Abstract
Atrial fibrillation is driven by spontaneous electrical activation emerging from the pulmonary veins. Catheter ablation using either radiofrequency or cryothermal energy electrically isolates these veins from the left atrium, both reducing the burden of atrial fibrillation episodes and improving the patient’s symptoms. Catheter ablation is superior to antiarryhthmic drugs when patients are carefully selected. Underlying medical problems – including obesity, hypertension and obstructive sleep apnoea – should be optimally treated before considering ablation. Although this treatment has the potential to cure patients of their symptoms, they should be aware of the important associated procedural complications.
KEYWORDS: Atrial fibrillation, classification, mechanisms, stroke, radiofrequency ablation, cryoablation, complications
Key points
Atrial fibrillation (AF) is caused by abnormal electrical wavefronts generated at the mouth of the pulmonary veins.
Catheter ablation is curative treatment for AF.
Catheter ablation is much more effective when performed early in the natural history of the disease.
Concomitant pathology causing AF, such as hypertension, mitral valve disease, obesity, obstructive sleep apnoea and left ventricular dysfunction, must be optimally managed before AF ablation.
Major complications can occur during ablation and are an important consideration when discussing the procedure with patients.
Introduction
Atrial fibrillation (AF) is a massive problem in the ageing UK population, and catheter ablation offers the current generation of physicians a curative treatment strategy. A discussion of ablation requires an understanding of the electrophysiology underlying AF, so in this article we review the types and mechanisms of AF, summarise the catheter ablation technologies currently available and discuss the evidence and guidelines supporting their use. We conclude by looking at future developments for this exciting field.
Types and mechanisms of AF
The natural history of AF is to progress from short paroxysms that self-terminate, to longer episodes that will not terminate spontaneously (ie are persistent) and finally to the point when the atria can no longer support sinus rhythm – ie permanent AF. The precise definitions are provided in Box 1. Longstanding persistent AF is a useful term when assessing the outcomes of AF ablation because it is associated with worse outcomes than paroxysmal AF or AF of short duration. Fig 1 summarises the mechanisms that are important in the initiation and maintenance of AF.
Box 1.
Types and classification of AF. Classification system according to the 2012 Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society expert consensus document.8 AF = atrial fibrillation. ECG = electrocardiograph.
| AF episode: AF that is documented by ECG monitoring and has a duration of at least 30 seconds, or if less than 30 seconds, is present continuously throughout the ECG monitoring tracing. To diagnose the presence of subsequent episodes of AF, sinus rhythm has to be documented by ECG monitoring between AF episodes. |
| Paroxysmal AF: Recurrent AF (≥2 episodes) that terminates spontaneously within 7 days. Episodes of AF of ≤48 hours’ duration that are terminated with electrical or pharmacological cardioversion should also be classified as paroxysmal AF episodes. |
| Persistent AF: Continuous AF that is sustained beyond seven days or episodes of AF in which a decision is made to electrically or pharmacologically cardiovert the patient after ≥48 hours. |
| Longstanding persistent AF: Continuous AF of greater than 12 months’ duration. |
| Permanent AF: Persistent AF where a joint decision by the patient and clinician has been made to not try and restore sinus rhythm. |
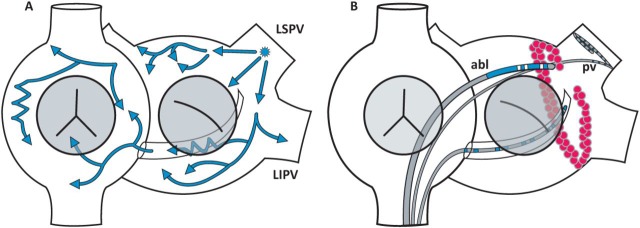
Fig 1.
Mechanism and ablation of AF. A cartoon of the right and left atria. Image A shows a focus of cells in the LSPV generating wavefronts (blue arrows) that break up into multiple wavefronts, resulting in the chaotic electrical activation that characterises AF. Image B shows an ABL delivering multiple ablation lesions (red dots) so that a line of conduction block is created around the mouth of the LIPV and LSPV. A catheter in the PV confirms that there is electrical silence within the pulmonary vein so that AF cannot be generated from these veins. ABL = ablation catheter; AF = atrial fibrillation; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; PV = pulmonary vein. Reproduced with permission from Dr Dominic Abrams, consultant cardiologist, Boston Children’s Hospital.
Paroxysmal AF is initiated primarily by ectopic beats originating from the pulmonary veins,1 although other anatomical sites of triggers are recognised, such as the superior vena cava.2 The pulmonary veins have unique ion channel distribution and electrophysiological properties such that automatic activity and local re-entry circuits within the muscular sleeves of these veins generate ectopic beats.3 A series of atrial ectopic beats emerging from the pulmonary veins at a high frequency (3–10 Hz) cannot be conducted across the rest of the atria in a 1:1 fashion, so electrical wavefronts break up causing activation of the atria to degenerate into a heterogeneous or chaotic pattern, easily recognised on the surface electrocardiogram as fibrillation waves. However, the long refractory periods of atrial tissue will not perpetuate these multiple wavefronts so that, when the pulmonary veins stop firing, AF terminates.
An episode of AF results in electrical (and later structural) remodelling of the atrial myocardium (shortening its refractory periods) and allows for the AF to be perpetuated even when the original triggers are removed (AF begets AF). There is widespread agreement on AF mechanisms up until this point. However, how persistent AF is sustained is the subject of two competing theories. The first is that there are multiple sites where a small group of cells generates high-frequency wavefronts. These ‘rotors’ are widely distributed across the atria but may be clustered around the entrances of the pulmonary veins, the left atrial appendage and the intra-atrial septum. The second is that multiple random wavefronts propagate around the atria bounded by lines of conduction block and available excitable myocardium. This opposes the rotor theory because sources of new wavefronts are not required. Another important factor is the local autonomic nerve supply of the heart, but further discussion of this is beyond the scope of this article.
The current rationale for catheter ablation to prevent future episodes of AF is set:
Electrically isolate the main source of triggers – the pulmonary veins.
If necessary, electrically isolate other sources of triggers – eg the superior vena cava.
Modify the substrate of the left and right atrium to prevent propagation of AF wavefronts and remove cells that may be acting as rotors.
For paroxysmal or persistent AF of short duration only the first step is required and the results are expected to be very good. If paroxysms of AF recur then step two might also be needed. Finally, for longstanding persistent AF, steps one and three will be required, with a lower success rate expected. It makes sense then to deal with AF at an early stage.
Catheter ablation technologies
Recent technological developments have had a big role in the evolution of catheter ablation of AF. Destroying (ablating) cells prevents them from propagating electrical activation. If a discrete line of ablation is delivered then conduction block will occur across the line. A circular line around the ostium of a pulmonary vein will therefore electrically isolate that vein from the left atrium it is attached to (Fig 1). The two main energies used for ablation are radiofrequency and cryothermal.
The radiofrequency ablation catheter used in AF ablation is a technological marvel. It is small enough to be delivered into the left atrium percutaneously via the right femoral vein and a transseptal hole made between the right and left atrium. The catheter is deflectable so it can be manipulated to any part of the complex geometry of the left atrium. It has a 4 mm platinum tip from which high frequency (20,000 Hz) radio waves are generated and conducted to a large indifferent electrode applied to the patient’s skin. The heating is concentrated at the tip of the catheter so ablation is confined to a small zone (lesions are usually the size of the tip of the catheter and 3–5 mm deep). Furthermore, the tip is recording at the endocardial surface the local electrical activation, the force that is being applied, the temperature and the impedance. All this information is updated in real time and relayed back to the operator. The catheter is not visualised by radiography but now by ubiquitous use of non-fluoroscopic three-dimensional mapping systems that allow for precise localisation of catheters and cardiac structures using electrical or magnetic fields created around the patient.
The commonest way to perform pulmonary vein isolation is to use the radiofrequency ablation catheter to create a series of ablation point lesions that overlap and form a line surrounding the mouth of the vein where it drains into the left atrium (Fig 1). The same catheter can then be used to create other lines of conduction block in the atria and target rotors, both of which are required for ablation of persistent AF. The difficulty with this technique is that it requires a reasonable level of operator skill, and, hence, extensive training is necessary. Furthermore, all the individual ablation lesions must be complete, because if even one lesion fails electrical wavefronts can break through the line and the arrhythmia can recur. A further problem is that procedures are long (up to 4 hours for longstanding persistent AF) and operators can tire standing in lead aprons for such a long time. This issue has been greatly helped by the development of robotic catheter navigation, which allows the operator to sit distant from the patient and away from the X-ray field, and to control the ablation catheter via a joy stick or computer mouse.4
Cryothermal energy – ie freezing – can also be used to ablate cells. Nitrous oxide (an inert gas in liquid form) is circulated in a closed circuit from the console along the catheter to the tip and back again. This circuit is insulated except at the tip where the temperature dramatically drops as low as –70°C, freezing the tissue that it is in contact with. Ice crystals form in the myocardial cells and as the crystals thaw again cell death is caused. Each ablation lesion takes much longer to form than for radiofrequency energy (3–5 min), so it is not feasible to produce multiple point lesions as described above. However, this technology lends itself to a one-shot approach, whereby the refrigerant is delivered to a pliable balloon inflated in the ostium of the pulmonary vein. The balloon occludes the vein (thus preventing the warming effect of blood flow) and ablation occurs where the equator of the balloon is in contact with the ostium of the vein. Thus, for a single 3–5 min freeze, a circular line of conduction block is produced. Similar tools have been developed using radiofrequency energy. However, these have not been as widely adopted as the cryoballoon.5 One-shot techniques have reduced the procedure time considerably, making it possible to perform an AF ablation in less than an hour, lending it to a day-case approach.
Current evidence on efficacy and safety of catheter ablation
There is a large amount of published data regarding outcomes of AF ablation. However, results need to be interpreted in light of study methods, patient selection, and endpoints reported. Several patient factors reduce the effectiveness of AF ablation, including duration of AF, age, concomitant cardiac disease, obesity, obstructive sleep apnoea, left atrium size and left ventricular function.6 Success of ablation is defined as freedom from AF at a particular timepoint, but this outcome can differ between studies depending on the duration of the blanking period (ie the period after ablation when recurrence of AF is censored), the frequency of arrhythmia monitoring, whether atrial tachycardia is thought to be a recurrence and the use of antiarrhythmic drugs. Because ablation is predominantly performed for patients’ symptoms, measures of quality of life, symptom scores and patient-reported outcomes should be considered. To date, there is no prospective evidence published that AF ablation improves prognosis or that it is safe to stop anticoagulation after an ablation if the CHA2DS2-Vasc score is greater than or equal to 1. There are retrospective data showing that after successful ablation the prognosis and stroke risk of patients are the same as those of a healthy matched population without AF.7 It must be remembered though that patients undergoing ablation are a highly selected group.
Broadly speaking, catheter ablation is more effective than antiarrhythmic drugs at maintaining sinus rhythm in patients with AF. Table 1 summarises the major studies that support the class 1 and 2a indications given to this treatment in international guidelines.8,9 It is important to recognise that most studies have been done in patients without comorbidities and with paroxysmal AF or AF of recent onset. The data in other patient populations, such as the very elderly or those with longstanding persistent AF or heart failure, are less robust.
Table 1.
Summary of RCTs and meta-analyses comparing catheter ablation of AF to medical therapy
| Study design | Study groups | n | Follow-up | AF freedom | Complications | Notes | |
|---|---|---|---|---|---|---|---|
| Oral 2006 12 | RCT comparing RFA with AAD for persistent AF | AAD
RFA |
69
77 |
12 months
12 months |
16%
66% |
–
0% |
77% crossed over from AAD to RFA group. 3% in RFA group remained on AAD. |
| The A4 study,
Jais 2008 13 |
RCT comparing RFA with AAD for paroxysmal AF (failed ≥1 AAD) | AAD
RFA |
59
53 |
12 months
12 months |
23%
89% |
–
3% |
63% crossed over from AAD to RFA group. 9% in RFA were treated with AAD. |
| Calkins 2009 14 | Meta-analysis of studies performed 1990–2006 | AAD
RFA |
3481
3481 |
12 (1–48) months
14 (2–30) months |
52%
71% |
–
5% |
Efficacy for RFA is quoted for ≥1 procedure off AAD. |
| Wilber 2010 15 | RCT comparing RFA with AAD for paroxysmal AF (failed ≥1 AAD) | AAD
RFA |
61
106 |
9 months
9 months |
16%
66% |
–
5% |
– |
| Cappato 2010 10 | Real world international multi-centre survey | RFA | 16,309 | 10±8 months | 75% (paroxysmal AF)
65% (persistent AF) |
5% | – |
| Andrade 2011 16 | Systematic review of cryoballoon ablation for AF | Cryoballoon | 1308 | 12 months | 73% | 6%* | 93% patients had paroxysmal AF. 6% of patients had persistent PNP with 0.4% persisting beyond 1 year. |
| MANTRA-PAF, Nielsen 2012 17 | RCT comparing RFA with AAD for treatment naive paroxysmal AF | AAD
RFA |
148
146 |
24 months
24 months |
44%
53% |
–
7% |
36% crossed over from AAD to RFA group. 9% in RFA remained on AAD after ablation. |
| STOP AF, Packer 2013 18 | RCT comparing cryoballoon ablation with AAD for paroxysmal AF (failed ≥1 AAD) | AAD
Cryoballoon |
82
163 |
12 months
12 months |
7%
70% |
–
3%* |
78% patients had paroxysmal AF.
Persistent PNP occurred in 11% with 1.5% persisting beyond 1 year. |
| RAAFT-2, Morillo 2014 19 | RCT comparing RFA with AAD for treatment naive paroxysmal AF | AAD
RFA |
61
66 |
24 months
24 months |
52%
72% |
–
9% |
Significantly improved quality of life in RFA group compared with the AAD group. |
| SARA, Mont 2014 20 | RCT comparing RFA with AAD for persistent AF (<1 year duration) | AAD
RFA |
48
98 |
12 months
12 months |
44%
70% |
–
6% |
– |
AAD = antiarrhythmic drug; AF = atrial fibrillation; PNP-phrenic nerve palsy; RCT = randomised controlled trial; RFA = radiofrequency ablation. *procedure related complications excluding phrenic nerve palsy.
Complications from AF ablation (Box 2) are caused by local or collateral damage during venous access, catheter manipulation and delivery of ablation, in the presence of heavy-duty systemic anticoagulation. A major complication is one that results in permanent injury or death, requires intervention for treatment, or prolongs or requires hospitalisation for more than 48 hours.8 In a worldwide survey involving 16,309 patients, major complications occurred in 4.5% of patients, with a mortality rate of 0.2%.10 In the published studies in Table 1, the major complication rate is 0–9%.
Box 2.
Major complications associated with catheter ablation for atrial fibrillation.
| Cardiac tamponade: Development of a pericardial effusion that results in haemodynamic compromise or requires urgent pericardiocentesis. It usually occurs during the procedure, but can present many days or weeks later. |
| Femoral vascular access complication: The commonest major complication. A haematoma, atrioventricular fistula or pseudoaneurysm requiring prolonged hospital stay, transfusion, percutaneous or surgical repair. |
| Stroke or transient ischaemic attack: A new neurological deficit typically within 24 hours but the high-risk period is up to 2 weeks after ablation. |
| Right phrenic nerve palsy: Common in cryoballoon ablation (5%). Absence of diaphragmatic movement during fluoroscopy as assessed by a sniff test. Rarely persists for more than 3 months and is usually asymptomatic. |
| Pulmonary vein stenosis: A reduction of diameter of one or more pulmonary veins, causing haemoptysis, breathlessness or recurrent chest infection. A greater than 50% narrowing causing symptoms requires intervention. |
| Atrio-oesophageal fistula: A rare but usually fatal consequence of ablation delivered to the posterior wall of the left atrium where, because of inflammation of the oesophagus, a connection forms. |
Who should be referred for AF ablation?
The presence of symptoms is a prerequisite for AF ablation. The international guidelines8,9 and those of the National Institute for Health and Care Excellence11 are remarkably consistent. It is the recommended treatment for patients who have paroxysmal AF when antiarrhythmic drugs are ineffective or not tolerated. It is reasonable to perform AF ablation as a first-line strategy (ie instead of drugs) for paroxysmal AF or for persistent AF of less than a year’s duration when antiarrhythmic drugs are ineffective or not tolerated. In patients who do not have symptoms, AF ablation should not be performed as a means to stop anticoagulation. AF ablation should not be considered until other comorbidities – particularly obstructive sleep apnoea, obesity, hypertension, hyperthyroidism, mitral valve disease and left ventricular dysfunction – are optimally treated.
What does the future hold?
The evolution of AF ablation will remain, for the foreseeable future, technology driven. Other energies for ablation, increased automation of catheter control, and new ways of recording and understanding the chaotic electrical activation of AF are all emerging to improve the efficacy and safety of this procedure. Such developments are costly, though, and it is a challenge to keep this treatment cost-effective. Until recently, AF ablation was provided at only a few specialist centres. However, now it can and should be introduced to any well equipped secondary care centre so that this highly effective intervention is made available to many more people.
References
- 1.Haissaguerre M. Jais P. Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Lin W-S. Tai C-T. Hsieh M-H, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–83. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 3.Nishida K. Datino T. Macle L. Nattel S. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J Am Coll Cardiol. 2014;64:823–31. doi: 10.1016/j.jacc.2014.06.1172. [DOI] [PubMed] [Google Scholar]
- 4.Ullah W. McLean A. Hunter RJ, et al. Randomized trial comparing robotic to manual ablation for atrial fibrillation. Heart Rhythm. 2014;11:1862–9. doi: 10.1016/j.hrthm.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Haegeli LM. Calkins H. Catheter ablation of atrial fibrillation: an update. Eur Heart J. 2014;35:2454–9. doi: 10.1093/eurheartj/ehu291. [DOI] [PubMed] [Google Scholar]
- 6.Takigawa M. Takahashi A. Kuwahara T. Long-term follow-up after catheter ablation of paroxysmal atrial fibrillation: the incidence of recurrence and progression of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:267–73. doi: 10.1161/CIRCEP.113.000471. [DOI] [PubMed] [Google Scholar]
- 7.Hunter RJ. McCready J. Diab I, et al. Maintenance of sinus rhythm with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart. 2012;98:48–53. doi: 10.1136/heartjnl-2011-300720. [DOI] [PubMed] [Google Scholar]
- 8.Calkins H. Kuck K-H. Cappato R, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632–96. doi: 10.1016/j.hrthm.2011.12.016. 2012. [DOI] [PubMed] [Google Scholar]
- 9.January CT. Wann LS. Alpert JS, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:2246–80. doi: 10.1016/j.jacc.2014.03.022. 2014. [DOI] [PubMed] [Google Scholar]
- 10.Cappato R. Calkins H. Chen S-A, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Care Excellence Atrial fibrillation: management. London: National Institute for Health and Care Excellence; 2014. [Google Scholar]
- 12.Oral H. Pappone C. Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–41. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 13.Jais P. Cauchemez B. Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 14.Calkins H. Reynolds MR. Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–61. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- 15.Wilber DJ. Pappone C. Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 16.Andrade JG. Khairy P. Guerra PG, et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011;8:1444–51. doi: 10.1016/j.hrthm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 17.Cosedis Nielsen J. Johannessen A. Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–95. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 18.Packer DL. Kowal RC. Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 19.Morillo CA. Verma A. Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2) JAMA. 2014;311:692. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 20.Mont L. Bisbal F. Hernández-Madrid A, et al. Catheter ablation vs antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study) Eur Heart J. 2014;35:501–7. doi: 10.1093/eurheartj/eht457. [DOI] [PMC free article] [PubMed] [Google Scholar]