Key points
The results of thyroid function tests (TFTs) must always be interpreted in light of the clinical status of the patient: hypothyroid, euthyroid or hyperthyroid
Awareness of the conditions and/or disorders that can be associated with different patterns of TFTs guides further investigation and management
Confounding factors that may might influence thyroid status (eg intercurrent non-thyroidal illness or medications) should be excluded before embarking on further biochemical, radiological or genetic testing
Screening for interference in thyroid hormone (T4 and T3) and/or thyrotropin (TSH) laboratory assays should be considered in any patient with apparently anomalous or discordant TFTs
Referral to a specialist laboratory and/or endocrine service is required when anomalous or discordant TFTs cannot be readily explained by confounding intercurrent illness, medication or assay interference
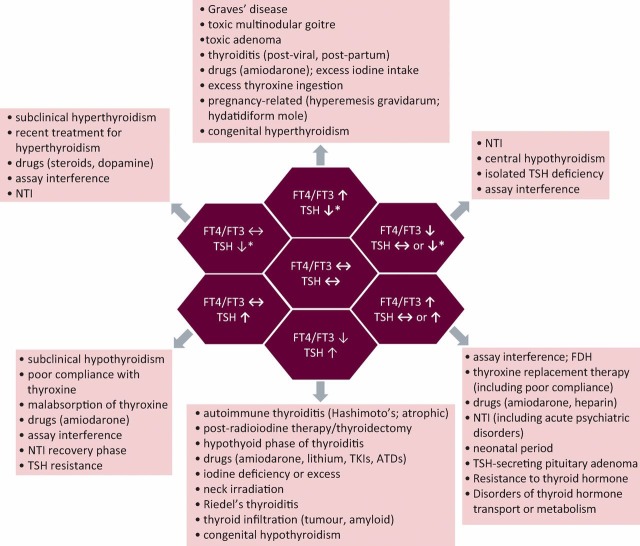
Disorders of thyroid function are common (estimated UK prevalence is 1–4%). Although thyroid disease in its most florid form is easily recognised, patients often manifest symptoms and/or signs that are non-specific, and present to clinicians in many different specialties.1,2 Accordingly, a high clinical index of suspicion is required, and confirmation of diagnosis usually depends on accurate measurement and interpretation of thyroid function tests (TFTs). In most cases the results of TFTs are straightforward and present a familiar pattern that is easy to recognise. However, in an important subgroup of patients, they can seem confusing, either by virtue of being discordant with the clinical picture (eg inability of supraphysiological levothyroxine therapy to suppress thyroid stimulating hormone (TSH; thyrotropin) in hypothyroidism) or because different assay results appear to contradict each other (eg raised thyroid hormone (TH) levels, but with non-suppressed TSH; or low TH levels with inappropriately normal or low TSH) (Fig 1). Here, we highlight the main causes of commonly encountered patterns of abnormal thyroid function, including so-called ‘anomalous/discordant TFTs’, and propose a simple strategy for their investigation.
Fig 1.
Schematic representation of different patterns of thyroid function tests and their causes. ATDs = antithyroid drugs; FDH = familial dysalbuminaemic hyperthyroxinaemia; FT3 = free triiodothyronine; FT4 = free thyroxine; NTI = non-thyroidal illness; TH = thyroid hormone; TKIs = tyrosine kinase inhibitors; TSH = thyroid-stimulating hormone (thyrotropin). *signifies that TSH can be either fully suppressed (eg as seen in classical primary hyperthyroidism) or partially suppressed (ie measureable, but below the lower limit of normal).
Interpreting thyroid function tests (TFTs)
General considerations
In any given individual, TH (thyroxine, T4; triiodothyronine, T3) levels remain relatively constant and reflect the ‘set-point’ of the hypothalamic–pituitary–thyroid (HPT) axis in that individual.3 Changes in thyroid status are typically associated with concordant changes in TH and TSH levels (eg raised T4 and T3 with suppressed TSH in thyrotoxicosis; low T4 and T3 with elevated TSH in hypothyroidism). However, the population reference ranges for TH are relatively wide (especially for T4) and changes in TH levels sufficient to render a patient hypo- or hyperthyroid might not necessarily be associated with numerically abnormal T4 or T3 levels (as occurs in so-called ‘subclinical’ hypo- or hyperthyroidism). Therefore, TSH has traditionally been recommended as a frontline screening test for thyroid dysfunction, because relatively modest changes in TH levels are associated with marked excursions in TSH. However, screening exclusively with TSH means that some patients will be misdiagnosed, whereas other conditions might be missed altogether (by virtue of returning a TSH result that falls within the reference range despite overt HPT dysfunction) (Box 1). Accordingly, many UK laboratories now routinely offer combination screening with T4 and TSH.
Box 1. Conditions in which measurement of TSH alone might be misleading.
It is also important to consider whether total (TT4 and TT3) or free (FT4 and FT3) TH levels are being measured. If the former, then changes in binding proteins can confound interpretation of results: T4 and T3 are heavily protein bound; thus, total, but not free, hormone measurements are affected by alterations in binding protein status (eg exogenous oestrogen therapy and pregnancy increase TT4 levels through elevation of T4-binding globulin (TBG)).
Specific considerations
In all patients in whom thyroid function testing is being considered, it is important to keep the following in mind.
Thyroid status. Ideally, results of TFTs should confirm one’s clinical suspicion, namely that the patient is euthyroid, hypothyroid or hyperthyroid. Reassessment of clinical status is particularly important when faced with an unexpected TFT result because it provides an important clue to the test (TSH, T4 and/or T3) that is likely to be discordant and guides further investigation (see below).
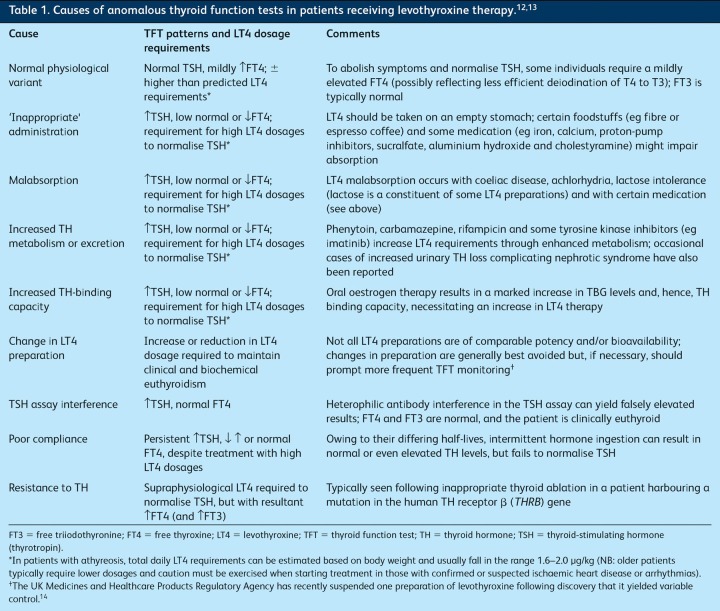
Levothyroxine therapy. Anomalous and/or discordant TFTs are not infrequently seen in patients being treated with levothyroxine (LT4) and can have several causes (Table 1).
Table 1.
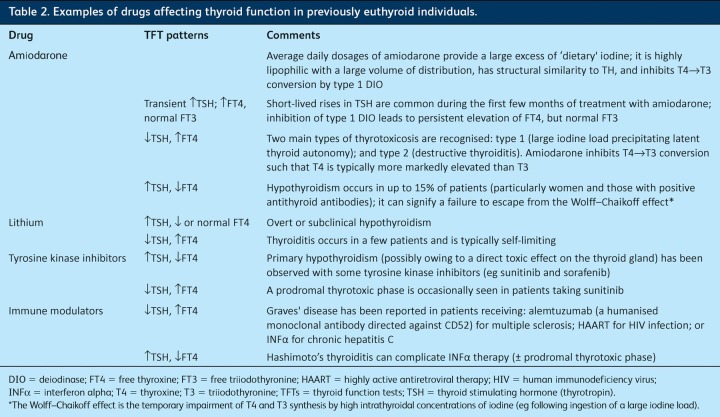
Medications. Several commonly prescribed drugs can cause thyroid dysfunction as an adverse effect and a careful medication history should be taken in all patients with thyroid disease (Table 2).4 Other agents can interfere with some laboratory measurements, producing an apparently discordant TFT picture. For example, both fractionated and unfractionated heparin activate endothelial lipoprotein lipase with hydrolysis of triglycerides and an increase in circulating free fatty acid levels that, in some individuals, leads to displacement of T4 and T3 from TBG, thus raising free (but not total) TH levels. Interestingly, such changes are not generally associated with clinical thyrotoxicosis, and TSH is usually normal.
Table 2.
Examples of drugs affecting thyroid function in previously euthyroid individuals.
Hypothalamic–pituitary disease. In the presence of suspected or confirmed central HPT dysfunction, TSH should not be considered to be a reliable marker of thyroid status and FT4 (±FT3) levels must be used to guide management. Common pitfalls in this setting include misinterpreting a low or undetectable TSH in a patient taking T4 as signifying over-replacement, or assuming that a normal TSH level equates with euthyroidism.5
Non-thyroidal illness (‘sick euthyroid syndrome’). Intercurrent illness can affect thyroid function in several different ways (Fig 1). Commonly, TSH is low normal or partially suppressed, with low or normal free TH. Therefore, it is generally advisable only to check thyroid function in those patients in whom primary disturbance of one or other part of the HPT axis is suspected.
Pregnancy. Physiological alterations during pregnancy lead to changes in TH and TSH levels. Accordingly, trimester-specific reference ranges for TH and TSH should be used whenever possible. Recently published guidelines offer useful advice on the management of suspected or confirmed thyroid dysfunction in pregnancy.6
Assay interference. In any patient in whom anomalous or discordant TFTs are not readily explained by the above, consideration must be given to whether one or other laboratory result could be erroneous. Genetic and acquired causes of interference in both TH and TSH assays are well recognised and can be screened for (eg cross-reacting heterophilic antibodies causing falsely low or elevated TSH are readily detected through TSH dilution studies, which return non-linear results in this context). It is important to note that simply sending a sample to another laboratory does not necessarily exclude assay interference (the same interference can affect different assay platforms) and specialist laboratory and/or endocrine advice should be sought in suspected cases.7
TFT patterns
Fig 1 illustrates the different patterns of TFTs that might be encountered in clinical practice.
Low TSH and high FT4 (and FT3)
This is the picture of primary hyperthyroidism and, in this context, TSH is usually undetectable (typically <0.03 mU/l for modern ultrasensitive TSH assays). In the UK, Graves’ disease (GD) and toxic multinodular goitre (MNG) are the two most common causes (Fig 1).
High TSH and low FT4 (and FT3)
This combination of TFTs suggests primary hypothyroidism and, in the UK, is most usually the result of autoimmune thyroiditis (Hashimoto’s disease or atrophic thyroiditis) or follows radioiodine or thyroidectomy.2 Other more rare causes are shown in Fig 1.
Low TSH and normal FT4 and/or FT3
Subclinical hyperthyroidism (eg owing to a ‘low-grade’ toxic multinodular goitre or adenoma) is characterised by apparently ‘normal’ TH levels, but low TSH.8 As described above, although within the reference range, TH levels in these individuals are higher than the normal set-point of the HPT axis, resulting in TSH suppression. Most endocrinologists consider that the term ‘subclinical hyperthyroidism’ should be reserved for those patients in whom TSH is fully suppressed, because this is the group for whom there is the greatest evidence of adverse sequelae (atrial fibrillation and osteoporosis) if TSH remains suppressed in the long term (especially postmenopausal women and patients older than 65 years of age).8 When TSH is not fully suppressed, a period of surveillance can reasonably be adopted, although emerging evidence suggests that even these individuals will have greater morbidity in the longer term. However, in a significant proportion of patients with subnormal TSH values, levels return to normal during follow-up without intervention. Non-thyroidal illness (NTI) is another common cause of transiently low (but not fully suppressed) TSH, with resolution following recovery,9 and emphasises the importance of not acting on a single TSH result. In cases of exogenous T4 administration, it is generally desirable to avoid full TSH suppression, with the notable exception of thyroid cancer treatment.
High TSH and normal FT4 (and FT3)
This pattern of results can signify so-called ‘subclinical hypothyroidism’.8 Measurement of antithyroid peroxidase (TPO) antibody titres is a useful adjunct to help guide decision-making (eg surveillance vs LT4 therapy) because positivity predicts a higher risk of subsequent progression to overt hypothyroidism. Again, confirmation that the TSH is persistently raised is generally advised, although in certain circumstances (eg pregnancy) T4 replacement can be instituted without delay. LT4 therapy should be particularly considered in: younger patients (especially when symptomatic); women who wish to become or who are pregnant; patients with positive TPO antibodies or a rising trend in serial TSH levels; and the presence of marked hypercholesterolaemia.8 Raised TSH with normal TH levels is also seen with assay interference and in patients taking exogenous T4, where it might reflect malabsorption, altered metabolism or poor compliance (Fig 1 and Table 1).
Low FT4 (and/or low FT3) with inappropriately normal or low TSH
This combination of TFTs is also seen in NTI and resolves with recovery.9 However, in the absence of a clear alternative diagnosis, central hypothyroidism must be considered and a full pituitary hormone profile, including assessment for secondary hypoadrenalism, is mandatory. T4 therapy in patients with untreated hypocortisolism can be life threatening and failure to recognise a large pituitary mass compressing the optic chiasm can result in irreversible visual loss.
High FT4 (±FT3) with inappropriately normal or high TSH
This unusual pattern of TFTs is most commonly accounted for by assay interference, confounding effects of drugs (eg amiodarone or heparin) or T4 replacement therapy (including non-compliance) (Table 1).7 Once these have been excluded, two rare but important conditions must be distinguished: resistance to TH (RTH) and a TSH-secreting pituitary adenoma (TSHoma), and referral to a specialist endocrine centre is advised.10,11
Conclusions
A structured approach, founded on careful clinical assessment, judicious use of TFTs and knowledge of the conditions and/or disorders that are associated with different TFT patterns enables most TFTs to be reliably interpreted with avoidance of inappropriate investigations and/or treatment.
References
- 1.Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ. 2006;332:1369–73. doi: 10.1136/bmj.332.7554.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801. [DOI] [PubMed] [Google Scholar]
- 3.Andersen S, Pedersen KM, Bruun NH, et al. Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87:1068–72. doi: 10.1210/jc.87.3.1068. [DOI] [PubMed] [Google Scholar]
- 4.Barbesino G. Drugs affecting thyroid function. Thyroid. 2010;20:763–70. doi: 10.1089/thy.2010.1635. [DOI] [PubMed] [Google Scholar]
- 5.Koulouri O, Auldin MA, Agarwal R, et al. Clin Endocrinol. 2011;74:744–9. doi: 10.1111/j.1365-2265.2011.03984.x. [DOI] [PubMed] [Google Scholar]
- 6.Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurnell M, Halsall DJ, Chatterjee VK. What should be done when thyroid function tests do not make sense. Clin Endocrinol. 2011;74:673–8. doi: 10.1111/j.1365-2265.2011.04023.x. [DOI] [PubMed] [Google Scholar]
- 8.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142–54. doi: 10.1016/S0140-6736(11)60276-6. [DOI] [PubMed] [Google Scholar]
- 9.Mebis L, Van den Berghe G. Thyroid axis function and dysfunction in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25:745–57. doi: 10.1016/j.beem.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Beck-Peccoz P, Persani L, Mannavola D, Campi I. TSH-secreting adenomas. Best Pract Res Clin Endocrinol Metab. 2009;23:597–606. doi: 10.1016/j.beem.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Gurnell M, Visser T, Beck-Peccoz PB, Chatterjee VKK. Resistance to thyroid hormone. In: Jameson JL, De Groot LJ, editors. Endocrinology. 6th edn. Philadelphia: Saunders Elsevier; 2010. pp. 1745–59. [Google Scholar]
- 12.Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab. 2009;23:781–92. doi: 10.1016/j.beem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. How do you approach the problem of TSH elevation in a patient on high-dose thyroid hormone replacement? Clin Endocrinol. 2009;70:671–3. doi: 10.1111/j.1365-2265.2009.03536.x. [DOI] [PubMed] [Google Scholar]
- 14.Medicines and Healthcare Products Regulatory Agency. 2012. www.mhra.gov.uk/NewsCentre/Pressreleases/CON143688 [Accessed 4 April 2013].