ABSTRACT
Despite the significant public health issue that it poses, only five medical treatments have been approved for Alzheimer’s disease (AD) and these act to control symptoms rather than alter the course of the disease. Studies of potential disease-modifying therapy have generally been undertaken in patients with clinically detectable disease, yet evidence suggests that the pathological changes associated with AD begin several years before this. It is possible that pharmacological therapy may be beneficial in this pre-clinical stage before the neurodegenerative process is established. Techniques providing earlier diagnosis, such as cerebrospinal fluid biomarkers and amyloid positron emission tomography neuroimaging, are key to testing this theory in clinical trials. Recent results from trials of agents such as aducanumab are encouraging but must also be interpreted with caution. Such medicines could potentially delay the onset of dementia and would therefore markedly reduce its prevalence. However, we currently remain a good distance away from clinically available disease-modifying therapy.
KEYWORDS: Alzheimer’s disease, pharmacological therapy, amyloid
Introduction
Dementia is a general term for a decline in cognitive ability severe enough to interfere with daily life. Alzheimer’s disease (AD) accounts for almost three-quarters of cases of dementia, with the remainder accounted for by vascular dementia (VaD), mixed Alzheimer’s and VaD, dementia with Lewy bodies, and frontotemporal dementia.
To many clinicians, the contrast between the significant advances in the last two decades in medical treatment for a wide range of illnesses, including targeted therapies, such as herceptin, for many cancers, as well as revolutionary combination drug therapies for HIV, and the lack of progress in the pharmacological treatment of dementia due to AD and other causes is surprising. However, it is increasingly clear that whereas the former illnesses are almost invariably well-defined and circumscribed disease entities, the syndrome of dementia is a multifactorial condition for most patients,1 and even within diagnostic entities such as AD, it is likely that there are sub-classifications of therapeutic significance.
Both of these findings have significant ramifications for treatment. The enmeshing of vascular disease and neurodegenerative illness in later life mean that dementia in later life is best viewed as a geriatric syndrome. The relevance of this conceptualisation is that geriatric syndromes, such as falls, rarely respond to single interventions, and pharmacological interventions are likely to succeed best in terms of one component of a package, which might include medication review, nutrition and exercise intervention, and cognitive stimulation/training, an approach supported by a recent multi-modal intervention study among older people at risk of developing dementia.2
Despite the significant public health issue that dementia poses, to date only five medical treatments have been approved for AD, involving only two classes of drugs, and these act to control symptoms rather than alter the course of the disease. Additionally, relatively few clinical trials have been undertaken in AD in the last decade, and these have had a 99.6% failure rate.3 As a result, the goal of disease-modifying therapy remains elusive, with the currently available medications acting to control symptoms only. In this review, we summarise current pharmacological treatment for AD, as well as highlighting potential future therapies, while reflecting on the underlying aetiological mechanisms on which these treatments are based.
Neurodegenerative pathways implicated in AD
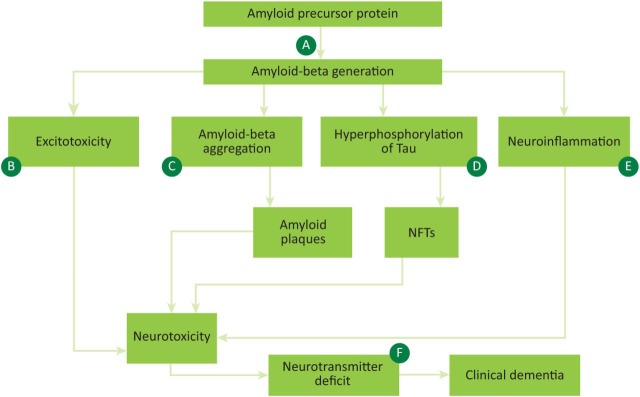
Several overlapping mechanisms have been proposed to explain the underlying pathology of AD, and both current and potential future treatments are based on modification of these pathways (Fig 1).
Fig 1.
Aetiology of Alzheimer's disease with therapeutic targets. A – secretase enzyme inhibitors; B – NMDA receptor modulators, eg memantine; C – immunotherapy, including immunisation and direct anti-amyloid therapy, including monoclonal antibodies; D – anti-tau therapy; E – anti-inflammatory treatments, including NSAIDs; F – anticholinesterase inhibitors, eg donepezil. APP = amyloid precursor protein; NFTs = neurofibrillary tangles; NMDA = N-methyl-D-aspartate; NSAIDs = non-steroidal anti-inflammatory drugs.
Amyloid cascade hypothesis
The amyloid hypothesis of AD began to gain traction in the 1990s, and centres on abnormal processing of the amyloid precursor protein (APP), leading to production of amyloid-beta (Aβ).4 Secretase enzymes cleave APP and aberrancy of this process, specifically mutations in gamma and beta-secretases, can lead to the abnormal production of Aβ.5 Aβ can then trigger a cascade leading to synaptic damage and neuron loss, and ultimately to the pathological hallmarks of AD: amyloid plaques and neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein, with resulting neurodegeneration (Fig 1).6
Tau hypothesis
Tau is a protein expressed in neurons that normally functions in the stabilisation of microtubules in the cell cytoskeleton.7 Hyperphosphorylation causes it to accumulate into these NFT masses inside nerve cell bodies. These tangles then aberrantly interact with cellular proteins, preventing them from executing their normal functions. Hyperphosphorylation occurs downstream of Aβ, with research suggesting that accumulation of Aβ may initiate this process.8 Additionally, there is evidence that toxic tau can enhance Aβ production via a feedback loop mechanism.9
Cholinergic hypothesis
An initial breakthrough in AD came in the 1970s with the demonstration of a cholinergic deficit in the brains of patients with AD, mediated by deficits in the enzyme choline acetyltransferase.10 This, along with the recognition of the role of acetylcholine in memory and learning, led to the cholinergic hypothesis of AD and stimulated attempts to therapeutically increase cholinergic activity. Cholinergic depletion is a late feature of the neurodegenerative cascade. Cholinesterase inhibitors block the cholinesterase enzyme, which breaks down acetyl choline at the synaptic cleft, potentiating cholinergic transmission.
Excitotoxicity
Excitotoxicity, defined as overexposure to the neurotransmitter glutamate, or overstimulation of its N-methyl-D-aspartate (NMDA) receptor, plays an important role in the progressive neuronal loss of AD.11 It is thought that loss of cholinergic neurons is affected by this process, resulting in excessive influx of calcium into cells.
Other important aetiological mechanisms
Vascular disease
While traditionally it was felt that vascular disease was the underlying factor in the development of VaD, it is clear that vascular burden also plays a role in AD pathogenesis, and that there is significant overlap between these two dementia subtypes.12 Vascular risk factors, such as high BMI, smoking, hypercholesterolemia and hypertension, have been associated with an increased risk of developing clinical AD.13 While vascular lesions such as cerebral amyloid angiopathy and white matter hyperintensities are common in patients with AD,14 hypertension is also associated with the development of specific neuropathological hallmarks of AD such as NFTs,15 and this association appears to be stronger when hypertension is present in mid rather than late life.16 Thus, vascular disease may directly affect amyloid plaques or NFTs by increasing their formation or reducing their elimination from the brain.17
Given these findings, it would seem to follow that control of vascular risk factors may slow the rate of decline of cognition in patients with AD; however, this is not yet supported by data from randomised controlled trials.18
Diabetes and hyperinsulinaemia
There is a well-established association between diabetes and AD19 and while vascular burden undoubtedly plays a role in this, it appears insulin dysregulation and abnormal central nervous system (CNS) insulin metabolism20 are also important independent factors. Insulin can cross the blood–brain barrier and is also produced in the CNS. Some studies have suggested a role for CNS insulin in controlling tau phosphorylation and protecting against Aβ accumulation via insulin degrading enzyme,21 while others have demonstrated lower levels of insulin in the cerebrospinal fluid (CSF) of people with AD.22 The potential pathway for this involves peripheral hyperinsulinaemia downregulating insulin uptake across the blood–brain barrier because of oversaturation above physiological levels.23 This results in the downregulation of insulin-degrading enzyme in the brain, which mediates amyloid clearance. Less insulin signalling may also enhance the activity of the enzyme glycogen synthase kinase 3, which can promote formation of tau and NFTs.24
These findings have led to the theory that drugs used in diabetes may be able to modify the pathophysiology of AD25 and a study of intranasal insulin as a therapy in mild cognitive impairment (MCI) and AD is currently being conducted after encouraging results from small pilot studies.26
Apolipoprotein gene
The Apo epsilon 4 (Apoe4) allele of the apolipoprotein (apo) gene, coding for a protein involved in cholesterol metabolism and lipid transport, has been identified as the primary genetic risk factor for AD.27 Individuals with one copy of the e4 allele have a three-fold higher chance of developing AD, while those with two alleles have an odds ratio of 14.9 for developing AD.28 The mechanism by which Apoe4 increases the risk of AD remains unclear but it may act via its effect on Aβ aggregation and clearance, thereby influencing the onset of Aβ deposition. Other proposed mechanisms include effects on synaptic function, neurotoxicity, hyperphosphorylation of tau, and neuroinflammation.29 It has been proposed that modulation of the Apo-related receptor at the blood–brain barrier may offer a therapeutic target to affect Aβ clearance, while other therapeutic options may include modulation of Apoe4 levels or converting Apoe4 to Apoe3, but clinical trials are currently lacking in this regard.30
Neuroinflammation
Neuroinflammation, an inflammatory response in the CNS characterised by accumulation of glial cells, appears to be a central event in AD pathophysiology.31 The brain was traditionally considered an ‘immune-privileged’ organ, isolated from the immune system by factors such as the blood–brain barrier and apparent inability of brain immune cells to mount an innate immune response,32 but opinion on this has subsequently shifted dramatically. In the 1990s, seminal epidemiological evidence suggested that anti-inflammatory drugs may have a protective effect in AD33 and neuroinflammatory cascades are now considered an important target for treatment in AD,34 and both amyloid plaques and NFTs may act as drivers for this immune response.
Unfortunately, meta-analyses have demonstrated no benefit of non-steroidal anti-inflammatory drugs, aspirin or steroids over placebo in patients with already symptomatic AD;35 however, some evidence suggests that naproxen may have a role in prevention of AD in healthy older people.36 It may be that the therapeutic window for such treatment occurs early in the disease process and as such, by the time symptoms emerge, this opportunity has been lost.37
Drug therapy
Much of the research in AD in the last decade has been directed towards disease-modifying therapy that will alter the course of the disease rather than act on symptoms alone, however the lack of effective disease-modifying drugs arising from these studies reflects the challenges involved in developing a therapeutic agent with potential to modify the course of a disease as complex as AD.38
Approved drug treatments
Cholinesterase inhibitors
Tacrine was the first-generation cholinesterase inhibitor but was limited by hepatotoxic side effects.39 Donepezil, rivastigmine and galantamine then followed, with the former probably the most widely used agent.
Efficacy appears similar between these different agents so choice should be based on cost, individual patient tolerance and physician experience.
Donepezil is prescribed at an initial dose of 5 mg in the evening, increased to 10 mg after one month if appropriate.40 Response is gauged by a rating of better memory, function or behaviour by the patient or carer: there is no point in trying to measure change with brief mental status schedules such as the mini mental state examination, as these are not designed to detect clinically relevant change. If there is no response after three months of treatment it is reasonable to consider stopping the medicine at that stage although opinions around this can differ. Common side effects are gastrointestinal, fatigue and muscle cramps, and all patients should have an electrocardiogram prior to commencing a cholinesterase inhibitor because of the risk of sick sinus syndrome and other conduction abnormalities. Care should be taken if considering commencing a cholinesterase inhibitor in a person with a history of peptic or duodenal ulcer disease. Small numbers of patients may exhibit an acute worsening of cognition or agitation on starting; in which case, the medicine should be stopped immediately.
Average effects on cognition and function are generally modest41 and response rates are variable, with around one-third of patients showing no benefit and a smaller proportion (around one-fifth) showing larger benefit. It is expected that about one-third of patients may not tolerate a cholinesterase inhibitor because of side effects.
Memantine
Memantine uncompetitively blocks the NMDA receptor42 and, thus, may be neuroprotective by preventing neuron loss, as well as improving symptoms by helping to restore function of damaged neurons (Fig 1).
Memantine is initially prescribed at a dose of 5 mg daily, increasing weekly by 5 mg to a maximum dose of 20 mg.40 It is generally well tolerated, with fewer side effects than cholinesterase inhibitors, although dizziness, headache, somnolence, constipation and hypertension can occur.
Memantine has been shown to have modest benefits in moderate to severe AD, with little evidence supporting its use in milder AD.43 Additionally, the addition of memantine to donepezil monotherapy may be beneficial in those with mid-stage AD or who are deteriorating cognitively.44 Neither memantine nor donepezil are beneficial in MCI.
While these medications represent our best current available pharmacological treatments in AD, they have relatively small average overall effect and do not alter the course of the underlying neurodegenerative process. It is likely that the down regulation of cholinergic transmission occurs too far downstream in this process for treatments such as cholinesterase inhibitors to exhibit such an effect.45 With this in mind, targeting the pathological process ‘upstream’ has also been the focus of much attention (Fig 1).
Potential future drug treatments
Anti-amyloid therapy
Until recently, several high-profile clinical trials of pharmacological agents targeted at modifying this amyloid cascade have been undertaken, with largely disappointing results. These agents generally had three different target sites: directly targeting Aβ, and either the gamma or beta-secretase enzymes involved in APP cleavage.46
Beta-secretase enzyme
Small molecule beta-secretase inhibitors have demonstrated reduced CSF beta-amyloid compared to controls. Phase II/III clinical trials of two agents, AZD3293 and MK-8931, are underway and due to be completed in 2019.47
Gamma-secretase enzyme
Phase III clinical trials of semagacestat, a small molecule gamma-secretase inhibitor,48 including over 3,000 patients, were discontinued in 2010 because of no improvement in cognition in the study group and worsening cognition at higher doses compared to controls.49 Incidence of skin cancer was also higher in the study group.
Tarenflurbil, related to the NSAID flurbiprofen, has been shown to reduce levels of Aβ by modulating the gamma-secretase enzyme, but demonstrated no improvement in cognition or function compared with placebo in phase III trials involving almost 1,700 patients.50
Immunisation
The initial human clinical trial of active immunisation against Aβ with the agent AN 1792 was stopped because of cases of meningoencephalitis in 6% of subjects.51 Subsequently, phase II trials of passive immunisation with intravenous immunoglobulin containing Aβ antibodies in a small number of patients with mild to moderate AD has been shown to be safe and potentially efficacious but phase III studies found no evidence for slowing progression of AD.52
Monoclonal antibodies
Bapineuzumab, a monoclonal antibody to Aβ,53 underwent a phase III clinical trial from 2007 to 2012 in patients with mild to moderate AD. It was shown to reduce the rate of amyloid accumulation in Apoe4 carriers but did not demonstrate any treatment effect on either cognitive or functional outcomes despite engaging its target.54
However, another monoclonal antibody, solanezumab, completed two phase III trials in 2012 and failed to reach predefined end points in patients with mild to moderate AD.55 Subsequently, pooled analysis showed that cognitive scores in a subgroup of patients with milder symptoms showed small benefits and an extension study, where those previously taking placebo crossed over to solanezumab, was undertaken. The difference in cognitive scores between these two groups was sustained for a further two years, suggesting that, while the absolute benefit was small, solanezumab had a potentially disease-modifying effect during the placebo-controlled phase.56
While this may represent the first evidence of disease modification on AD, results must be interpreted with caution57 and EXPEDITION 3, a placebo-controlled trial in mild AD, is ongoing with the aim of clarifying the results seen in this cohort.
Another monoclonal antibody, aducanumab, has shown promising results in patients with pre-clinical and mild AD. Phase Ib studies of 165 patients with pre-clinical/mild AD, demonstrated dose-dependent reductions in brain Aβ, as well as dose-dependent slowing of mini mental state examination and clinical dementia rating at one year and phase III studies are commencing soon.58
Tau-targeted therapy
Tau-targeted strategies that are currently in clinical trials include agents to prevent hyperphosphorylation, as well as those targeting microtubule stability and aggregation.59 Both lithium and valproic acid may act to inhibit tau phosphorylation60 but randomised controlled trials of these agents were negative.61 More recently, a phase II clinical trial of methylthioninium, a tau aggregation inhibitor, has demonstrated minor benefits in cognition in patients with both mild and moderate AD after 50 weeks therapy and there are plans to proceed to phase III trials.62
Future directions
Studies of potentially disease-modifying therapy up to now have generally been undertaken in patients with clinically detectable, established disease, while mounting evidence suggests that the pathological changes associated with dementia begin to occur several years before the emergence of the clinical syndrome.63 It is possible then that pharmacological therapy may be more beneficial in this pre-clinical stage before the neurodegenerative process has been established. Techniques to provide earlier diagnosis are key to testing this theory in clinical trials, facilitating trials in presymptomatic phases.
Early diagnosis
Currently, earlier diagnosis of AD is primarily based on CSF and neuroimaging biomarkers, reflected in new research diagnostic criteria for AD.
Cerebrospinal fluid biomarkers
Reflecting the underlying neuropathology of AD, CSF markers of amyloid and tau are reliable diagnostic tools to detect dementia. CSF Aβ42 is decreased in patients with AD, possibly because of deposition of the peptide in plaques.64 However, a decreased ratio of Aβ42/Aβ40 appears to be a more reliable marker than Aβ42 alone. Elevated total tau is a very sensitive marker for detection of AD but is also increased in other dementias, including VaD and frontotemporal dementia, while elevated phosphorylated tau, the major component of NFTs, is more specific than total tau. Combinations of these CSF markers have been used to improve diagnostic potential in early stages of AD, for example, MCI patients with both low Aβ42 and high tau levels were shown to have a substantially increased risk of developing AD.65 Despite this, the discriminatory power of CSF biomarkers in the differential diagnosis remains somewhat suboptimal as a lone diagnostic test66 and current strategies for presymptomatic evaluation involve combining results with neuroimaging findings.
Neuroimaging
Traditionally, structural neuroimaging in AD was used to rule out alternative diagnoses when presentations were atypical, eg brain tumours. However, functional imaging modalities such as 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), are now able to detect loss of neuronal function in asymptomatic individuals by measuring cerebral metabolic rates of glucose metabolism (CMRglc), a surrogate marker for neuronal activity.67 Patients with early AD demonstrate reduced CMRglc in parietotemporal, frontal and posterior cingulate cortices.68 These changes have also been shown to precede the onset of symptoms in individuals genetically at risk for AD,69 as well as in patients with MCI.70 However, there is some overlap of the hypometabolic regions found in AD with those found in other dementia subtypes, and the additional use of amyloid PET, which can estimate amyloid plaque surface area, improves diagnostic accuracy.71
Re-evaluating research diagnostic criteria
The need for presymptomatic diagnosis of AD has dictated that new research diagnostic criteria have been proposed, formalising the view that AD exists on a continuum from the presymptomatic phase, to a symptomatic, pre-dementia phase (MCI) and then to AD.72 In these new criteria, biomarkers are used to establish the presence of presymptomatic AD in research subjects with no or very subtle overt symptoms. Three pre-clinical stages of AD are proposed for research purposes: asymptomatic amyloidosis, asymptomatic amyloidosis with neurodegeneration, amyloidosis with neurodegeneration and subtle cognitive decline.73
These diagnostic criteria will facilitate a more formalised approach to the diagnosis of presymptomatic AD and provide a framework for studying early interventions in AD. While this definition of presymptomatic AD is certainly useful to, and will be primarily used in the research environment, we must also be mindful of the ethical implications of presymptomatic diagnosis of a disease with relatively ineffective available medical treatment.
Better trial design
Better selection of patients for clinical trials may yield more favourable clinical outcomes. For example, the ‘mild to moderate’ AD group may be too heterogeneous, and treatment effects within subgroups could be lost, as seen in the solanezumab trials. As well as earlier diagnosis, advanced biomarker analysis may also facilitate better selection of study subjects by allowing selection of patients with more uniform underlying pathology for targeted trials. This individualised approach would mirror several cancer therapies where bespoke treatment is targeted at specific patients, using markers such as HER2.
Conclusion
Given the rising prevalence of dementia, and the relative inadequacy of current available pharmacological treatment, the need to develop and implement new therapies is pressing. Recent results from trials of agents in AD with potential disease-modifying effects are encouraging but must also be interpreted with caution. Such medicines could potentially delay the onset of dementia and would therefore markedly reduce its prevalence and impact;74 however, currently we remain a good distance away from clinically available disease-modifying therapy. One would hope, however, that with advancing neuroimaging techniques and biochemical biomarkers, and an enhanced understanding of the underlying pathological processes involved, that this becomes a realistic goal in the near future.
In addition, while focus on the development of new therapies is very welcome, we must also be mindful that dementia is a multifaceted, complex disease, which by its nature directs a need for a multidisciplinary approach to care. Our focus in managing patients with dementia must remain well rounded and holistic, concentrating not just on pharmacological therapy but also on the complex biopsychosocial aspects of caring for this group of patients.
References
- 1.Brayne C. Davis D. Making Alzheimer’s and dementia research fit for populations. Lancet. 2012;380:1441–3. doi: 10.1016/S0140-6736(12)61803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ngandu T. Lehtisalo J. Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–63. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 3.Cummings JL. Morstorf T. Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy J. Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MP. LeVine H. Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19:311–23. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrano-Pozo A. Frosch MP. Masliah E. Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandelkow EM. Mandelkow E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998;8:425–7. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- 8.Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–8. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 9.Huang HCC. Jiang ZFF. Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer’s disease. J Alzheimers Dis. 2009;16:15–27. doi: 10.3233/JAD-2009-0960. [DOI] [PubMed] [Google Scholar]
- 10.Whitehouse PJ. The cholinergic deficit in Alzheimer’s disease. J Clin Psychiatry. 1998;59(Suppl 13):19–22. [PubMed] [Google Scholar]
- 11.Lipton SA. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res. 2005;2:155–65. doi: 10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- 12.Ravona-Springer R. Davidson M. Noy S. Is the distinction between Alzheimer’s disease and vascular dementia possible and relevant? Dialogues Clin Neurosci. 2003;5:7–15. doi: 10.31887/DCNS.2003.5.1/rravonaspringer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasnain M. Vieweg WV. Possible role of vascular risk factors in Alzheimer’s disease and vascular dementia. Curr Pharm Des. 2014;20:6007–13. doi: 10.2174/1381612820666140314153440. [DOI] [PubMed] [Google Scholar]
- 14.Kalaria RN. Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S115–23. doi: 10.1097/00002093-199912003-00017. [DOI] [PubMed] [Google Scholar]
- 15.Petrovitch H. White LR. Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 16.Tolppanen AMM. Solomon A. Soininen H. Kivipelto M. Midlife vascular risk factors and Alzheimer’s disease: evidence from epidemiological studies. J Alzheimers Dis. 2012;32:531–40. doi: 10.3233/JAD-2012-120802. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien JT. Markus HS. Vascular risk factors and Alzheimer’s disease. BMC Med. 2014;12 doi: 10.1186/s12916-014-0218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenti R. Pantoni L. Markus HS. Treatment of vascular risk factors in patients with a diagnosis of Alzheimer’s disease: a systematic review. BMC Med. 2014;12:160. doi: 10.1186/s12916-014-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu FPP. Lin KPP. Kuo HKK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luchsinger JA. Tang MXX. Shea S. Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–92. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 21.Farris W. Mansourian S. Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100:4162–7. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craft S. Peskind E. Schwartz MW, et al. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–8. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 23.Banks WA. Jaspan JB. Huang W. Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18:1423–9. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- 24.Martinez A. Gil C. Perez DI. Glycogen synthase kinase 3 inhibitors in the next horizon for Alzheimer’s disease treatment. Int J Alzheimers Dis. 2011;2011:280502. doi: 10.4061/2011/280502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarchoan M. Arnold SE. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes. 2014;63:2253–61. doi: 10.2337/db14-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craft S. Baker LD. Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corder EH. Saunders AM. Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 28.Farrer LA. Cupples LA. Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- 29.Kim J. Basak JM. Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu CCC. Liu CCC. Kanekiyo T. Xu H. Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–18. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morales I. Guzmán-Martínez L. Cerda-Troncoso C. Farías GA. Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014;8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carson MJ. Doose JM. Melchior B. Schmid CD. Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGeer PL. Schulzer M. McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–32. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 34.Heneka MT. Carson MJ. El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaturapatporn D. Isaac MGEKNG. McCleery J. Tabet N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst Rev. 2012;2:CD006378. doi: 10.1002/14651858.CD006378.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breitner JC. Baker LD. Montine TJ, et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement. 2011;7:402–11. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hensley K. Neuroinflammation in Alzheimer’s disease: mechanisms, pathologic consequences, and potential for therapeutic manipulation. Alzheimers Dement. 2010;21:1–14. doi: 10.3233/JAD-2010-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salomone S. Caraci F. Leggio GMM, et al. New pharmacological strategies for treatment of Alzheimer’s disease: focus on disease modifying drugs. Br J Clin Pharmacol. 2012;73:504–17. doi: 10.1111/j.1365-2125.2011.04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manning FC. Tacrine therapy for the dementia of Alzheimer’s disease. Am Fam Physician. 1994;50:819–26. [PubMed] [Google Scholar]
- 40.National Institute for Health and Care Excellence Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease. London: NICE; 2011. Available online at www.nice.org.uk/guidance/ta217/chapter/3-The-technologies. [Accessed 22 December 2015] [Google Scholar]
- 41.Birks JS. Harvey R. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev. 2003;(3):CD001190. doi: 10.1002/14651858.CD001190. [DOI] [PubMed] [Google Scholar]
- 42.Robinson DM. Keating GM. Memantine: a review of its use in Alzheimer’s disease. Drugs. 2006;66(11):1515–34. doi: 10.2165/00003495-200666110-00015. [DOI] [PubMed] [Google Scholar]
- 43.Areosa SA. Sherriff F. Memantine for dementia. Cochrane Database Syst Rev. 2003;(3):CD003154. doi: 10.1002/14651858.CD003154. [DOI] [PubMed] [Google Scholar]
- 44.Atri A. Molinuevo JL. Lemming O, et al. Memantine in patients with Alzheimer’s disease receiving donepezil: new analyses of efficacy and safety for combination therapy. Alzheimers Res Ther. 2013;5:6. doi: 10.1186/alzrt160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mufson EJ. Counts SE. Perez SE. Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev Neurother. 2008;8:1703–18. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghezzi L. Scarpini E. Galimberti D. Disease-modifying drugs in Alzheimer’s disease. Drug Des Dev Ther. 2013;7:1471–8. doi: 10.2147/DDDT.S41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassar R. BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimers Res Ther. 2014;6:89. doi: 10.1186/s13195-014-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henley DB. May PC. Dean RA. Siemers ER. Development of semagacestat (LY450139), a functional gamma-secretase inhibitor, for the treatment of Alzheimer’s disease. Exp Opin Pharmacother. 2009;10:1657–64. doi: 10.1517/14656560903044982. [DOI] [PubMed] [Google Scholar]
- 49.Doody RS. Raman R. Farlow M, et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med. 2013;369:341–50. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 50.Green RC. Schneider LS. Amato DA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–64. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson SR. Bishop GM. Lee HGG. Münch G. Lessons from the AN Alzheimer vaccine: lest we forget. Neurobiol Aging. 2004;25:609–15. doi: 10.1016/j.neurobiolaging.2003.12.020. 1792. [DOI] [PubMed] [Google Scholar]
- 52.Loeffler DA. Intravenous immunoglobulin and Alzheimer’s disease: what now? J Neuroinflammation. 2013;10:70. doi: 10.1186/1742-2094-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prins ND. Scheltens P. Treating Alzheimer’s disease with monoclonal antibodies: current status and outlook for the future. Alzheimers Res Ther. 2013;5:56. doi: 10.1186/alzrt220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salloway S. Sperling R. Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–33. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doody RS. Thomas RG. Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–21. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 56.Siemers ER. Sundell KL. Carlson C, et al. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement. 2016;12:110–20. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 57.McCartney M. Margaret McCartney: The “breakthrough” drug that’s not been shown to help in Alzheimer’s disease. BMJ. 2015;351:h4064. doi: 10.1136/bmj.h4064. [DOI] [PubMed] [Google Scholar]
- 58.National Institute on Aging US Department of Health and Human Services. BIIB037 in prodromal or mild Alzheimer’s disease. Bethesda, MD: NIA; 2016. Available online at www.nia.nih.gov/alzheimers/clinical-trials/biib037-prodromal-or-mild-alzheimers-disease. [Accessed 5 April 2016] [Google Scholar]
- 59.Wischik CM. Harrington CR. Storey JM. Tau-aggregation inhibitor therapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88:529–39. doi: 10.1016/j.bcp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Tariot PN. Aisen PS. Can lithium or valproate untie tangles in Alzheimer’s disease? J Clin Psychiatry. 2009;70:919–21. doi: 10.4088/jcp.09com05331. [DOI] [PubMed] [Google Scholar]
- 61.Hampel H. Ewers M. Bürger K, et al. Lithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry. 2009;70:922–31. [PubMed] [Google Scholar]
- 62.Wischik CM. Staff RT. Wischik DJ, et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J Alzheimers Dis. 2015;44:705–20. doi: 10.3233/JAD-142874. [DOI] [PubMed] [Google Scholar]
- 63.Braak H. Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–7. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 64.Anoop A. Singh PK. Jacob RS. Maji SK. CSF Biomarkers for Alzheimer’s disease diagnosis. Int J Alzheimers Dis. 2010;2010:606802. doi: 10.4061/2010/606802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hertze J. Minthon L. Zetterberg H, et al. Evaluation of CSF biomarkers as predictors of Alzheimer’s disease: a clinical follow-up study of 4.7 years. J Alzheimers Dis. 2010;21:1119–28. doi: 10.3233/jad-2010-100207. [DOI] [PubMed] [Google Scholar]
- 66.Engelborghs S. Le Bastard N. The impact of cerebrospinal fluid biomarkers on the diagnosis of Alzheimer’s disease. Mol Diagn Ther. 2012;16:135–41. doi: 10.1007/BF03262201. [DOI] [PubMed] [Google Scholar]
- 67.Mosconi L. Berti V. Glodzik L, et al. Pre-clinical detection of Alzheimer’s disease using FDG-PET, with or without amyloid imaging. J Alzheimers Dis. 2010;20:843–54. doi: 10.3233/JAD-2010-091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mosconi L. Berti V. Glodzik L, et al. Pre-clinical detection of Alzheimer’s disease using FDG-PET, with or without amyloid imaging. J Alzheimers Dis. 2010;20:843–54. doi: 10.3233/JAD-2010-091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kennedy AM. Frackowiak RS. Newman SK, et al. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer’s disease. Neurosci Lett. 1995;186:17–20. doi: 10.1016/0304-3940(95)11270-7. [DOI] [PubMed] [Google Scholar]
- 70.Chételat G. Desgranges B. de la Sayette V, et al. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–7. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 71.Vandenberghe R. Adamczuk K. Van Laere K. The interest of amyloid PET imaging in the diagnosis of Alzheimer’s disease. Curr Opin Neurol. 2013;26:646–55. doi: 10.1097/WCO.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 72.Jack CR. Albert MS. Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–62. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Croisile B. Auriacombe S. Etcharry-Bouyx F. Vercelletto M. National Institute on Aging (US), Alzheimer Association. [The new 2011 recommendations of the National Institute on Aging and the Alzheimer’s Association on diagnostic guidelines for Alzheimer’s disease: Preclinal stages, mild cognitive impairment, and dementia] Rev Neurol (Paris) 2012;168:471–82. doi: 10.1016/j.neurol.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 74.Sloane PD. Zimmerman S. Suchindran C, et al. The public health impact of Alzheimer’s disease, 2000-2050: potential implication of treatment advances. Annu Rev Public Health. 2002;23:213–31. doi: 10.1146/annurev.publhealth.23.100901.140525. [DOI] [PubMed] [Google Scholar]