was supplied by a panel of sunlamps (FS-20, Westinghouse, The Q-Panel Co., Cleveland) filtered with 0.08-mm cellulose diacetate films to eliminate any radiation below 290 nm (Sisson and Caldwell, 1975; Krizek and Koch, 1979). The spectral irradiance at leaf height under the lamps was determined with an spectroradiometer (model 742, Optronic Laboratories, Orlando, FL) equipped with a dual holographic grating. It was modified for maintaining constant temperature by the addition of Peltier heat exchange units and interfaced with a printing calculator (model 85, Hewlett-Packard, Palo Alto, CA; Murali and Teramura, 1986). In mixed light experiments, the radiation sources were placed as directly over the plant material as possible. Fluence rates were adjusted by changing the distance of the lamps to the plants. The fluence rates used in the UV-B region were adjusted to give the desired biologically effective UV-B (UV-BBE) dose using the generalized plant response action spectrum (Caldwell, 1971; Green et al., 1980; Coohill, 1989) normalized at 300 nm. For example, for 6 h of chase time at 1.24 μmol m−2 s−1 UV-B, the total UV-BBE received by the plant material would be equivalent to approximately 11.5 kJ m−2.
Thylakoid (Membrane) Preparation
Thylakoids were prepared according to standard methods (Elich et al., 1992) using ice-cold solutions. The final thylakoid pellets were suspended in a small volume of buffer A (10 mm Tricine {N-[2-hydroxy-1,1-Bis(hydroxy{methyl)ethyl]glycine}-NaOH, pH 7.8, 100 mm sorbitol, 10 mm MgCl2, and 10 mm NaCl) and chlorophyll concentrations determined in 80% (w/v) acetone (Arnon, 1949).
Electrophoresis and Immunoblots
Thylakoid proteins were solubilized in 1× sample buffer (Mattoo et al., 1981) for 1 h at room temperature. The samples were loaded on the basis of 1 μg of chlorophyll per lane and fractionated at room temperature on 10% to 20% (w/v) acrylamide gradient gels containing 5 m urea. The gels were either dried and exposed to an x-ray film or electrotransferred to nitrocellulose membranes for at least 5 h at 0.2 A. The blots were then treated using D1 or D2 specific antibodies. The blots were air dried and exposed to x-ray film. The rates of D1 and D2 degradation were quantified by densitometric scans of the x-ray films (Greenberg et al., 1987), while the steady-state level of each protein was immunologically determined (Callahan et al., 1989). The level of LHCII or the 43-kD PSII protein was taken as an internal control to normalize the data. The in vivo half-life times were determined for each individual chase time point, by comparison with the 0 h time point, and assuming first-order kinetics (Greenberg et al., 1989). The reciprocal of the half-life time was used (compare with Figures) as a measure of degradation rate.
ACKNOWLEDGMENTS
We thank Dr. Brenda Shirley for the gifts of some Arabidopsis mutants and for comments on the manuscript, and Dr. Richard Sayre for the gift of D2 antibodies.
LITERATURE CITED
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu TS, Jansen MAK, Greenberg BM, Gaba V, Malkin S, Mattoo AK, Edelman M. Amplified degradation of photosystem II D1 and D2 proteins under a mixture of photosynthetically active radiation and UV-B radiation: dependence on redox status of photosystem II. Photochem Photobiol. 1999;69:553–559. [Google Scholar]
- Barber J, Nield J, Morris EP, Zheleva D, Hankamer B. The structure, function and dynamics of photosystem two. Physiol Plant. 1997;100:817–827. [Google Scholar]
- Bharti AK, Khurana JP. Mutants of Arabidopsis as tools to understand the regulation of phenylpropanoid pathway and UV-B protection mechanisms. Photochem Photobiol. 1997;65:765–776. doi: 10.1111/j.1751-1097.1997.tb01923.x. [DOI] [PubMed] [Google Scholar]
- Caldwell MM. Solar UV irradiation and the growth and development of higher plants. In: Giese AC, editor. Photophysiology. Vol. 6. New York: Academic Press; 1971. pp. 131–177. [Google Scholar]
- Caldwell MM, Robberecht R, Flint SD. Internal filters: prospects for UV-acclimation in higher plants. Physiol Plant. 1983;58:445–450. [Google Scholar]
- Callahan FE, Wergin WP, Nelson N, Edelman M, Mattoo AK. Distribution of thylakoid proteins between stromal and granal lamellae in Spirodela. Plant Physiol. 1989;91:629–635. doi: 10.1104/pp.91.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen YP, Bornman JF. The response of bean plants to UV-B radiation under different irradiances of background visible light. J Exp Bot. 1990;41:1489–1495. [Google Scholar]
- Chapple CCS, Vogt T, Ellis BE, Somerville CR. An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell. 1992;4:1413–1424. doi: 10.1105/tpc.4.11.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coohill TP. Ultraviolet action spectra (280 nm to 380 nm) and solar effectiveness spectra for higher plants. Photochem Photobiol. 1989;50:451–457. [Google Scholar]
- Elich TD, Edelman M, Mattoo AK. Identification, characterization, and resolution of the in vivo phosphorylated form of the D1 photosystem II reaction center protein. J Biol Chem. 1992;267:3523–3529. [PubMed] [Google Scholar]
- Fiscus EL, Booker FL. Is increased UV-B a threat to crop photosynthesis and productivity? Photosynth Res. 1995;43:81–92. doi: 10.1007/BF00042965. [DOI] [PubMed] [Google Scholar]
- Friso G, Barbato R, Giacometti GM, Barber J. Degradation of the D2 protein due to UV-B irradiation of the reaction center of photosystem II. FEBS Lett. 1994;339:217–221. doi: 10.1016/0014-5793(94)80419-2. [DOI] [PubMed] [Google Scholar]
- González R, Mepsted R, Wellburn AR, Paul ND. Non-photosynthetic mechanisms of growth reduction in pea (Pisum sativum L.) exposed to UV-B radiation. Plant Cell Environ. 1998;21:23–32. [Google Scholar]
- Green AES, Cross KR, Smith LA. Improved analytical characterization of ultraviolet skylight. Photochem Photobiol. 1980;31:59–65. [Google Scholar]
- Greenberg BM, Gaba V, Canaani O, Malkin S, Mattoo AK, Edelman M. Separate photosensitizers mediate degradation of the 32-kDa photosystem II reaction center protein in the visible and UV spectral regions. Proc Natl Acad Sci USA. 1989;86:6617–6620. doi: 10.1073/pnas.86.17.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BM, Gaba V, Mattoo AK, Edelman M. Identification of a primary in vivo degradation product of the rapidly-turning-over 32 kD protein of photosystem II. EMBO J. 1987;6:2865–2869. doi: 10.1002/j.1460-2075.1987.tb02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BM, Wilson MI, Gerhardt KE, Wilson KE. Morphological and physiological responses of Brassica napus to ultraviolet-B radiation: photomodification of ribulose-1,5-bisphosphate carboxylase/oxygenase and potential acclimation processes. J Plant Physiol. 1996;148:78–85. [Google Scholar]
- Jansen MAK, Gaba V, Greenberg BM. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci. 1998;3:131–135. [Google Scholar]
- Jansen MAK, Gaba V, Greenberg BM, Mattoo AK, Edelman M. UV-B driven degradation of the D1 reaction-center protein of photosystem II proceeds via plastosemiquinone. In: Yamamoto HY, Smith CM, editors. Photosynthetic Responses to the Environment. Rockville, MD: American Society of Plant Physiologists; 1993. pp. 142–149. [Google Scholar]
- Jansen MAK, Gaba V, Greenberg BM, Mattoo AK, Edelman M. Low threshold levels of ultraviolet-B in a background of photosynthetically active radiation trigger rapid degradation of the D2 protein of photosystem II. Plant J. 1996a;9:693–699. [Google Scholar]
- Jansen MAK, Greenberg BM, Edelman M, Mattoo AK, Gaba V. Accelerated degradation of the D2 protein of photosystem II under ultraviolet radiation. Photochem Photobiol. 1996b;63:814–817. [Google Scholar]
- Jordan BR. The effects of ultraviolet-B radiation on plants: a molecular perspective. Adv Bot Res. 1996;22:97–161. [Google Scholar]
- Krizek DT, Koch EJ. Use of regression analysis to estimate UV spectral irradiance from broad band radiometer settings under FS-40 fluorescent sunlamps filtered with cellulose acetate. Photochem Photobiol. 1979;30:451–457. [Google Scholar]
- Landry LG, Chapple CCS, Last RL. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol. 1995;109:1159–1166. doi: 10.1104/pp.109.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry LG, Stapleton AE, Lin J, Hoffman P, Hays JB, Walbot V, Last RL. An Arabidopsis photolyase mutant is hypersensitive to ultraviolet-B radiation. Proc Natl Acad Sci USA. 1997;94:328–332. doi: 10.1073/pnas.94.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ou-Lee T-M, Raba R, Amundson RG, Last RL. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell. 1993;5:171–179. doi: 10.1105/tpc.5.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Gitz IIIDC, McClure JW. Effects of UV-B on flavonoids, ferulic acid, growth and photosynthesis in barley primary leaves. Physiol Plant. 1995;93:725–733. [Google Scholar]
- Lois R, Buchanan BB. Severe sensitivity to ultraviolet radiation in an Arabidopsis mutant deficient in flavonoid accumulation. Planta. 1994;194:504–509. [Google Scholar]
- Mattoo AK, Giardi M-T, Raskind A, Edelman M. Dynamic metabolism of photosystem II reaction center proteins and pigments. Physiol Plant. 1999;107:454–461. [Google Scholar]
- Mattoo AK, Hoffman-Falk H, Pick U, Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the “proteinaceous shield” regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci USA. 1981;78:1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo AK, Marder J, Edelman M. Dynamics of the photosystem II reaction center. Cell. 1989;56:241–246. doi: 10.1016/0092-8674(89)90897-0. [DOI] [PubMed] [Google Scholar]
- Murali NS, Teramura AH. Effects of ultraviolet-B irradiance on soybean: VI. Influence of phosphorus nutrition on growth and flavonoid content. Physiol Plant. 1985;63:413–416. [Google Scholar]
- Murali NS, Teramura AH. Effectiveness of UV-B radiation on the growth and physiology of field-grown soybean modified by water stress. Photochem Photobiol. 1986;48:653–657. [Google Scholar]
- Posner HB. Aquatic vascular plants. In: Witt FA, Wessels NK, editors. Methods in Developmental Biology. New York: Cromwell; 1967. pp. 301–317. [Google Scholar]
- Reuber S, Bornman JF, Weissenböck G. A flavonoid mutant of barley (Hordeum vulgare L.) exhibits increased sensitivity to UV-B radiation in the primary leaf. Plant Cell Environ. 1996;19:593–601. [Google Scholar]
- Rozema J, vandeStaaij J, Björn LO, Caldwell M. UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol. 1997;12:22–28. doi: 10.1016/s0169-5347(96)10062-8. [DOI] [PubMed] [Google Scholar]
- Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koorneef M, Ausubel FM, Goodman HM. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995;8:659–671. doi: 10.1046/j.1365-313x.1995.08050659.x. [DOI] [PubMed] [Google Scholar]
- Sisson WB, Caldwell MM. Lamp/filter systems for simulation of solar UV irradiance under reduced atmospheric ozone. Photochem Photobiol. 1975;21:453–456. [Google Scholar]
- Somerville CR, Ogren WL. Isolation of photorespiration mutants in Arabidopsis thaliana. In: Edelman M, Hallick RB, Chua N-H, editors. Methods in Chloroplast Molecular Biology. New York: Elsevier Biomedical Press; 1982. pp. 129–138. [Google Scholar]
- Strid A, Chow WS, Anderson JM. UV-B damage and protection at the molecular level in plants. Photosynth Res. 1994;39:475–489. doi: 10.1007/BF00014600. [DOI] [PubMed] [Google Scholar]
- Teramura AH, Sullivan JH. Effects of UV-B radiation on photosynthesis and growth of terrestrial plants. Photosynth Res. 1994;39:463–473. doi: 10.1007/BF00014599. [DOI] [PubMed] [Google Scholar]
- Tevini M, Braun J, Fieser G. The protective function of the epidermal layer of rye seedlings against ultraviolet-B radiation. Photochem Photobiol. 1991;53:329–333. [Google Scholar]
- Vass I, Sass L, Spetea C, Bakou A, Ghanotakis DF, Petrouleas V. UV-B induced inhibition of photosystem II electron transport studied by EPR and chlorophyll fluorescence: impairment of donor and acceptor side components. Biochemistry. 1996;35:8964–8973. doi: 10.1021/bi9530595. [DOI] [PubMed] [Google Scholar]
- Wilson MI, Greenberg BM. Protection of the D1 photosystem II reaction center protein from degradation in ultraviolet radiation following adaptation of Brassica napus L. to growth in ultraviolet-B. Photochem Photobiol. 1993;57:556–563. [Google Scholar]