Abstract
ST segment elevation myocardial infarction remains a significant contributor to morbidity and mortality worldwide, despite a declining incidence and better survival rates. It usually results from thrombotic occlusion of a coronary artery at the site of a ruptured or eroded plaque. Diagnosis is based on characteristic symptoms and electrocardiogram changes, and confirmed subsequently by raised cardiac enzymes. Prognosis is dependent on the size of the infarct, presence of collaterals and speed with which the occluded artery is reopened. Mechanical reperfusion by primary percutaneous coronary intervention is superior to fibrinolytic therapy if delivered by an experienced team in a timely fashion. Post-reperfusion care includes monitoring for complications, evaluation of left ventricular function, secondary preventive therapy and cardiac rehabilitation.
KEYWORDS: ST elevation myocardial infarction, primary percutaneous coronary intervention, fibrinolysis
Key points
ST elevation myocardial infarction remains an important cause of morbidity and mortality worldwide.
It is the result of an occlusive thrombus at the site of a disrupted coronary plaque.
Successful treatment is based on prompt diagnosis and institution of reperfusion therapy – ‘time is muscle’.
For the majority of patients, mechanical reperfusion with primary percutaneous coronary intervention is superior to fibrinolysis, provided it is delivered by an experienced team in a timely fashion.
Prognosis post ST segment elevation myocardial infarction is dependent on left ventricular function, lifestyle modifications and adherence to secondary preventive therapy.
Introduction
ST segment elevation myocardial infarction (STEMI) remains a major cause of premature death worldwide and, despite recent advances, controversies persist regarding its optimal management. Most STEMI are caused by atherosclerotic plaque rupture with vessel occlusion due to secondary thrombosis, with the extent of subsequent myocardial injury dependent on the area of myocardium subtended by the culprit vessel, duration of occlusion and presence of collaterals. Therefore, expeditious restoration of vessel patency represents the cornerstone of treatment of this condition, and although widespread uptake of primary percutaneous coronary intervention (PPCI) has significantly improved outcomes, debate continues regarding optimal antithrombotic/anticoagulant and interventional strategies employed.
Incidence and pathophysiology
STEMI accounted for 39% (31,653 patients) of all hospital admissions due to myocardial infarction in the UK (excluding Scotland).1 The unadjusted 30-day mortality rate for STEMI patients was 8.1% during the period 2013–14, compared with 12.4% ten years earlier (2003–2004);1 such an impressive decline can be attributed to improvements in emergency medical response, adoption of effective reperfusion strategies and widespread use of secondary preventive pharmacotherapy.
STEMI is most frequently triggered by acute thrombotic coronary artery occlusion at the site of a ruptured pre-existing atherosclerotic plaque, with plaque erosion and calcific nodules occurring in smaller proportions (approximately 30% and 5%, respectively). While historically ‘vulnerable’ plaques that have ruptured may appear mild or moderate at coronary angiography, in reality they are much more severe, as demonstrated in postmortem histological and intravascular imaging studies,2 likely due to positive remodeling, where the vessel expands in response to plaque enlargement and encroachment on the lumen.
Soft, or lipid-rich, plaques with a thin fibrous cap are particularly prone to rupture in response to repetitive cycles of shear stress, leading to exposure of the lipid core, localised platelet aggregation, fibrin deposition and formation of a propagating thrombus that eventually occludes the vessel. Coronary occlusion for more than 20 minutes results in irreversible damage to cardiac myocytes, and nearly half of potentially salvageable myocardium is lost within the first hour.3 The extent of myocardial cell death is clearly dependent on the size of the myocardial territory supplied by the culprit artery, duration of the occlusion, and the presence of a collateral circulation; intuitively, larger myocardial infarcts are more likely to cause death or cardiac failure.
Diagnosis and triage
Diagnosis of STEMI is based on history and electrocardiogram (ECG) changes. Although STEMI may be silent or present with sudden cardiac death, the majority of patients present with typical ischaemic-type chest discomfort accompanied by ST segment elevation on the 12-lead ECG, often with reciprocal ST segment depression in other leads. Atypical clinical presentations are well recognised, particularly in diabetic and elderly patients, and there are several less common ECG variants that either indicate, or are highly suggestive of STEMI. The latter include anterior ST depression with dominant R waves and upright T waves in V1–V3 (true posterior STEMI), new/presumed new left bundle branch block (although specificity is somewhat poor)4 and isolated ST elevation in lead aVR with widespread ST depression in other leads (which may be indicative of left main coronary artery occlusion).5 Echocardiography may be a useful tool in ruling out acute STEMI by demonstrating absence of regional wall motion abnormalities.
Raised cardiac biomarkers confirm a diagnosis of STEMI. Troponin measurements are favoured over other biomarkers because of superior sensitivity and specificity; however, the decision to administer reperfusion therapy should not await the results of cardiac enzyme assays owing to the need to institute such therapy emergently.
Management
The key priority in the management of STEMI is rapid restoration of vessel patency in order to maximise myocardial salvage (‘time is muscle’).6 Given this time dependence, reperfusion therapy is recommended in patients who present within 12 hours of symptom onset and have persistent ST elevation or new left bundle branch block. Beyond 12 hours, reperfusion therapy is only recommended in patients with ongoing ischaemic chest pain and persisting ST segment elevation, or in those with cardiogenic shock.7
Fibrinolysis or PPCI?
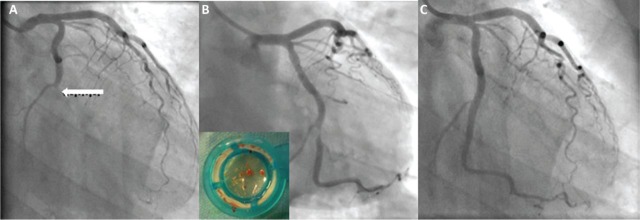
In the 1980s/1990s, reperfusion was achieved pharmacologically with fibrinolytic agents, but their unpredictable efficacy and less favourable outcomes have led to adoption of mechanical reperfusion with PPCI (Fig 1) as the preferred reperfusion strategy in many countries, including the UK.8 In 2013–14, 98.5% of STEMI patients in England underwent PPCI, compared with less than 5% a decade earlier, thereby demonstrating the success of the national implementation of the PPCI strategy.1 Current UK recommendations are a maximum call-to-balloon time (time between call for help and balloon inflation) of ≤150 minutes and a door-to-balloon time (time between arrival at PCI-capable centre and first balloon inflation/device deployment) of ≤90 minutes.1,9 In situations where there is an expected delay of >120 minutes before PPCI can be delivered, or when patients present very early (within 2 hours) after symptom onset, fibrinolytic therapy may be considered in preference to PPCI because of equivalent outcomes;7 however, in England in 2013–14, fibrinolytic therapy for STEMI was only utilised in 35 cases (2%).1 In the recent STREAM trial, patients presenting early (within 3 hours of symptom onset), and where a delay of ≥1 hour to PPCI was expected, were randomised to early fibrinolysis followed by routine coronary angiography and subsequent revascularisation within 6–24 hours or PPCI. Despite STREAM demonstrating equivalence in clinical outcomes between the two strategies,10 the potential for confusion between choices of therapy for individual patients has led most geographies (the UK included) to settle on a single strategy for all patients, usually PPCI. Nevertheless, PPCI must be delivered in a timely fashion, and therefore encouraging direct admission to PPCI-capable centres, minimising inter-hospital delays (average 40 minutes in 20129) and promoting early presentation through public awareness campaigns are all key to the success of such a strategy.
Fig 1. Mechanical reperfusion (with primary percutaneous coronary intervention) to an occluded circumflex artery. A – complete occlusion of circumflex artery (arrow). B – residual stenosis at site of plaque rupture following removal of thrombus (inset). C – widely patent artery following stent implantation.
Antiplatelet therapy and preloading
In the treatment of STEMI in general, extensive evidence favours use of dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor blocker,11 with additional indications still where PPCI is performed and stents implanted. However, considerable debate persists over which P2Y12 inhibitor to use and when it should be administered. Generally, UK and European practice has included administration of loading doses of aspirin (300 mg) and clopidogrel (600 mg) by paramedic crews in the pre-hospital setting. However, with more rapidly acting and potent P2Y12 inhibitors now available, in areas with short ambulance transfer times, or in healthcare systems such as the USA where there is early availability of coronary artery bypass graft surgery (CABG), DAPT administration may be delayed until hospital arrival or even following delineation of coronary anatomy at angiography.
The newer P2Y12 agents, ticagrelor and prasugrel, have a more rapid onset of antiplatelet activity and a more predictable and reliable platelet inhibition. Both have demonstrated efficacy in the PPCI setting, albeit in subgroup analyses of larger studies encompassing acute coronary syndrome (ACS) cohorts that included STEMI patients.12,13 Owing to differences in the proportion of STEMI patients in each study and the variation in timing of drug administration (prasugrel after angiography and ticagrelor after clopidogrel preloading in STEMI cases), the trials cannot usefully be directly compared. However, despite the STEMI subgroups being underpowered, similar results were obtained to those in the overall studies in terms of reduction in hard endpoints, although not always achieving statistical significance. Importantly in this high-risk subset, both agents reduced the incidence of stent thrombosis without increasing non-CABG-related bleeding. At present, data support use of prasugrel only in ACS patients (including STEMI) who undergo PCI, whereas ticagrelor can be used in all ACS patients, regardless of management strategy (PCI, CABG or medically managed).
Despite their potency and speed of action, a recent small study in 50 STEMI patients (RAPID study),14 demonstrated, somewhat surprisingly, that pre-hospital loading with ticagrelor and prasugrel produces insufficient platelet inhibition in nearly half of patients at 2 hours, with effective platelet inhibition only achieved at a mean of 3 hours for prasugrel and 5 hours for ticagrelor, partly due to the intestinal absorption-delaying effects of opioid analgesics. However, the ATLANTIC study found that administering ticagrelor in the pre-hospital setting was associated with a lower incidence of definite stent thrombosis compared with in-hospital administration and was as safe in terms of bleeding events.15 On the basis of these data, administration of DAPT by ambulance crew ‘in the field’ should probably be encouraged.
Owing to increased bleeding risk observed in the pivotal trials, prasugrel is contraindicated in patients with a previous history of cerebrovascular disease and not recommended in patients 75 years and over or weighing less than 60 kg. Furthermore, both prasugrel and ticagrelor are contraindicated in patients with previous intracranial haemorrhage. In patients unsuitable for either of these agents, a loading dose of clopidogrel at 600 mg can be used; although there is no direct evidence from randomised studies for this loading dose in PPCI, data from the OASIS-7 trial of 600 mg vs 300 mg clopidogrel loading dose in ACS patients suggested a significant reduction in the composite endpoint of cardiovascular death, myocardial infarction or stroke at 30 days, as well as in stent thrombosis, with the higher dose.16
Given the clear lack of consensus, there is considerable variation in loading strategy: some centres (with short transfer times) may favour no preloading at all with a P2Y12 inhibitor, others administer clopidogrel pre-hospital to all with the option of subsequent switching after angiography or PPCI, and others still have opted for pre-hospital loading with one of the more potent agents, and clopidogrel only when these are contraindicated. Given the available data, all of these are reasonable therapeutic strategies.
PPCI: procedural considerations
As for DAPT choice and timing, there are controversies surrounding several procedural issues for PPCI, summarised in Table 1. Perhaps the least contentious is the emergence of data establishing transradial access as superior to the transfemoral route in patients undergoing PPCI, with a lower risk of bleeding/vascular complications and reduced mortality.17,18 The potential for further reduction in bleeding events in STEMI patients has resulted in considerable interest in the direct thrombin inhibitor bivalirudin as an alternative to heparin, either alone or when combined with glycoprotein IIb/IIIa inhibitors (GPI), which themselves have fallen out of favour except in cases with angiographic evidence of large thrombus burden. Bivalirudin use in other PCI settings has hinted at improved net patient outcomes given equipoise for ischaemic events and reduced bleeding. However, randomised trials comparing bivalirudin with unfractionated heparin have been confounded by heterogeneity in trial design, including endpoint definitions (eg major bleeding), variation in anticoagulant doses, use of contemporary potent antiplatelet agents, access site choice, use of concomitant GPI and variation in use of post-procedure bivalirudin infusion.18–22 In HORIZONS-AMI, EUROMAX, BRIGHT and MATRIX, bivalirudin was generally superior to unfractionated heparin in terms of reduced bleeding but was associated with higher rates of acute stent thrombosis in 3 out of the 4 studies. All studies used higher rates of GPI in the comparator heparin arm and some continued the bivalirudin infusion post-PPCI. The HEAT-PPCI trial was a randomised, controlled, open-label, single-centre UK trial that compared bivalirudin with heparin in truly contemporary PPCI practice (majority radial access, novel antiplatelet agents and only bailout GPI use) and did not use a post-procedure infusion. The occurrence of the composite primary endpoint, including re-infarction and all-cause mortality, was higher in the bivalirudin group as was the incidence of stent thrombosis. Furthermore, and most importantly, there was no difference in major bleeding between the groups, although the dose of heparin used was lower (average 70 IU/kg) in comparison with other trials. Overall, it is still unclear which anticoagulant is truly superior and use of other therapies may influence local choices: at present, both heparin alone and bivalirudin should be regarded as viable options.
Table 1. Current contoversies in PPCI practice.
| Controversy | Contention | Studies for | Studies against | Notes |
|---|---|---|---|---|
| Arterial access route? | Radial access reduces bleeding, mortality | RIVAL 17, MATRIX 38 | ||
| Optimal procedural anticoagulant? | Bivalirudin reduces bleeding, mortality | HORIZONS-AMI 19, BRIGHT 22, MATRIX 18 | HEAT-PPCI | Confusion regarding confounding effects of heparin dose, DAPT preloading. |
| Thrombectomy during PPCI? | Manual aspiration thrombectomy improves outcomes | TAPAS 39 | TASTE 24, TOTAL 40 | TAPAS underpowered; powered studies and meta-analyses show no benefit. |
| Culprit-only or multivessel PPCI? | PCI to all lesions improves outcomes | PRAMI 41, CVLPRIT 29 | Confounding by selection bias (multivessel PPCI only in ‘attractive’ lesions) |
DAPT = dual antiplatelet therapy; PPCI = primary percutaneous coronary intervention.
Although initially showing hope for reduced need for potent anticoagulation,23 the routine use of manual aspiration thrombectomy in PPCI has now been shown in two large-scale trials (TASTE and TOTAL) not to result in any clinical benefits, and may even increase stroke risk, compared with conventional PCI.24,25
Concerns over increased stent thrombosis rates with drug-eluting stent use have not materialised, with newer generation drug-eluting stents being shown to be both safer and more efficacious than bare metal stents in the PPCI setting.26
Finally, the debate over treatment of non-culprit ‘bystander’ lesions continues: 30–50% of STEMI patients have significant multivessel disease27 and current guidelines advocate revascularisation of non-culprit lesions during the index PPCI procedure only in patients who are haemodynamically unstable, or have ongoing ischaemia despite successful culprit vessel PPCI.7 However, recent studies (eg PRAMI and CVLPRIT) have rekindled this debate by demonstrating the safety and efficacy of a complete revascularisation strategy at the time of PPCI, although neither trial was sufficiently powered to detect a mortality benefit.28,29 A recent meta-analysis has shown that complete revascularisation at the time of PPCI results in a reduction in major adverse cardiac events rates, largely driven by a decrease in repeat revascularisation, without firm evidence for a reduction in death or myocardial infarction.30 Forthcoming studies, including COMPLETE, will inform this debate further.
Other avenues showing early promise in STEMI include deferred compared with immediate stenting following re-establishment of flow in the culprit artery,31 use of systemic hypothermia (COOL AMI pilot trial),32 bone marrow-derived cell therapy (BAMI trial)33 and ischaemic preconditioning.34
Post-revascularisation care
All patients should initially be monitored on a coronary care unit,7 and should receive secondary preventative therapies post-STEMI, including DAPT, beta blockers, angiotensin-converting-enzyme (ACE) inhibitors and high-dose statin. Initiation of beta blockers and ACE inhibitors is recommended early (within 24 hours), unless there are specific contraindications. In patients with a left ventricular ejection fraction (LVEF) <40% and heart failure or diabetes, eplerenone is also recommended.7 Smoking cessation advice should be given and all eligible patients should be offered an exercise-based cardiac rehabilitation programme. All patients should have transthoracic echocardiography to assess LV function.
The optimal duration of DAPT post-PPCI is debatable. Current European Society of Cardiology guidelines recommend 12 months, although a shorter duration (<6 months) may also be safe.35,36 Pending further data, it is reasonable to recommend shorter duration DAPT in high bleeding risk patients and extended DAPT in low bleeding risk patients with high ischaemic risk. In patients with an indication for oral anticoagulation (OAC), eg atrial fibrillation, guidelines recommend triple therapy (DAPT + OAC), but the optimal regimen remains unknown. Commonly, DAPT + OAC are given for 6 weeks, followed by OAC and antiplatelet monotherapy for life. The preferred P2Y12 blocker in patients requiring OAC is clopidogrel.
STEMI may be complicated by cardiogenic shock in approximately 6% of patients.37 Such patients have a 50% mortality rate and will require early, maximal revascularisation, inotropic support and possibly a mechanical assist device. Other recognised complications of STEMI include acute mitral regurgitation, ventricular rupture, tamponade, LV thrombus/aneurysm, pericarditis and arrhythmias. Patients with impaired LV function need repeat echocardiography at least 40 days post-STEMI to assess their eligibility for an implantable cardioverter-defibrillator. All patients with STEMI should undergo early and late (pre-discharge) risk stratification using a validated model, such as TIMI or GRACE. Low-risk patients can be safely discharged 72 hours post-PCI.7 Current Driver and Vehicle Licensing Agency (DVLA) regulations prohibit driving for 1 week, provided no other urgent (<4 weeks) revascularisation is planned and LVEF is ≥40% prior to hospital discharge (for ordinary car or motorcycle licences). Follow-up should be arranged with a cardiologist for ongoing care in the outpatient setting.
Conclusions
Management of STEMI remains an area of intense debate and interest; despite multiple clinical trials, there are still many questions to be answered regarding several aspects of care from pre-hospital management to the PPCI procedure itself. What is certain is that the impressive mortality and morbidity reductions already associated with PPCI are only likely to be improved further by such developments.
References
- 1.Myocardial Ischaemia National Audit Project How the NHS cares for ischaemia with heart attack. London: MINAP; 2014. Available online at http://www.ucl.ac.uk/nicor/audits/minap/documents/annual_reports/minap-public-report-2014. [Accessed 6 April 2016] [Google Scholar]
- 2.Stone GW. Narula J. The myth of the mild vulnerable plaques. JACC Cardiovasc Imaging. 2013;6:1124–6. doi: 10.1016/j.jcmg.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Reimer KA. Lowe JE. Rasmussen MM. Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–94. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 4.Brown AJ. Hoole SP. McCormick LM, et al. Left bundle branch block with acute thrombotic occlusion is associated with increased myocardial jeopardy score and poor clinical outcomes in primary percutaneous coronary intervention activations. Heart. 2013;99:774–8. doi: 10.1136/heartjnl-2012-303194. [DOI] [PubMed] [Google Scholar]
- 5.Roffi M. Patrono C. Collet JP, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2015;37:267–315. doi: 10.1093/eurheartj/ehv320. 2016. [DOI] [PubMed] [Google Scholar]
- 6.De Luca G. Suryapranata H. Ottervanger JP. Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–5. doi: 10.1161/01.CIR.0000121424.76486.20. [DOI] [PubMed] [Google Scholar]
- 7.Task Force on the management of STseamiotESoC Steg PG. James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 8.Keeley EC. Boura JA. Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 9.B. NICOR. National Audit of Percutaneous Coronary Interventional Procedures Public Report. 2012. Available online at www.hqip.org.uk/national-programmes/a-z-of-nca. [Accessed 6 April 2016] [Google Scholar]
- 10.Armstrong PW. Gershlick AH. Goldstein P, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368:1379–87. doi: 10.1056/NEJMoa1301092. [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS. Cannon CP. Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–89. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 12.Montalescot G. Wiviott SD. Braunwald E, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373:723–31. doi: 10.1016/S0140-6736(09)60441-4. [DOI] [PubMed] [Google Scholar]
- 13.Wallentin L. Becker RC. Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 14.Parodi G. Valenti R. Bellandi B, et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol. 2013;61:1601–6. doi: 10.1016/j.jacc.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Montalescot G. van ’t Hof AW. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:2339. doi: 10.1056/NEJMc1412729. [DOI] [PubMed] [Google Scholar]
- 16.Mehta SR. Tanguay JF. Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233–43. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 17.Jolly SS. Yusuf S. Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–20. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 18.Valgimigli M. Frigoli E. Leonardi S, et al. Bivalirudin or Unfractionated Heparin in Acute Coronary Syndromes. N Engl J Med. 2015;373:997–1009. doi: 10.1056/NEJMoa1507854. [DOI] [PubMed] [Google Scholar]
- 19.Stone GW. Witzenbichler B. Guagliumi G, et al. Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet. 2011;377:2193–204. doi: 10.1016/S0140-6736(11)60764-2. [DOI] [PubMed] [Google Scholar]
- 20.Shahzad A. Kemp I. Mars C, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet. 2014;384:1849–58. doi: 10.1016/S0140-6736(14)60924-7. [DOI] [PubMed] [Google Scholar]
- 21.Zeymer U. van ’t Hof A. Adgey J, et al. Bivalirudin is superior to heparins alone with bailout GP IIb/IIIa inhibitors in patients with ST-segment elevation myocardial infarction transported emergently for primary percutaneous coronary intervention: a pre-specified analysis from the EUROMAX trial. Eur Heart J. 2014;35:2460–7. doi: 10.1093/eurheartj/ehu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y. Guo J. Zheng Y, et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA. 2015;313:1336–46. doi: 10.1001/jama.2015.2323. [DOI] [PubMed] [Google Scholar]
- 23.Svilaas T. Vlaar PJ. van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–67. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 24.Lagerqvist B. Frobert O. Olivecrona GK, et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111–20. doi: 10.1056/NEJMoa1405707. [DOI] [PubMed] [Google Scholar]
- 25.Jolly SS. Cairns JA. Yusuf S, et al. Stroke in the TOTAL trial: a randomized trial of routine thrombectomy vs. percutaneous coronary intervention alone in ST elevation myocardial infarction. Eur Heart J. 2015;36:2364–72. doi: 10.1093/eurheartj/ehv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bangalore S. Amoroso N. Fusaro M. Kumar S. Feit F. Outcomes with various drug-eluting or bare metal stents in patients with ST-segment-elevation myocardial infarction: a mixed treatment comparison analysis of trial level data from 34 068 patient-years of follow-up from randomized trials. Circ Cardiovasc Interv. 2013;6:378–90. doi: 10.1161/CIRCINTERVENTIONS.113.000415. [DOI] [PubMed] [Google Scholar]
- 27.Muller DW. Topol EJ. Ellis SG. Sigmon KN. Lee K. Califf RM. Multivessel coronary artery disease: a key predictor of short-term prognosis after reperfusion therapy for acute myocardial infarction. Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study Group. Am Heart J. 1991;121:1042–9. doi: 10.1016/0002-8703(91)90661-z. [DOI] [PubMed] [Google Scholar]
- 28.Wald DS. Morris JK. Wald NJ. Investigators P. Preventive angioplasty in myocardial infarction. N Engl J Med. 2014;370:283. doi: 10.1056/NEJMc1314696. [DOI] [PubMed] [Google Scholar]
- 29.Gershlick AH. Khan JN. Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. Journal of the American College of Cardiology. 2015;65(10):963–72. doi: 10.1016/j.jacc.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bangalore S. Toklu B. Wetterslev J. Complete versus culprit-only revascularization for ST-segment-elevation myocardial infarction and multivessel disease: a meta-analysis and trial sequential analysis of randomized trials. Circulation Cardiovascular interventions. 2015;8(4) doi: 10.1161/CIRCINTERVENTIONS.114.002142. [DOI] [PubMed] [Google Scholar]
- 31.Carrick D. Oldroyd KG. McEntegart M, et al. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI) J Am Coll Cardiol. 2014;63:2088–98. doi: 10.1016/j.jacc.2014.02.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.clinicaltrials.gov COOL AMI EU Pilot Trial to Assess Cooling as an Adjunctive Therapy to Percutaneous Intervention in Patients With Acute Myocardial Infarction. Available online at https://clinicaltrials.gov/ct2/show/NCT02509832. [Accessed 12 February 2016] [DOI] [PubMed] [Google Scholar]
- 33.clinicaltrials.gov. BAMI The Effect of Intracoronary Reinfusion of Bone Marrow-derived Mononuclear Cells(BM-MNC) on All Cause Mortality in Acute Myocardial Infarction. Available online at https://clinicaltrials.gov/ct2/show/NCT01569178. [Accessed 12 February 2016]
- 34.White SK. Frohlich GM. Sado DM, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:178–88. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Navarese EP. Andreotti F. Schulze V, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ. 2015;350:h1618. doi: 10.1136/bmj.h1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmerini T. Sangiorgi D. Valgimigli M, et al. Short- versus long-term dual antiplatelet therapy after drug-eluting stent implantation: an individual patient data pairwise and network meta-analysis. J Am Coll Cardiol. 2015;65:1092–102. doi: 10.1016/j.jacc.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg RJ. Spencer FA. Gore JM. Lessard D. Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009;119:1211–9. doi: 10.1161/CIRCULATIONAHA.108.814947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valgimigli M. Gagnor A. Calabro P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–76. doi: 10.1016/S0140-6736(15)60292-6. [DOI] [PubMed] [Google Scholar]
- 39.Vlaar PJ. Svilaas T. van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–20. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 40.Jolly SS. Cairns JA. Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387:127–35. doi: 10.1016/S0140-6736(15)00448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wald DS. Morris JK. Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–23. doi: 10.1056/NEJMoa1305520. [DOI] [PubMed] [Google Scholar]