Abstract
Recently, vascular endothelial growth factor receptor (VEGFR)-targeted tyrosine kinase inhibitors (TKIs) have become available for the treatment of recurrent or metastatic thyroid cancer. However, a number of clinical challenges that impact the use of VEGFR-targeted TKI in daily clinical practice have arisen. Toxicity is considerable, to the extent that most physicians hesitate to start VEGFR-targeted TKI and prefer to continue a watch-and-wait approach until the patient’s disease markedly worsens. This delayed use of VEGFR-targeted TKI leads to a higher incidence of serious adverse events than was reported in clinical trials. Moreover, the watch-and-wait approach has several demerits, including a worsening of quality of life, worsening of outcomes in patients of older age or with follicular thyroid cancer and increased risk of brain metastasis or bleeding. Thus, optimal timing for the start of VEGFR-targeted TKI requires careful consideration. Moreover, management of VEGFR-targeted TKI toxicities requires appropriate supportive care, well-organised infrastructure in the outpatient clinic and patient education. Future treatment will progress to precision medicine based on molecular testing. Promotion of precision medicine requires the establishment of a system of easy access to molecular testing and the promotion of translational research for the development of new drugs.
Keywords: thyroid cancer, vegfr-targeted tki
Key questions.
What is already known about this subject?
Recently, vascular endothelial growth factor receptor (VEGFR)-targeted tyrosine kinase inhibitors (TKIs) have become available for the treatment of recurrent or metastatic thyroid cancer. Generally, patients with rapid tumor growth and/or symptomatic disease are considered candidates for immediate use of a VEGF-targeted TKI. For patients who have neither rapidly progressive nor symptomatic disease, most physicians hesitate to start VEGF-targeted TKI and prefer to continue a watch-and-wait approach until the patient’s disease has markedly worsened.
Because the clinical outcome of patients with recurrent or metastatic thyroid cancer remains limited, quality of life during treatment is markedly important.
What does this study add?
The watch-and-wait approach comes with several demerits, including a worsening of the patient’s quality of life; worsening of outcomes in patients of older age or with follicular thyroid cancer; and increased risk of brain metastasis or bleeding. Careful consideration of the optimal timing of VEGFR-targeted TKI is needed.
Management of toxicities with VEGFR-targeted TKI is based on three essential initiatives: the provision of appropriate supportive care including the drug holiday in accordance with the timing of the adverse events, well-organized infrastructure in the outpatient clinic and patient education.
Key questions.
How might this impact on clinical practice?
While concern over toxicities warrants hesitation before initiating VEGF-targeted TKI in patients without rapidly progressive or symptomatic disease, optimal timing should also consider the demerits of the watch-and-wait approach. Furthermore, the opportunity to commence VEGF-targeted TKI should not be missed in cases where the watch-and-wait approach would produce poor clinical outcomes or exacerbate risk factors for bleeding.
Because the clinical outcome of patients with recurrent or metastatic thyroid cancer remains limited, quality of life during treatment is markedly important. Ideally, treatment extends survival while ensuring a good quality of life. Management of toxicities with VEGFR-targeted TKI improve the quality of survival of cancer patients.
Introduction
Surgery is the primary mode of therapy for all histological types of thyroid cancer. For patients with recurrent or metastatic differential thyroid cancer (DTC) who are not amenable to salvage surgery, radioactive iodine therapy (RAI) is considered the standard of care. However, 25%–50% of patients with locally advanced or metastatic DTC become RAI-refractory.1 Several cytotoxic drugs have demonstrated clinical activity but with limited response duration as well as considerable toxicity.2–4 Available treatment options for recurrent or metastatic thyroid cancer have therefore been limited. Recently, however, successful phase 3 trials5–8 brought in the approval of vascular endothelial growth factor receptor (VEGFR)-targeted tyrosine kinase inhibitors (TKIs) for RAI-refractory DTC and medullary thyroid carcinoma (MTC). Nevertheless, a number of clinical challenges that impact the use of VEGFR-targeted TKI in daily clinical practice remain. Toxicity is considerable, leading most physicians to hesitate to start VEGFR-targeted TKI and preferring instead to continue a watch-and-wait approach until marked disease worsening. This delayed use of VEGFR-targeted TKI in turn leads to a higher incidence of serious adverse events than was seen in clinical trials. This review discusses the optimal use of VEGFR-targeted TKI for thyroid cancer patients and the management of toxicities.
Radioactive iodine therapy
RAI has been used in the management of patients with DTC since the 1940s. RAI is administered after thyroidectomy in patients with DTC for any or all of three reasons: to ablate residual normal thyroid tissue (remnant ablation), to provide adjuvant therapy for subclinical micrometastatic disease and to clinically treat apparent residual or metastatic thyroid cancer.
For patients with recurrent or metastatic DTC who achieve complete remission after receiving RAI, the survival rate is markedly better, with a 10-year overall survival (OS) of 92%.1 Even in patients who do not achieve complete remission, survival is better than in patients without iodine uptake, indicating that DTC patients with iodine uptake should be considered to initially receive RAI. In other words, VEGFR-targeted TKIs should not be given before iodine uptake by the tumour is confirmed. Repeat RAIs are required for most patients with DTC who do not achieve complete remission after receiving RAI. Furthermore, 25%–50% of patients with locally advanced or metastatic DTC become RAI-refractory.
Cytotoxic drugs
No phase 3 trial of cytotoxic drugs for the treatment of thyroid cancer has appeared, and the clinical benefits of these agents thus remain unclear. Several cytotoxic drugs have demonstrated clinical activity but with limited response duration and considerable toxicity.2–4 Cytotoxic drugs are therefore not preferred for first-line therapy for recurrent or metastatic thyroid cancer. Nevertheless, cytotoxic drugs may be a treatment option for selected patients who have progressive disease that is refractory or not amenable to VEGFR-targeted TKI.
Clinical trials of VEGFR-targeted TKI for thyroid cancer
VEGFR-targeted TKIs for recurrent or metastatic thyroid cancer, including RAI-refractory DTC and MTC, have been investigated in phase 3 trials (table 1). In the pivotal phase 3 trials, including the DECISION5 and SELECT trials,6 evidence of RAI-refractory disease was defined to include at least one of the following: one or more measurable lesions without iodine uptake on any radioiodine scan; one or more measurable lesions that had progressed per Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1 criteria within 13 or 14 months of RAI despite radioiodine avidity at the time of treatment; or cumulative dose of iodine-131 >600 mCi.
Table 1.
Results of phase 3 trials of VEGFR-targeted TKI for thyroid cancer
| Histology | Study | Drug | No. of patients | ORR | PFS | HR (95% CI) P values |
| DTC | DECISION5 | Sorafenib placebo | 419 | 12 0.5 |
10.5 5.8 |
0.59 (0.46 to 0.76) p<0.0001 |
| SELECT6 | Lenvatinib placebo | 392 | 65 1.5 |
18.3 3.6 |
0.21 (0.14 to 0.31)* p<0.001 | |
| MTC | ZETA7 | Vandetanib placebo | 331 | 45 13 |
Not reached 19.3 |
0.46 (0.31 to 0.69) p<0.001 |
| EXAM8 | Cabozantinib placebo | 330 | 28 0 |
11.2 4.0 |
0.28 (0.19 to 0.40) p<0.0001 |
*99% CI.
DTC, differentiated thyroid cancer; MTC, medullary thyroid cancer; ORR, overall response rate; PFS, progression-free survival.
Sorafenib is an oral kinase inhibitor of VEGFR-1, VEGFR-2, VEGFR-3, RET, RAF and platelet-derived growth factor receptor β. The DECISION trial was an international phase III clinical trial of sorafenib compared with placebo in patients with progressive, locally advanced, or metastatic RAI-refractory DTC.5 Results showed that median progression-free survival (PFS), the primary endpoint, was significantly longer in the sorafenib group than in the placebo group (10.8 months vs 5.8 months, HR 0.59, 95% CI 0.45 to 0.76, p <0.0001). Objective response rate (ORR) was 12.2% in the sorafenib group compared with 0.5% in the placebo group (p<0.0001). OS did not differ significantly between groups (HR 0.80, 95% CI 0.54 to 1.19; p=0.14). Dose interruptions, reductions or withdrawals because of adverse events occurred in 66.2%, 64.3% and 18.8% of patients, respectively. Hand–foot skin reaction was the most common reason for sorafenib dose interruption (26.6%), reduction (33.8%) and withdrawal (5.3%), and the most frequent treatment-emergent adverse events were hand–foot skin reaction (76.3%), diarrhoea (68.6%), alopecia (67.1%) and rash or desquamation (50.2%). In the sorafenib group, seven deaths were attributable to underlying disease, two to unknown causes and one each to lung infection, chronic obstructive lung disease and myocardial infarction.
Lenvatinib is an oral, multitargeted TKI of VEGFR1–3, fibroblast growth factor receptors 1–4, platelet-derived growth factor receptor α, rearranged during transfection (RET) and KIT. In the SELECT trial, a randomised trial of lenvatinib versus placebo in patients with progressive, locally advanced or metastatic RAI-refractory DTC, lenvatinib demonstrated significantly improved PFS and ORR in patients with RAI-refractory DTC versus placebo (median PFS 18.3 months vs 3.6 months; HR 0.21, 99% CI 0.14 to 0.31, p<0.001; ORR 64.8% vs 1.5%, p<0.001).6 The OS difference between groups was not statistically significant (HR 0.73, 95% CI 0.50 to 1.07; p=0.1032); this difference did improve when a potential crossover bias was considered (rank- preserving structural failure time model; HR 0.62, 95% CI 0.40 to 1.00, bootstrapped p=0.051). For lenvatinib, major treatment-emergent adverse events (>40%, all grades) were hypertension (67.8%), fatigue/asthenia (64.4%), diarrhoea (59.4%), decreased appetite (50.2%), decreased weight (46.4%) and nausea (41.0%). Discontinuation due to adverse events occurred in 37 (14.2%) of lenvatinib-treated patients. In the lenvatinib arm, 20 (7.7%) patients experienced fatal treatment-emergent adverse events. Of these, six deaths (2.3%) were considered by the investigator as treatment-related, including one case each of pulmonary embolism, haemorrhagic stroke and general physical health deterioration.
In a subset analysis of patients with anaplastic thyroid cancer (ATC) in a phase 2 study of lenvatinib for patients with all histological types of thyroid cancer, clinical activity was observed in the majority of patients.9 ORR was 24%, median PFS was 7.4 months and median OS of 10.6 months. Based on these results, lenvatinib is now available for ATC in Japan.
Vandetanib is an oral kinase inhibitor of EGFR, VEGFR2 and RET. In a phase III trial (ZETA trial), a randomised trial of vandetanib versus placebo in patients with unresectable locally advanced or metastatic MTC, significant prolongation of PFS was observed for patients receiving vandetanib compared with placebo (HR 0.46; 95% CI 0.31 to 0.69; p<0.001).7 Vandetanib also showed significant advantages compared with placebo in the secondary efficacy endpoints of ORR, disease control rate and calcitonin and carcinoembryonic antigen (CEA) biochemical response rates. Most common grade 3 or 4 adverse events were diarrhoea (11%), hypertension (9%), ECG QT prolongation (8%) and fatigue (6%). Thirty-one patients discontinued treatment during the randomised phase because of an adverse event, 28 (12%) receiving vandetanib and 3 (3%) receiving placebo.
Cabozantinib is an oral kinase inhibitor of MET, VEGFR2 and RET. In a phase III trial (EXAM), cabozantinib demonstrated significant improvement in PFS for patients with documented radiographic progression of metastatic MTC (median PFS 11.2 months vs 4.0 months; HR 0.28, 95% CI 0.19 to 0.40; p<0.001).8 ORR was 28% for cabozantinib. The most frequently reported grade 3 or 4 adverse events related to cabozantinib were diarrhoea (15.9%), palmarplantar erythrodysesthesia (12.6%) and fatigue (9.3%) and resulted in dose reductions in 79% and holds in 65% of patients. Adverse events led to treatment discontinuation in 16% of cabozantinib-treated and 8% of placebo-treated patients.
Indications for VEGFR-targeted TKI for patients with thyroid cancer
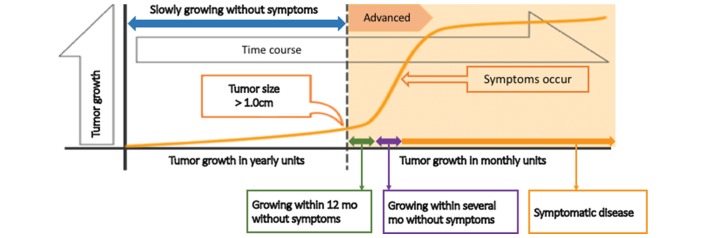
Any consideration of indications for VEGF-targeted TKI must weigh the relative merits of VEGF-targeted TKI. The appropriate timing for the start of VEGF-targeted TKI is controversial because, apart from ATC, the tumour growth of thyroid cancers is slower than that of other cancers, even if the tumour becomes radioiodine-refractory (figure 1). In fact, patients having indolent disease with a tumour size of less than 1 cm will experience no symptoms and have a good quality of life. In other words, these patients with indolent disease do not require tumour shrinkage by anticancer drugs. Furthermore, toxicities due to VEGF-targeted TKI worsen quality of life. In this regard, pivotal phase 3 trials, including the DECISION and SELECT studies, requested disease progression by RECIST within 13 or 14 months as a requirement of study enrolment.
Figure 1.
Appropriate timing for the start of TKI treatment. TKI, tyrosine kinase inhibitor.
Generally, patients with rapid tumour growth and/or symptomatic disease are considered candidates for immediate use of a VEGF-targeted TKI, while those with disease progression by RECIST within 12 months but no symptomatic disease are candidates for a watch-and-wait approach. Even if patients have neither rapidly progressive nor symptomatic disease, however, immediate use of a VEGF-targeted TKI is required in selected patients.
The watch-and-wait approach has both merits and demerits. Merits include reduced cost by the delayed start and awareness of symptom improvement by VEGF-targeted TKI for symptomatic patients. Demerits include the risk of worsening quality of life. For example, spinal cord paralysis and compression leads to an extremely poor quality of life. Furthermore, the watch-and-wait approach increases risk of brain metastasis, against which TKI has no beneficial effect. Furthermore, a subanalysis of the SELECT study suggested that the watch-and-wait approach could worsen outcomes in older patients and those patients with follicular thyroid cancer (FTC).
Age is considered the most important prognostic factor for metastatic thyroid cancer mortality, which markedly increases among patients aged more than 60 years.10 In a subanalysis of the SELECT study,11 no difference in OS was observed between the lenvatinib and placebo arm in patients aged less than 65 years. Furthermore, OS did not significantly differ among age groups in the lenvatinib arm. In contrast, OS did significantly differ between the two arms in patients aged more than 65 years (HR 0.53, 95% CI 0.31 to 0.91; p=0.020). In other words, lenvatinib significantly improved OS over placebo in elderly patients, and delayed use of lenvatinib worsens patient outcomes in elderly patients.
FTC tends to occur in an older population than other differentiated thyroid cancers. Incidence is reported to peak between 40 and 60 years, compared with the earlier peaking of papillary thyroid cancer (PTC) incidence at 30–50 years. Given that FTC typically occurs in older patients, it is more commonly associated with an aggressive clinical course, distant metastases and higher mortality than PTC.12 In a subanalysis of the SELECT study, although no difference in OS was seen between the two arms in patients with PTC (HR 0.92, 95% CI 0.60 to 1.41; p=0.708), OS was significantly better in the lenvatinib arm than the placebo arm among those with FTC (HR 0.41, 95% CI 0.18 to 0.97; p<0.035).13 In other words, lenvatinib demonstrated survival benefits in patients with FTC, indicating that delayed use would worsen OS in these patients. In other words, one of the demerits of the watch-and-wait approach is worsened outcomes in elderly patients or patients with FTC.
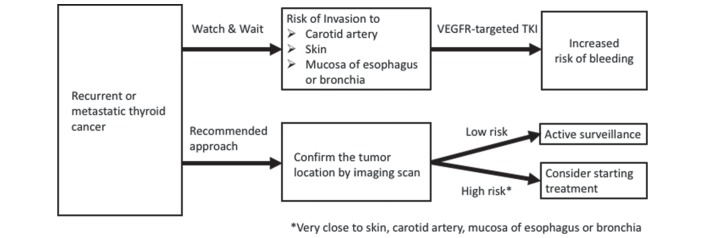
The watch-and-wait approach also increases the risk of bleeding. In fact, a number of serious and fatal cases of bleeding following VEGF-targeted TKI for thyroid cancer has been recently reported.14 15 In accordance with tumour growth, the watch-and-wait approach may increase the risk of invasion to the carotid artery, skin and mucosa of the oesophagus or bronchia, leading to increased risk of bleeding. Evaluation of the risk of bleeding by imaging-based confirmation of tumour location is therefore mandatory (figure 2). If the patient has high risk factors of bleeding, including a tumour located very close to the skin, carotid artery and mucosa of the oesophagus, the initiation of VEGF-targeted TKI should be considered. If the patient has a low risk of bleeding, active surveillance should be considered. While concern over toxicities warrants hesitation before initiating VEGF-targeted TKI in patients without rapidly progressive or symptomatic disease, optimal timing should also consider the demerits of the watch-and-wait approach. Furthermore, the opportunity to commence VEGF-targeted TKI should not be missed in cases where the watch-and-wait approach would produce poor clinical outcomes or exacerbate risk factors for bleeding.
Figure 2.
Evaluation of risk of bleeding according to tumour growth. TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
Management of adverse events related to VEGFR-targeted TKI
Because the clinical outcome of patients with recurrent or metastatic thyroid cancer remains limited, quality of life during treatment is markedly important. Ideally, treatment extends survival while ensuring a good quality of life. To improve the quality of survival of patients with cancer, three considerations are essential. First is adequate supportive care for toxicities. Second is patient education, because early contact with medical providers is essential for early recognition, leading to optimal management. Last is the infrastructure of your institution, because it is necessary to accept calls from patients 24 hours a day, every day.
VEGF-targeted TKI causes a number of adverse events, including hypertension, hand–foot syndrome, anorexia, fatigue, diarrhoea, proteinuria, thrombosis and myocardial ischaemia. The procedures for these adverse events have been reported.16 While most adverse events are manageable using established procedures, management of the proteinuria related to this treatment has not been established, and although drug withdrawal results in a significant reduction, persistence is common. A reduction in intraglomerular pressure with angiotensin II receptor blockers or ACE inhibitors may improve proteinuria. Although proteinuria is typically an asymptomatic event, the three complications of oedema, hyperlipidaemia and hypercoagulability should be also considered. Dietary sodium restriction and loop diuretics are available for oedema. While the mechanisms of hyperlipidaemia remain unclear, one possibility is that VEGF-targeted TKI enhances the hepatic synthesis of lipoproteins related to the fall in oncotic pressure. If hyperlipidaemia occurs, a 3-hydroxy-3-methylglutarl coenzyme A reductase inhibitor should be taken. Hypercoagulability also increases the risk of arterial and venous thrombosis, which sometimes lead to fatal events. Information about the risk of these events is therefore mandatory, even if they are rare. Because the persistence of proteinuria is common several weeks after drug withdrawal, patients in a Japanese phase 2 study with grade 2 proteinuria were allowed to continue lenvatinib, demonstrating no concern about serious renal dysfunction.17 Drug withdrawal is required when either an increase in creatinine level or leg oedema occurs but is not required when grade 2 proteinuria occurs without increased creatinine or leg oedema. When grade 3 or worse proteinuria occurs, drug withdrawal is preferred regardless of any increase in creatinine level or leg oedema. Continuous proteinuria is not life threatening, and drug discontinuation due to this adverse event alone is not necessary. Weighing the relative advantages of drug continuation, we consider that continuation is more beneficial than discontinuation for patients with recurrent of metastatic thyroid cancer, whose outcomes remain limited.
When these adverse events become severe, quality of life deteriorates, leading to increased risk of drug discontinuation. Reducing the severity of adverse events requires both early recognition and appropriate management.
Severe or intolerable toxicities require frequent dose interruptions and modifications. When severe or intolerable adverse events arise within 1 week after starting VEGF-targeted TKI, either the same or a reduced dose could be given. If a patient restarts administration at the same dose as before, severe or intolerable adverse events will again recur within 1 week, which would be intolerable for the patient. When administration is restarted at a reduced dose, administration could take longer, leading to higher dose intensity than that with the original higher dose. Because higher dose intensity is associated with better clinical activity in the treatment of VEGF-targeted TKI,18 dose reduction is preferred in patients who develop severe adverse events within 1 week after starting treatment. When severe adverse events occur several weeks after starting VEGF-targeted TKI, the same dose will remain tolerable for several further weeks. Furthermore, the same dose will allow the maintenance of dose intensity, whereas dose reduction would of course not. Drug withdrawal before the development of severe or intolerable adverse events is also preferred as a means of avoiding extended drug withdrawal, which is associated with tumour regrowth. The restart of VEGF-targeted TKI within 2 weeks is also therefore preferred. If tumour regrowth is faster and leads to a worsening of quality of life, the period of drug withdrawal should be shorter.
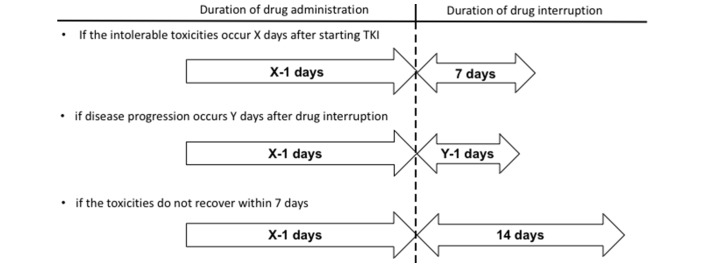
Finally, when adverse events that worsen quality of life occur, the drug holiday should be established in accordance with the timing of the adverse events (figure 3). Namely, if severe or intolerable toxicities occur X days after starting VEGF-targeted TKI, the duration of drug administration should be X−1 days. As I mentioned above, extended drug withdrawal is associated with tumour regrowth, and the duration of drug interruption should be 7 days initially. If disease progression occurs Y days after the drug interruption, the interruption should be shortened to Y−1 days. If the toxicities do not resolve within 7 days, the duration of drug interruption should be extended to 14 days. Restart of drug administration within 2 weeks is preferable given concerns about tumour regrowth.
Figure 3.
Planned drug holidays according to timing of the appearance of intolerable toxicities. TKI, tyrosine kinase inhibitor.
Patient education is of critical importance. This is because early contact with medical providers is essential for early recognition, leading to optimal management. Patient education should start prior to therapy onset and continue throughout therapy at every contact point the clinician has with the patient. In particular, the clinician should inform the patient about adverse reaction profiles. Given that mild adverse events are manageable, however, the problem of adverse events should not be overemphasised. While both patient and family need to understand who and when to call about adverse reactions, they both find it challenging to remember the clinician’s explanation and take appropriate action. A leaflet to make them aware of possible side effects should therefore be provided. For example, our institute provides a flow chart-type leaflet, which consist of yes or no questions to guide patients on how to take supportive medicine for adverse events and when to call the hospital.19 This could reduce non-adherence and improve patient judgement during chemotherapy, leading to a decrease in emergency hospital admissions.
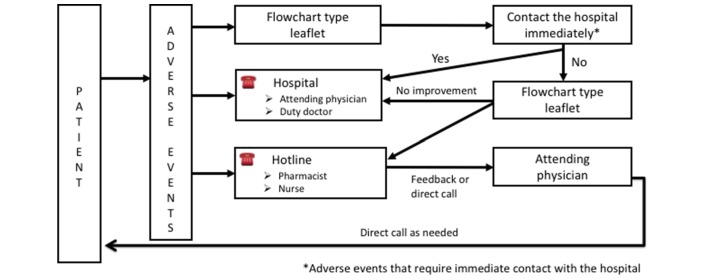
Multidisciplinary team (MDT) care improves adherence to best practice in patients with thyroid cancer who receive VEGF-targeted TKI. Furthermore, MDT care is essential for optimal management of adverse events and complications. Careful monitoring of adverse events and an easy-access consultation system are essential. Therefore, each institution is required to establish the appropriate infrastructure for patients with cancer at the outpatient clinic. For example, our institute has established ‘Telephone follow-up’, ‘hot line’ and ‘pharmacist outpatient clinic for oral anti-cancer drug’ initiatives. After a physician obtains informed consent for treatment and subsequently prescribe a VEGF-targeted TKI, the pharmacist explains again about possible adverse events, guidance on blood pressure self-measurement and guidance on self-management of toxicities. Several days after the start of the VEGF-targeted TKI, pharmacists proactively check adverse events and drug adherence by telephone, called ‘Telephone follow-up’. Patients with any questions or concerns related to treatment and adverse events can consult a pharmacist or nurse at the outpatient clinic centre by emergency phone call—called the ‘Hot line’—and finally, when a patient next comes to visit, a pharmacist at the outpatient clinic initially checks both adverse events and adherence and sends feedback to the physician, allowing physicians to assess these issues before the actual consultation. Management of toxicities in the outpatient clinic for early recognition of adverse events is shown in figure 4. If an adverse event occurs, three lines of action are available. First is the flow chart-type leaflet that a patient refers to initially: when an adverse event that requires contact with the hospital occurs, the patient is required to call the hospital immediately. The second is direct call to the physician, while the third is a hot line; the latter is helpful for the early recognition of adverse events, because some patients hesitate to call the attending physician directly. The person in charge of the hotline sends feedback through the electronic record or by direct call to the attending physician; the attending physician then calls the patient directly as warranted.
Figure 4.
Management schema for toxicities in the outpatient clinic.
Recent advances in development of new drugs for thyroid cancer
The recent identification of a number of molecular abnormalities has led to the idea that molecular screening for specific genetic alterations in thyroid tumours may provide the opportunity for efficient, targeted therapy. In fact, several molecular targeted drugs have emerged for the treatment of thyroid cancer.
The most common driver mutation is BRAF mutation,20 with a reported incidence of 44% in PTC and 24% in ATC.21 Vemurafenib was investigated in a phase 2 study for BRAF-mutated PTC,22 where it demonstrated promising clinical activity regardless of previous VEGFR-targeted TKI (overall response: 38.5% in patients who had not previously received VEGFR-targeted TKI vs 27.3% in patients who had previously received these agents). The BRAF inhibitor dabrafenib was investigated in a phase 2 study for BRAF-mutated PTC23 and showed clinical activity in the majority of patients. ORR was 29%, and median treatment duration was 8.4 months.
While BRAF inhibitors have demonstrated promising activity, tumour resistance has limited their benefits. Reactivation of the mitogen-activated protein kinase (MAPK) pathway through RAS is a major contributor to tumour resistance in BRAF mutant melanoma. In transgenic mouse models of BRAF V600–mutant ATC, combined inhibition of BRAF and MAPK kinase (MEK) kinases enhances antitumour activity compared with single-agent BRAF inhibitors.24 These rationales in turn lead to the idea of adding a MEK inhibitor to a BRAF inhibitor as a means of overcoming resistance. At the last American Society of Clinical Oncology (ASCO) meeting, the results of a randomised phase 2 study of dabrafenib with or without trametinib for BRAF-mutated PTC was reported. Addition of the MEK inhibitor to the BRAF inhibitor slightly improved ORR (54% vs 50%) and median PFS (15.1 months versus 11.4 months).25 Although this improvement was inferior to the investigators’ initial expectations, this combination demonstrated promising activity for ATC. The results of a subset analysis of this combination for patients with ATC showed an overall response of 69% in the intent-to-treat population and 73% in patients whose BRAF mutation were centrally confirmed.26 Median duration of response, PFS and OS were not reached as a result of a lack of events, with 12 month estimates of 90%, 79% and 80%, respectively.
Tropomyosin receptor kinase (Trk) receptors are expressed in human neuronal tissue and play an essential role in both the physiology of development and the function of the nervous system. This receptor family comprises three transmembrane proteins, which are referred to as Trk A, B and C and encoded by the NTRK1, NTRK2 and NTRK3 genes, respectively. Somatic rearrangement of the NTRK1 gene in PTC was observed in less than 12%. NTRK3 fusion is more frequent in radiation-related tumours. Larotectinib (LOXO-101), a pan-Trk inhibitor with highly selective activity against the Trk kinase family, demonstrated promising clinical activity for patients with Trk fusion cancers, including thyroid cancer.27 The overall response rate was 75% (95% CI 61 to 85) according to independent review and 80% (95% CI 67 to 90) according to investigator assessment. At 1 year, 71% of the responses were ongoing and 55% of the patients remained progression free. Furthermore, clinical development of a new drug against acquired NTRK resistance mutation has also been long ongoing. LOXO195 is a selective TRK TKI designed to overcome acquired resistance mediated by recurrent kinase domain mutations identified in multiple patients who have developed resistance to TRK TKIs. Clinical cases have been reported involving patients who developed disease progression due to acquired NTRK resistance mutations and subsequently experienced a rapid clinical response to therapy after starting LOXO195.28
Until now, most available medical treatment has been designed for average patients. As a result of this ‘one-size-fits-all’ approach, treatment can be very successful for some patients but not for others. Precision medicine, however, is an innovative approach that takes into account individual difference in the patient’s genes, environment and lifestyle. Future treatment will move on to precision medicine based on molecular testing. However, the screening of molecular abnormalities has not been attempted, because molecular testing is not reimbursed, so that most patients have no access to it. To promote precision medicine, we have to establish a system of easy access for molecular testing. To develop new drugs, furthermore, we have to promote translational research, which provides both the gene profile of the individual tumour and an understanding of the mechanism of drug resistance.
Conclusion
VEGF-targeted TKIs are now available for thyroid cancer. For patients who have neither rapidly progressive nor symptomatic disease, most physicians hesitate to start VEGF-targeted TKI and prefer to continue a watch-and-wait approach until the patient’s disease has markedly worsened. The watch-and-wait approach comes with several demerits, however, including a worsening of the patient’s quality of life, worsening of outcomes in patients of older age or with FTC and increased risk of brain metastasis or bleeding. Careful consideration of the optimal timing of VEGFR-targeted TKI is needed. Management of toxicities with VEGFR-targeted TKI is based on three essential initiatives: the provision of appropriate supportive care, well-organised infrastructure in the outpatient clinic and patient education.
Future treatment will move on to precision medicine based on molecular testing. To promote precision medicine, we have to establish a system that provides easy access to molecular testing and promotes translational research for the development of new drugs.
Footnotes
Funding: The author has not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MT reports grants and personal fees from Bayer and Eisai within the submitted work and grants and personal fees from Merck Serono, MSD, Pfizer, Astra Zeneca, Ono Pharmaceutical and Bristol-Myers Squibb, personal fees from Otsuka and grants from Boehringer Ingelheim, Novartis and NanoCarrier outside the submitted work.
Patient consent: Not required.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1.Durante C, Haddy N, Baudin E, et al. . Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 2006;91:2892–9. 10.1210/jc.2005-2838 [DOI] [PubMed] [Google Scholar]
- 2.Sherman SI. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin Oncol 2010;22:464–8. 10.1016/j.clon.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 3.Hadoux J, Schlumberger M. Chemotherapy and tyrosine-kinase inhibitors for medullary thyroid cancer. Best Pract Res Clin Endocrinol Metab 2017;31:335–47. 10.1016/j.beem.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Tiedje V, Stuschke M, Weber F, et al. . Anaplastic thyroid carcinoma: review of treatment protocols. Endocr Relat Cancer 2018;25:R153–R161. 10.1530/ERC-17-0435 [DOI] [PubMed] [Google Scholar]
- 5.Brose MS, Nutting CM, Jarzab B, et al. . Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014;384:319–28. 10.1016/S0140-6736(14)60421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlumberger M, Tahara M, Wirth LJ, et al. . Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621–30. 10.1056/NEJMoa1406470 [DOI] [PubMed] [Google Scholar]
- 7.Wells SA, Robinson BG, Gagel RF, et al. . Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134–41. 10.1200/JCO.2011.35.5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elisei R, Schlumberger MJ, Müller SP, et al. . Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639–46. 10.1200/JCO.2012.48.4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahara M, Kiyota N, Yamazaki T, et al. . Lenvatinib for anaplastic thyroid cancer. Front Oncol 2017;7:25 10.3389/fonc.2017.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins RJ, Wan Q, Grewal RK, et al. . Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab 2006;91:498–505. 10.1210/jc.2005-1534 [DOI] [PubMed] [Google Scholar]
- 11.Brose MS, Worden FP, Newbold KL, et al. . Effect of age on the efficacy and safety of lenvatinib in radioiodine-refractory differentiated thyroid cancer in the phase III SELECT Trial. J Clin Oncol 2017;35:2692–9. 10.1200/JCO.2016.71.6472 [DOI] [PubMed] [Google Scholar]
- 12.Grebe SK, Hay ID. Follicular thyroid cancer. Endocrinol Metab Clin North Am 1995;24:761–801. [PubMed] [Google Scholar]
- 13.Ando Y, Elisei R, Schlumberger M, et al. . Subgroup analysis according to differentiated thyroid cancer histology in the phase 3 (SELECT) trial of lenvatinib. Sapporo: Japan Society of Medical Oncology (JSMO) Annual Meeting 2015, 2015:1–5. [Google Scholar]
- 14.Masaki C, Sugino K, Saito N, et al. . Lenvatinib induces early tumor shrinkage in patients with advanced thyroid carcinoma. Endocr J 2017;64:819–26. 10.1507/endocrj.EJ17-0104 [DOI] [PubMed] [Google Scholar]
- 15.Resteghini C, Locati LD, Bossi P, et al. . Do not throw the baby out with the bathwater: SELECT a personalized, de-escalated lenvatinib schedule allows response in locally advanced DTC while controlling major drug-related bleeding. Ann Oncol 2017;28:2321–2. 10.1093/annonc/mdx251 [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S, Kiyota N, Tahara M. Optimal use of lenvatinib in the treatment of advanced thyroid cancer. Cancers Head Neck 2017;2:7 10.1186/s41199-017-0026-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Till JE. 1.3 Quality of Survival In: Innovations in radiation oncology. 25, 1988. [Google Scholar]
- 18.Ye X, Zhu Y, Cai J. Relationship between toxicities and clinical benefits of newly approved tyrosine kinase inhibitors in thyroid cancer: a meta-analysis of literature. J Cancer Res Ther 2015;11(Suppl 2):185–90. 10.4103/0973-1482.168182 [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Enokida T, Kobayashi T, et al. . Evaluation of the impact of a flowchart-type leaflet for cancer inpatients. SAGE Open Med 2014;2:205031211453125 10.1177/2050312114531256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 2013;13:184–99. 10.1038/nrc3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer 2005;12:245–62. 10.1677/erc.1.0978 [DOI] [PubMed] [Google Scholar]
- 22.Brose MS, Cabanillas M, Cohen EE, et al. . An open-label, multicenter phase 2 study of the BRAF inhibitor vemurafenib in patients with metastatic or unresectable papillary thyroid cancer (PTC) positive for the BRAF V600 mutation and resistant to radioactive iodine (NCT012866753, NO25530). Eur J Cancer 2013;49:S13. [Google Scholar]
- 23.Falchook GS, Millward M, Hong D, et al. . BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid 2015;25:71–7. 10.1089/thy.2014.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFadden DG, Vernon A, Santiago PM, et al. . p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc Natl Acad Sci U S A 2014;111:E1600–9. 10.1073/pnas.1404357111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah MH, Wei L, Wirth LJ, et al. . Results of randomized phase II trial of dabrafenib versus dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma. J Clin Oncol 2015;35:abstr 6022. [Google Scholar]
- 26.Subbiah V, Kreitman RJ, Wainberg ZA, et al. . Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol 2018;36:7–13. 10.1200/JCO.2017.73.6785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drilon A, Laetsch TW, Kummar S, et al. . Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018;378:731–9. 10.1056/NEJMoa1714448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drilon A, Nagasubramanian R, Blake JF, et al. . A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov 2017;7:963–72. 10.1158/2159-8290.CD-17-0507 [DOI] [PMC free article] [PubMed] [Google Scholar]