Abstract
This paper describes three elderly patients who were admitted to hospital with aspiration pneumonia. They were kept nil by mouth (NBM) for a number of days, while being given intravenous hydration initially and enteral feeding subsequently. During that time they deteriorated and appeared to be dying, so the Liverpool Care Pathway (LCP) for the dying was used to support their care. Artificial nutrition and hydration were stopped. They quickly improved and the LCP was discontinued. Two of the patients deteriorated again on reintroduction of enteral feeding and/or intravenous fluids, only to improve a second time following withdrawal of feeding and fluids. Vulnerable elderly patients should not be made NBM except as a last resort. Clinicians should be alert to the possibility of refeeding syndrome and overhydration as reversible causes of clinical deterioration, particularly in frail elderly patients. Use of the LCP in these patients provided a unique opportunity to witness the positive effects of withdrawal of excessive artificial nutrition and hydration.
KEY WORDS: Liverpool Care Pathway, dying, elderly, overhydration, refeeding syndrome
Key Points
Vulnerable elderly patients should not be made nil by mouth except as a last resort
Clinicians need to actively exclude reversible causes of deterioration in patients before diagnosing dying
Clinicians should be alert to the possibility of refeeding syndrome as a reversible cause of clinical deterioration, particularly in frail elderly patients
Overprovision of fluid, particularly saline, can lead to deterioration in undernourished patients, especially elderly patients, who may handle excess salt and water badly due to reduced reserve capacity
When used appropriately, the Liverpool Care Pathway can be a beneficial and valuable clinical pathway
‘If a convalescent while taking nourishment remains weak, it is a sign that the body is being overnourished’
Hippocratic Aphorism, 4th century BC
Case 1
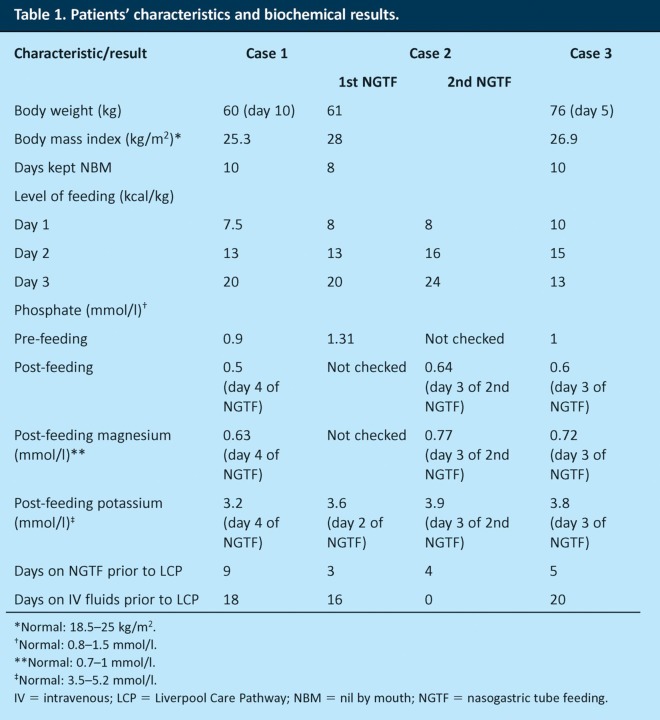
A 92-year-old lady with multiple comorbidities was admitted to hospital from a residential home with community-acquired pneumonia. She was judged to be at high risk of aspiration and so was made nil by mouth (NBM) pending review by a speech and language therapist (SLT). Her body weight, body mass index (BMI) and history of weight change were not recorded on admission. She was given intravenous (IV) antibiotics and IV fluids at a rate that cannot be determined by retrospective examination of the medical notes. On day 4 of her admission she was reviewed by an SLT, who advised to continue keeping her NBM and consider an alternative route of feeding. On day 9, the patient was reviewed by a dietitian. The physiotherapist who treated her commented that she had grossly oedematous arms and legs. On day 10, the patient received nasogastric tube feeding (NGTF) and was started on a low-energy feeding regimen (30 ml/hour for 16 hours) to avoid refeeding syndrome (RFS) (Table 1). Intravenous fluids were continued for 1 day, and on day 2 of NGTF, serum magnesium was found to be low; 8 mmol of magnesium were given intravenously. On day 3 of NGTF, the patient’s respiratory function deteriorated, her antibiotics were changed and the feeding rate was increased to 75 ml/hour. The patient’s phosphate had fallen to 0.5 mmol/l. A further 6 days later, the patient pulled out her feeding tube on the day that she was judged clinically to be dying. Intravenous fluids were discontinued and the LCP was used to support her care. Within 4 days she had dramatically improved, was no longer oedematous or breathless, and was asking for a cup of tea. The LCP was discontinued. However, the patient continued to be at high risk of aspiration, so she was again kept NBM; as she refused NGTF, IV fluids were restarted to avoid dehydration. Two days later, the patient deteriorated again and seemed clinically to be dying. She was again supported by the LCP, and IV fluids were stopped. Ten days later, the patient had improved so much that she started to eat and drink for pleasure – despite the high risk of aspiration. The patient was eventually discharged to a nursing home, where she died 10 months later.
Table 1.
Patients’ characteristics and biochemical results.
Case 2
An 86-year-old man with dementia and Parkinson’s disease was admitted to hospital from a nursing home with suspected aspiration pneumonia. He was overweight with a BMI of 28 kg/m2. He was started on antibiotics and about 2 l of IV 0.9% sodium chloride daily. On day 6, he was found to be at high risk of aspiration and so was made NBM, with the SLT suggesting that an alternative route of feeding be considered. On day 14, NGTF was commenced using a low-energy feeding regimen (25 ml/hour for 20 hours) with supplemental vitamins (Pabrinex) in view of his refeeding risks (Table 1). The feed was increased to 40 ml/hour on day 2 of NGTF while he continued on IV fluids. His serum phosphate level was not checked. By day 3 of NGTF, the patient was judged clinically to be dying and the LCP was used to support his care. Intravenous fluids and NGTF were stopped. Three days later he had improved significantly, so his care needs were re-evaluated and the LCP was discontinued. He was restarted on NGTF, but not on IV fluids. Once again, a low-energy feed was given, increasing daily (25 ml/hour for 20 hours on day 1, increased to 50 ml/hour on day 2 and 75 ml/hour on day 3). On day 4, his condition deteriorated again. The NGTF was discontinued, and the LCP was used for a second time. His serum phosphate at this stage was low (0.64 mmol/l). He improved once more and managed to resume oral intake of food and fluids, so the LCP was discontinued 6 days later, although, unfortunately, he died suddenly 1 week later.
Case 3
An 87-year-old man with advanced Alzheimer’s disease was admitted to hospital with suspected aspiration pneumonia. He was started on antibiotics and IV fluids, about 1 l of 5% dextrose and 1 l of 0.9% sodium chloride daily. He was also started on a diet of thickened fluids only, while he awaited assessment by an SLT. When weighed on day 5, he had lost 14 kg (15.5% of his body weight) since a previous admission 6 weeks earlier, but remained of normal overall weight (76 kg). On day 6, due to a high risk of aspiration, he was made NBM by an SLT and was reviewed by a dietitian. Repeated attempts to insert a nasogastric tube were unsuccessful, and later attempts were delayed due to a high international normalised ratio. Eventually, on day 16, he was started on NGTF on a low-energy feeding regimen (25 ml/hour for 10 hours, followed by 50 ml/hour for 10 hours) due to the risk of refeeding syndrome (Table 1). In addition, 1 l of 0.9% sodium chloride was given, as well as thiamine 100 mg three times daily and vitamin B (Compound Strong) orally. On day 2 of NGTF, the feeding rate was increased (50 ml/hour for 10 hours followed by 75 ml/hour for 10 hours) and IV fluids continued. On day 3 of NGTF, the patient deteriorated, with serum phosphate of 0.6 mmol/l. The feeding rate was reduced to 50 ml/hour for 20 hours, and 1 l of 0.9% sodium chloride and 8 mmol/l of magnesium were given intravenously. On day 5 of NGTF, the patient was judged clinically to be dying. The NGTF and IV fluids were stopped, and the LCP was used to support his care. Five days later, his condition had improved; he started to eat and drink for pleasure, and the LCP was discontinued. One litre of 0.9% sodium chloride was given subcutaneously daily for 11 days. He died suddenly 6 weeks later.
Comment
The LCP has been introduced in many NHS hospitals and hospices in the UK to help healthcare professionals provide better care for imminently dying patients. This includes individual assessment and regular monitoring of ongoing clinical interventions.1 The focus is on comfort and includes consideration of discontinuation of artificial feeding and intravenous hydration, which happens in most cases.2 The pathway has become a matter of considerable debate recently in the lay press. Many of the issues raised concern patient and family consent and information given before using the pathway. Some of the pathway’s critics suggest that it is used to withdraw drugs, fluids and food and hence to hasten death. However, it is not uncommon for patients placed on the LCP to improve unexpectedly. In hospitals, at least 3% of patients supported by the LCP improve clinically.3 This has been attributed to difficulties in estimating prognosis and diagnosing dying, especially during the last few days of someone’s life.4 Although this may be true in most cases, those presented in this paper alert us to the possibility that withdrawal of excessive feeding and hydration can contribute to unanticipated clinical improvement.
The patients presented in this paper were admitted for pneumonia, which was possibly related to aspiration in the community. After admission, they continued to be at high risk of aspiration and were made NBM to prevent such an event and further deterioration. However, starvation – even for a few days – is known to produce pronounced effects on body composition, micronutrient status and electrolyte/water balance (Box 1), which put patients at risk of fluid overload and refeeding syndrome.5 A recent report by the Royal College of Physicians in London commented that ‘Nil by mouth should be a last resort, not the initial default option’.6 It also highlighted the need for decisions regarding artificial nutrition to be made early, which would help avoid the development of a risk of refeeding syndrome. A holistic assessment of patients may show that it is sometimes appropriate for them to eat and drink for pleasure, especially towards the end of life, despite the high risk of aspiration.
Box 1. Effect of starvation on the body.
Refeeding syndrome is a condition that results from overprovision of food to a malnourished individual; it is due to severe electrolyte and water imbalance following reintroduction of food and manifests within a few days of starting feeding.7,8 Fluid intolerance, hypophosphataemia, hypomagnesaemia, hypokalaemia and hyperglycaemia are common in patients with RFS, with clinical consequences including heart failure, respiratory failure, seizures and sudden death.9 However, RFS is often unrecognised, because the signs and symptoms are non-specific and biochemical changes may be minimal. The National Institute for Health and Clinical Excellence (NICE) guidelines in England suggested that patients at high risk of RFS should generally be started on 10 kcal/kg/day, with feeds increased gradually over 5–7 days,10 although others debate that the guidelines are overcautious.11 However, in the three patients reported here, even this level of feeding was possibly detrimental.
Excess administration of IV fluids may also have been a contributing factor to the deterioration of these patients given their risk of fluid intolerance resulting from starvation initially and RFS later. Inadequate documentation of prescription and administration of IV fluids precludes us from making definitive comment on the extent to which excess IV fluids contributed to their general decline. However, the clinical development of gross oedema in the arms and legs in case 1 suggests that the patient was overhydrated. A recent consensus for fluid prescribing in surgical patients recommends particular caution when prescribing normal saline.12 The sodium needed to meet maintenance requirements in a normal adult is only around 1 mmol/kg/day, which means that 0.5 l/day of 0.9% sodium chloride solution (which contains 154 mmol sodium per litre) is adequate for most patients. Elderly medical patients initially may need extra fluid resuscitation due to dehydration or sepsis, but comorbidities and age-related decline in organ function increase vulnerability to overhydration.
The clinical deterioration of these patients to the point that they seemed to be dying led to the decision to use the LCP to support their care. Regular patient review, as required by the LCP, provided an opportunity to witness the positive effects of withdrawing excessive feeding and hydration and, in that respect, was of clear benefit to these patients. This shows that, paradoxically, a period of reduced fluid and nutrition might actually be beneficial to a few patients. The possibility that fluid overload or refeeding issues might be complicating the clinical picture should therefore be considered carefully (albeit mostly then rejected) before accepting that any patient is actually in the dying phase.
Our observations emphasise the need for clinicians to actively exclude reversible causes of deterioration in patients before dying is diagnosed. They also suggest that:
NICE guidance about the prevention and treatment of refeeding syndrome may not be overcautious
overprovision of fluids, particularly saline, can lead to deterioration in undernourished, particularly elderly, medical patients, who handle excess salt and water badly due to reduced reserve capacity
the education of healthcare professionals on nutritional and fluid prescribing issues is critical in ensuring good patient care.
Funding
This work received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Acknowledgments
The authors are very grateful to Jane Berg and Dr Martin Craig Gannon for their comments on drafts of this article.
Permissions
Permissions cannot be obtained as patients are deceased, and relatives have not been approached to obtain permission, as this could potentially cause distress to them; however, the patients are not identifiable.
References
- 1.Ellershaw JE, Wilkinson S, editors. Care of the dying: a pathway to excellence. Oxford: Oxford University Press; 2003. [DOI] [Google Scholar]
- 2.Marie Curie Palliative Care Institute Liverpool. National care of the dying audit – hospitals (NCDAH). Round 3. Generic report 2011/2012. Liverpool: Marie Curie Palliative Care Institute Liverpool; 2012. www.liv.ac.uk/media/livacuk/mcpcil/documents/NCDAH-GENERIC-REPORT-2011-2012-FINAL.doc-17.11.11.pdf [Accessed 25 April 2013] [Google Scholar]
- 3.Edmonds P, Burman R, Prentice W. End of life care in the acute hospital setting. BMJ. 2009;339:b5048. doi: 10.1136/bmj.b5048. [DOI] [PubMed] [Google Scholar]
- 4.Christakis NA, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–73. doi: 10.1136/bmj.320.7233.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keys A, Brozek J, Henschel A, editors. et al. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 6.Royal College of Physicians, British Society of Gastroenterology. Oral feeding difficulties and dilemmas: a guide to practical care, particularly towards the end of life. London: RCP; 2010. [Google Scholar]
- 7.Hearing S. Refeeding syndrome. BMJ. 2004;328:908–9. doi: 10.1136/bmj.328.7445.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336:1495–8. doi: 10.1136/bmj.a301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition. 2001;17:632–7. doi: 10.1016/S0899-9007(01)00542-1. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence. Nutrition support in adults: oral nutrition support, enteral tube feeding and parenteral nutrition. London: NICE; 2006. www.nice.org.uk/page.aspx?o=cg032 [Accessed 25 April 2013]. [PubMed] [Google Scholar]
- 11.De Silva A, Smith T, Stroud M. Attitudes to NICE guidance on refeeding syndrome. BMJ. 2008;337:a680. doi: 10.1136/bmj.a680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell-Tuck J, Gosling P, Lobo DN, et al. British consensus guidelines on intravenous fluid therapy for adult surgical patients: GIFTASUP. Redditch: BAPEN; 2008. www.bapen.org.uk/pdfs/bapen_pubs/giftasup.pdf [Accessed 25 April 2013]. [DOI] [PubMed] [Google Scholar]