Abstract
In 2009, the National Confidential Enquiry into Patient Outcome and Death (NCEPOD) report identified significant deficiencies in the management of acute kidney injury (AKI) in hospitals in the UK. Many errors arose from failure to recognise patients with AKI and those at risk of developing AKI. Currently, there is no universally accepted risk factor assessment for identifying such patients on admission to acute medical units (AMUs). A multicentre prospective observational study was performed in the AMUs of 10 hospitals in England and Scotland to define the risk factors associated with AKI and to assess quality of care. Data were collected on consecutive acute medical admissions over two separate 24-h periods. Acute kidney injury was present in 55/316 (17.7%) patients, with sepsis, hypovolaemia, chronic kidney disease (CKD) and diabetes mellitus identified as the major risk factors. Deficiencies in patient care were identified, reinforcing the continuing need to improve the management of AKI.
KEY WORDS: Acute kidney injury (AKI), risk factors, acute medical units (AMUs)
Introduction
Acute kidney injury (AKI) commonly occurs in hospitalised patients,1 but its true prevalence has been difficult to capture because of the lack of a universal definition. However, new definitions that have allowed a more standardised approach have been proposed.2–4 These new definitions are based on an increase in concentration of creatinine in serum or reduction in urine output. These definitions reflect the fact that even a relatively small increase in concentration of creatinine in serum is associated with adverse patient outcomes,5,6 including increases in length of hospital stay, mortality and costs. More recently, attention has focussed on the longer term consequences of AKI, with the severity and duration of an episode predicting progression to chronic kidney disease.7 It therefore is important that patients with AKI, or those at risk of developing it, are recognised at the earliest opportunity following hospital admission and that early management is directed at minimising further injury or the development of complications. Unfortunately, evidence indicates that this is not done reliably in hospitals in the UK. In 2009, the National Confidential Enquiry into Patient Outcome and Death (NCEPOD) report identified widespread deficiencies in the recognition and management of AKI, with only 50% of patients receiving good care.8 There was a failure to recognise patients at risk of developing AKI and consequently a failure to take simple steps to minimise risk, including correction of hypovolaemia, cessation of nephrotoxic drugs, charting of fluid balance and monitoring of serum biochemistry.
Acute medicine is the fastest growing medical specialty in the UK, with acute medical units (AMUs) representing the hospital entry point for most unscheduled medical admissions, many of which involve elderly patients with multiple comorbidities. Prevention and management of AKI in this setting will go a long way to addressing the deficiencies described in the NCEPOD report.8 However, there is a paucity of data regarding the prevalence of community-acquired AKI (c-AKI) in patients admitted to the AMU and the associated risk factors. This study aimed to define the prevalence of c-AKI in emergency admissions to AMUs in 10 hospitals and to determine the key risk factors associated with c-AKI on admission. A clear understanding of which patients are at greatest risk of AKI is a prerequisite for prompt recognition and effective management of this patient cohort.
In addition to risk factor modelling, we also assessed the reliability of four simple clinical measures that may prevent or manage AKI. This part of the study identified deficiencies in patient care, reinforcing the continuing need to improve the management of AKI.
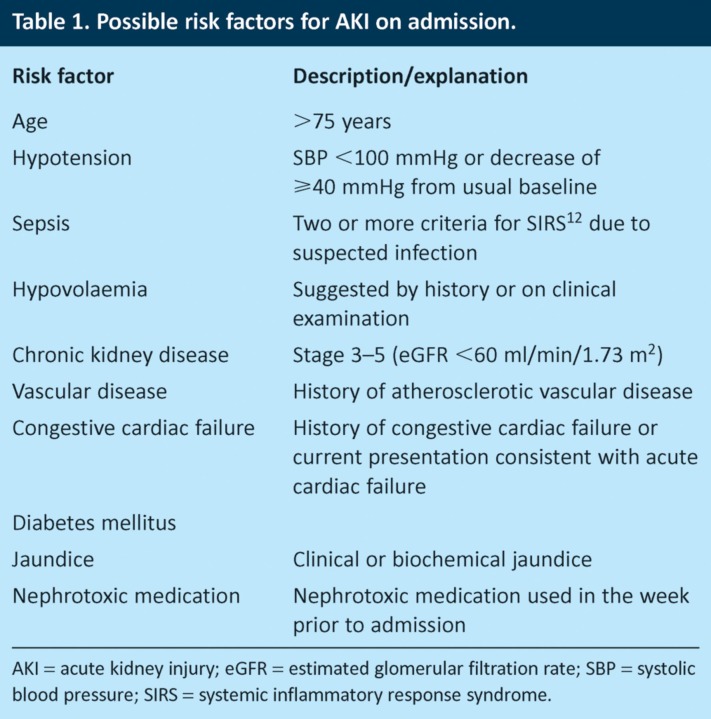
Methods
The study was conducted over two separate 24-hour periods at a total of 10 AMUs in England and Scotland (see Acknowledgements). The contributing hospitals included seven district general hospitals (serving populations of between 120,000 and 300,000) and three teaching hospitals. The mean number of admissions per day was 21.1 (standard deviation 11.8, range 4–44). Consecutive acute medical admissions over this period were included, with the exception of patients already on renal replacement therapy or receiving end-of-life care. Ten potential risk factors for AKI were identified (Table 1) based on a literature review of factors shown to increase the risk of AKI and expert consensus of a group of nephrologists and acute physicians. Nephrotoxic medications were defined as agents known to have potential adverse effects on renal function either through direct toxicity or by impairment of renal perfusion, with recognition that their toxicity may depend on the clinical context. Common nephrotoxic agents included angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), non-steroidal anti-inflammatory drugs (NSAIDs), aminoglycosides, radiocontrast agents and diuretics. Case notes, observation charts, laboratory results and drug charts were used to obtain data on demographics and serum biochemistry and to determine whether or not the patient had any of the 10 potential risk factors for AKI.
Table 1.
Possible risk factors for AKI on admission.
This study was conducted in two phases. In the pilot study (phase 1), data on risk factors and demographics were collected for 69 patients. Phase 2, which included 247 patients, repeated the data collection in phase 1 and also collected data about the clinical management of AKI (Box 1). Clinicians at each hospital were asked to determine whether there was clear documentation that specific measures, such as the administration of intravenous fluids, were indicated and whether these steps were implemented. If this was not apparent from the available information, ‘unknown’ was recorded for each intervention. Fluid assessment is a challenging clinical skill, with a relative paucity of published evidence about the optimal approach,9 and examination findings must be interpreted in the context of a patient’s history and relevant laboratory results. It is thus difficult to define tight criteria for intravenous fluid requirements (although documented hypovolaemia was considered one such indication). In order to improve the reliability of this measure, data collection was either performed by or discussed with a senior clinician in each hospital. Indications for charting of fluid balance were considered to be AKI, hypovolaemia, hypotension and sepsis.
Box 1. Appropriate management steps for AKI.
Acute kidney injury was defined according to the International Kidney Disease: Improving Global Outcomes (KDIGO) classification.2 The baseline level of serum creatinine was defined as per the Renal Association guidelines,10 using a reference measure of creatinine in serum within 1 week of admission when possible (3 months to 1 year is acceptable).
Univariable analyses were conducted to explore the association between AKI and each of the potential risk factors using Pearson’s chi-square test. Multivariable logistic regression models were run to study the effect of variables that maintained a significant association with the outcome. Backwards step selection was used to remove variables that did not improve the model fit, which were determined using likelihood ratio tests. The area under the receiver operating characteristic (ROC) curve of the final model was 0.837. Logistic regression used robust standard errors to adjust for evidence of clustering of patients at the hospital level. Results are presented as odds ratios (OR) and 95% confidence intervals (CI). Tests of statistical significance were set at p=0.05. Where stated, p values are two sided. All statistical analyses were performed using Stata IC (version 12.1) or StatsDirect software.
Results
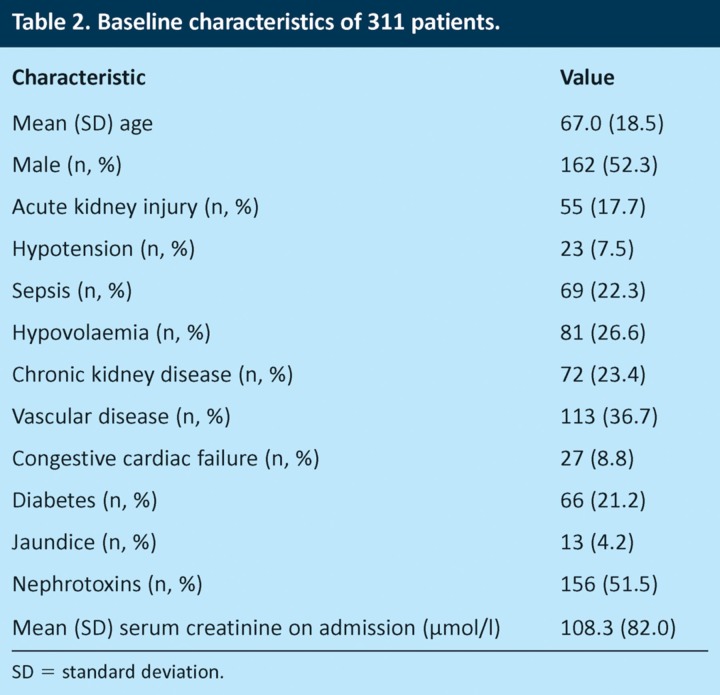
Data were collected on 316 patients who met the inclusion criteria. Five records were excluded from the final dataset due to incomplete and missing data. Table 2 shows the baseline characteristics of the sample. The mean age was 67 (standard deviation (SD) 18.5) years, and 162 (52.3%) patients were male. The prevalence of c-AKI in the study population was 17.7%.
Table 2.
Baseline characteristics of 311 patients.
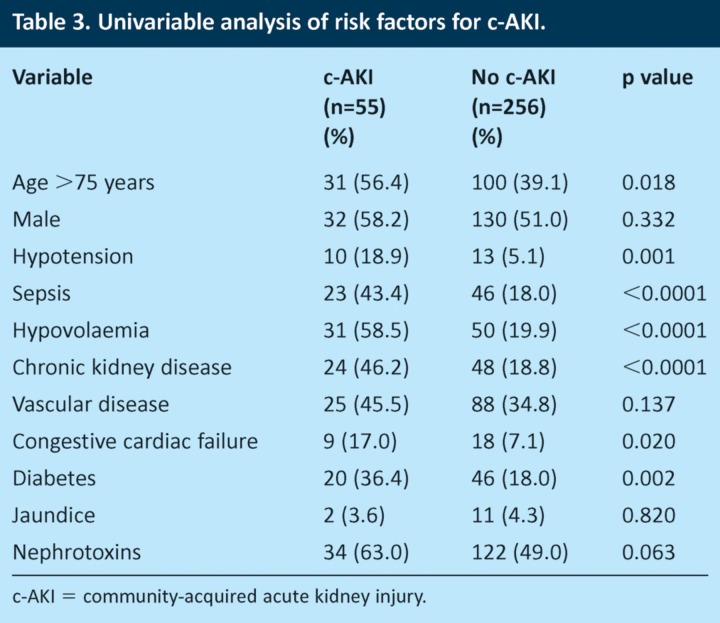
Univariable analyses (Table 3) showed that patients with c-AKI were older than those without c-AKI (56.4% aged ≥75 years with c-AKI compared with 39.1% without c-AKI, p = 0.018). Patients with c-AKI were also more likely than those without c-AKI to have clinical evidence of hypotension (18.9% vs 5.1%, p=0.001), sepsis (43.4% vs 18.0%, p≤0.0001), hypovolaemia (58.5% vs 19.9%, p≤0.0001) and CKD (46.2% vs 18.8%, p≤0.0001). Patients with c-AKI were also more likely to have congestive cardiac failure (17.0% vs 7.1%, p=0.02) and diabetes (36.4% vs 18.0%, p=0.002).
Table 3.
Univariable analysis of risk factors for c-AKI.
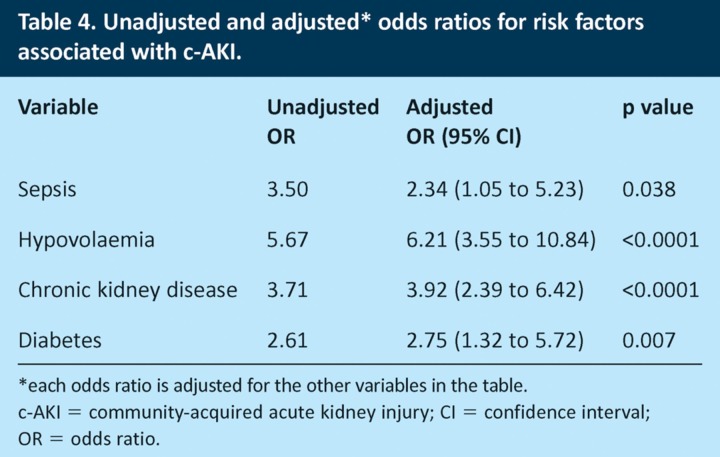
When the statistically significant risk factors were analysed together using logistic regression, only hypovolaemia, sepsis, diabetes and CKD remained significant (Table 4). After adjustment, clinical evidence of hypovolaemia was associated with a 6.2-fold increase in the odds ratio of c-AKI and sepsis with a 2.3-fold increase. Diabetes and CKD were associated with 2.8- and 3.9-fold increases in the odds ratio of c-AKI, respectively.
Table 4.
Unadjusted and adjusted* odds ratios for risk factors associated with c-AKI.
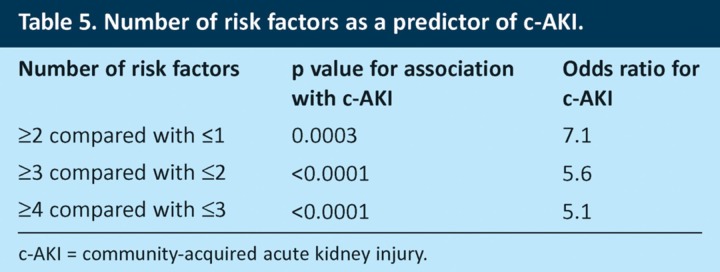
When results for the 287 patients who had no missing values for any risk factors were analysed, 84.6% of these patients had at least one risk factor for AKI on admission and 65.8% had ≥2 risk factors (Fig 1A). The total number of risk factors for an individual patient was strongly predictive of the risk of c-AKI (Fig 1B). Patients with ≥2 risk factors had a 7.1-fold increased risk of having AKI on admission compared to those with ≤1 risk factor (Table 5).
Fig 1.
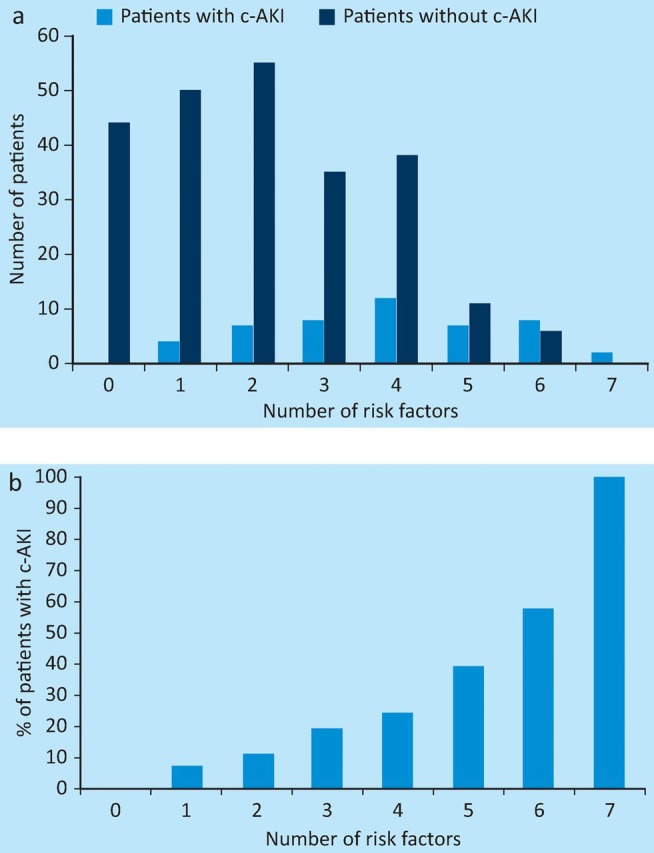
Distribution of number of risk factors for patients with and without c-AKI (a) and correlation between c-AKI and number of risk factors (b). c-AKI = community-acquired acute kidney injury.
Table 5.
Number of risk factors as a predictor of c-AKI.
More than 50% of acute medical patients were taking potentially nephrotoxic medication prior to admission, principally ACE inhibitors, ARBs and diuretics. Interestingly, the use of these medicines was not independently associated with AKI on admission (p=0.06). The interaction between the use of nephrotoxic agents and the other risk factors was explored by combining nephrotoxic agents with each of the other risk factors in turn in a model combining two exposure variables. We found no significant association between nephrotoxin use and AKI on admission when any of the other risk factors were also present, although there was a trend towards an association when nephrotoxin use was combined with hypovolaemia (p=0.07).
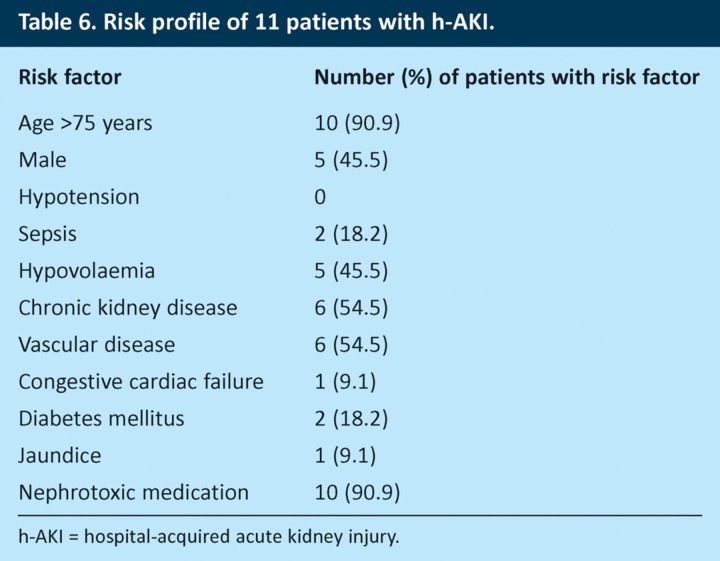
Eleven patients who did not have AKI on admission developed it within 72 hours and were therefore considered to have hospital-acquired AKI (h-AKI). Table 6 summarises the risk profiles of these patients. It is noteworthy that almost all of these patients were older than 75 years (90.9%) and on nephrotoxic medications (90.9%). Nine (81.8%) of the 11 patients had both of these risk factors. Chronic kidney disease, vascular disease and hypovolaemia were also common in these patients (54.5%, 54.5% and 45.5%, respectively).
Table 6.
Risk profile of 11 patients with h-AKI.
Phase 2 of the study (n=247) assessed the use of four simple interventions that could prevent, treat or manage AKI:
correction of hypovolaemia
cessation of potentially nephrotoxic drugs
charting of fluid balance
monitoring of serum biochemistry.
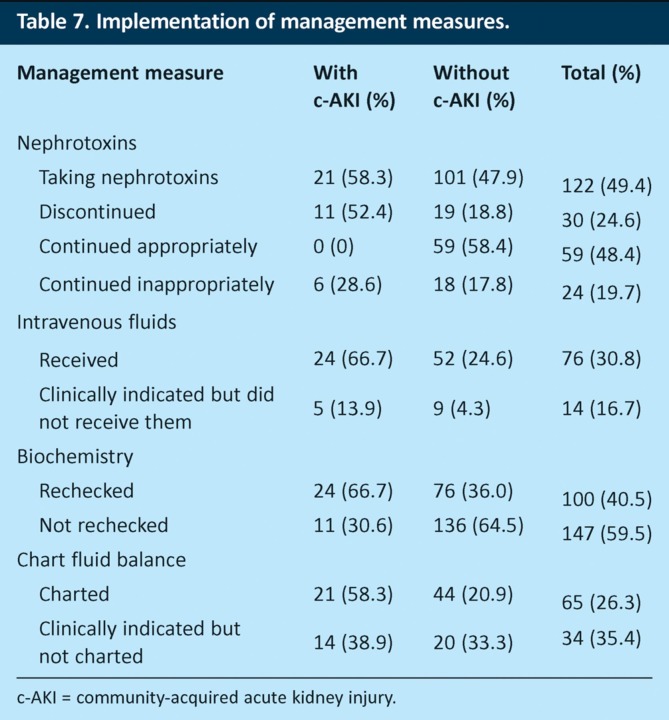
The evidence showed failure to perform these steps in a significant number of patients. Of the 247 patients in phase 2, 122 (49.4%) were taking potentially nephrotoxic medications at the time of admission. Nephrotoxic agents were continued in 85 (69.7%) patients. For 24 (19.7%) of these patients, the drugs were continued despite a documented clinical indication for stopping them (AKI, hypovolaemia, hypotension or sepsis). This included six patients with AKI on admission (Table 7). Nineteen (7.7%) patients were excluded from the analysis as data collectors were unable to determine with certainty whether or not nephrotoxins should have been stopped.
Table 7.
Implementation of management measures.
Seventy-six (30.8%) patients received intravenous fluids. Fourteen (16.7%) of the 84 patients determined to have a clinical indication for intravenous fluids did not receive them. Ninety-six (38.9%) patients had clinical indications for charting of fluid balance, but such charts were not made for 34 (35.4%) of these patients. Fluid-balance charts were also not recorded for 14 (38.9%) of 36 patients with biochemical evidence of AKI on admission, and serum biochemistry was not rechecked within 24 hours in 11 (30.6%) of patients with AKI on admission.
Discussion
Acute kidney injury can be considered as community acquired (present at the time of or apparent within 24 hours of hospital admission) or hospital acquired (developing ≥24 hours after hospital admission). Patients with AKI on admission need to be recognised promptly, with appropriate steps taken to ameliorate progression of the injury and reduce the associated short- and long-term complications. It is equally important to recognise the development of h-AKI following admission, as highlighted in the NCEPOD report.8 Currently, no validated AKI risk calculators are available for patients with undifferentiated acute medical illness. This study has identified the prevalence and significance of a number of previously described risk factors associated with patients admitted with AKI. It is important to identify these risk factors, as patients admitted to the AMU are increasingly elderly, with several comorbidities and multiple medications. This study confirms that AKI is a common problem in this patient population, affecting 17.7% of patients at the time of admission and a further 3.5% over the next 72 hours. As expected, risk factors for c-AKI are also common, with a median of two risk factors for unselected admissions and four for patients with c-AKI. Hypovolaemia, CKD, sepsis and diabetes mellitus had the strongest association with c-AKI, with odds ratios of 6.21, 3.92, 2.34 and 2.75, respectively. The number of risk factors for an individual patient was strongly associated with the likelihood of having AKI on admission (Fig 1B), with ≥2 risk factors giving an odds ratio of 7.1 compared to ≤1 risk factor.
More than half of the patients surveyed were on potentially nephrotoxic medications at the time of admission; there was no significant association between the use of nephrotoxic medications and the presence of c-AKI, although there was a trend towards an association in patients who also had hypovolaemia. The pharmacological effects of drugs such as ACE inhibitors and diuretics suggest that they are likely to increase the risk of AKI during intercurrent illness, and this inter-relationship is commonly seen in clinical practice. This study may have been underpowered to detect this association. It is also noteworthy that more than 90% of patients who developed h-AKI within 72 hours of admission were on nephrotoxic medications. Numbers were too small to allow statistical analysis, but eight of the 11 patients with h-AKI were elderly and on nephrotoxic drugs, which suggests that this cohort may be at particular risk. Further research is needed to evaluate this finding and to define more fully the risk factors for h-AKI.
Although it is now 3 years since the publication of the NCEPOD report in 2009, the data presented indicate that there is a continuing problem with the management of patients with AKI. Four simple interventions that could prevent, treat or manage AKI were examined. A limitation of the study is that assessment of the clinical indications for each intervention was based only on what was documented for each patient, but our audit tool gave clinicians the option to record ‘unknown’ if they felt there was insufficient information to make a decision. Data collection was performed by (or discussed with) senior clinicians at each site.
Charting of fluid balance was the least reliable process, being omitted for 35.4% of patients for whom a clinical indication for charting was documented, including 38.9% of patients with AKI. Nephrotoxic agents were continued in 19.7% of patients who had documented indications for stopping them, including six patients with AKI on admission. Intravenous fluids were not given to 16.7% of patients in whom they were indicated, and serum biochemistry was not rechecked within 24 hours in 30.6% of patients with AKI on admission. These figures show that a significant number of patients who have AKI or are at risk of developing it do not receive adequate management. This may in part be due to a lack of understanding about risk factors for AKI in acute medical patients and hence a failure to recognise these patients when they present with a range of other acute illnesses. Muniraju et al recently found that 30% of trainee doctors in the UK were unable to name more than two risk factors for AKI.11
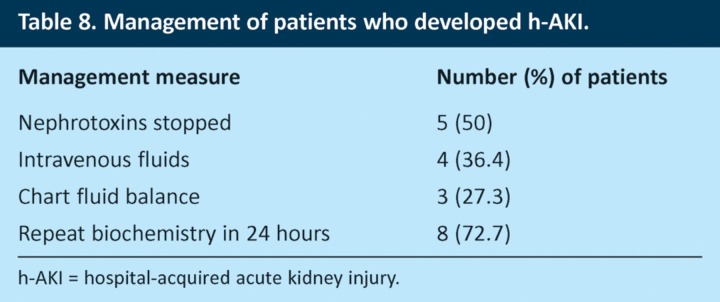
The NCEPOD report found that quality of care was worse for patients who developed AKI after admission than for those who were admitted with AKI.8 Our study followed patients only for 72 hours after admission and so will not have identified all the patients who subsequently developed h-AKI. However, we found 11 patients who developed h-AKI within the first 3 days after admission. Interestingly, 10 of these patients were on nephrotoxic medications and 10 were older than 75 years. The mean number of risk factors for AKI in this group was 3.9, which suggests that their increased risk of h-AKI could be identified on admission. Despite this, fluid balance was charted in only three (27.3%) patients, and nephrotoxins were discontinued in only five (50%) patients (Table 8). These results emphasise the importance of improving risk assessment for AKI in acute medical patients.
Table 8.
Management of patients who developed h-AKI.
A further potential limitation of this study is that several of the contributing hospitals had small numbers of medical admissions (six of the 10 centres had fewer than 20 admissions per day and one of these contributed only four patients over a 24-hour period). It will be important to confirm that these results are generalisable to larger hospitals.
Conclusion
This study identified several key risk factors that are associated with c-AKI. The presence of two or more risk factors correlates with a significantly increased risk of having c-AKI on admission. An important question will be whether these same risk factors are associated with patients developing h-AKI, and further work is required to clarify this. With an area under the ROC curve of 0.837, our data suggest that it may be possible to develop a weighted AKI risk calculator to be applied to patients with undifferentiated acute medical problems to identify those at increased risk. Such a tool could be used in the community and in the hospital setting and could be linked to a simple response algorithm or ‘AKI care bundle’, prompting both primary care physicians and hospital-based clinicians to review medications, assess volume status and monitor kidney function. Such an approach could be an important step towards ensuring reliable recognition and management of patients at risk of AKI.
Acknowledgements
Hospitals (and clinicians) contributing data to this study were: Dumfries and Galloway Royal Infirmary (Dr Sian Finlay); Grantham and District Hospital (Drs Laura James and Shirin Boardman); Leighton Hospital, Mid Cheshire (Dr Shirley Hammersley); Princess Royal University Hospital, London (Dr Anita Banerjee); Queen Elizabeth Hospital, King’s Lynn (Dr D Bhattacharjee); Queen Margaret Hospital, Dunfermline (Dr Laura Clark); Royal Victory Infirmary, Newcastle (Drs Jane Atkinson and Satyam Veeratterapillary); Western General Hospital, Edinburgh (Dr Matthew King); Sheffield University Teaching Hospital; and Wishaw General Hospital, Lanarkshire (Dr Ali Harmouche). The authors also acknowledge the significant contribution of Heather Barrington (Dumfries) to the statistical analysis.
References
- 1.Naikar SS, Liu KD, Chertow GM. The incidence and prognostic significance of acute kidney injury. Curr Opin Nehrol Hypertens. 2007;16:227–36. doi: 10.1097/MNH.0b013e3280dd8c35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. www.kdigo.org/clinical_practice_guidelines/AKI.php [Accessed 25 April 2013]. [DOI] [Google Scholar]
- 3.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy, and information technology needs. The second international consensus conference of Acute Dialysis Quality Initative (ADQI) Group. Crit Care. 2004;8:R204–2. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network (AKIN): report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 7.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–9. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Confidential Enquiries into Patient Outcome and Death (NCEPOD) Adding insult to injury. A review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury. London: NCEPOD; 2009. [Google Scholar]
- 9.Academy of Medical Royal Colleges. Acute kidney injury: a competency framework. Defining the role of the clinician. London: AOMRC; 2011. [Google Scholar]
- 10.Renal Association. Clinical practice guidelines: acute kidney injury. 5th edn. Petersfield: Renal Association; 2010. [DOI] [PubMed] [Google Scholar]
- 11.Muniraju TM, Lillicrap MH, Horrocks JL, et al. Diagnosis and management of acute kidney injury: deficiencies in the knowledge base of non-specialist, trainee medical staff. Clin Med. 2012;12:216–21. doi: 10.7861/clinmedicine.12-3-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]