Abstract
Background
Neonates with inborn errors of metabolism (IEM) often develop hyperammonemia which, if not corrected quickly, may result in poor neurologic outcomes. As pharmacologic therapy cannot rapidly lower ammonia levels, dialysis is frequently required. Both hemodialysis (HD) and standard-dose continuous renal replacement therapy (CRRT) are effective; however, HD may be followed by post-dialytic ammonia rebound, and standard-dose CRRT may not effect a rapid enough decrease in ammonia levels.
Case-Diagnosis/Treatment
We present two cases of IEM-associated neonatal hyperammonemia in which we employed a biphasic, high-dose CRRT treatment strategy, initially using dialysate flow rates of 5,000 mL/h (approximately 40,000 mL/h/1.73 m2) in order to rapidly decrease ammonia levels, then decreasing the dialysate flow rates to 500 mL/h (approximately 4,000 mL/h/1.73 m2) in order to prevent ammonia rebound.
Conclusions
This biphasic dialytic treatment strategy for neonatal hyperammonemia effected rapid ammonia reduction without rebound and accomplished during a single dialysis run without equipment changes.
Keywords: Hyperammonemia, Neonate, Hemodialysis, Pediatrics, Inborn errors of metabolism
Introduction
Neonates with inborn errors of metabolism (IEM), including organic acidemias and urea cycle disorders (UCD), frequently develop hyperammonemia. The initial presentation of acute hyperammonemia usually includes changes in mental status, such as lethargy, which may progress to seizures, coma, and even death [1]. Patients with neonatal hyperammonemia are at significant risk for irreversible neurological damage [1, 2], and the duration of hyperammonemia has been correlated with the degree of cognitive impairment [1, 3]. As such, timely intervention is of critical importance in order to rapidly decrease ammonia levels and improve neurological outcomes.
Pharmacologic therapy with ammonia-scavenging medications, such as sodium phenylacetate and sodium benzoate, provides a pathway by which excessive ammonia may be removed. However, dialysis is often used initially as it can rapidly and efficiently lower serum ammonia levels. As neonates with peak ammonia levels of >500 umol/L have poorer survival rates [4], it is recommended that planning for dialysis should begin when ammonia levels reach 400 umol/L [5]. Dialysis treatment options usually include hemodialysis (HD) and continuous renal replacement therapy (CRRT). Alternatively, in centers that lack the ability or expertise to perform extracorporeal therapy, peritoneal dialysis (PD) can be utilized; however, PD clears ammonia at a lower rate than hemodialytic modalities [6, 7].
Although ammonia removal is most efficient with HD, there may be post-dialysis rebound hyperammonemia and, consequently, a requirement for repeated HD sessions or CRRT [7]. As such, the ideal dialytic treatment for severe hyperammonemia should include high initial clearance rates to rapidly reduce ammonia levels, followed by continued, lower clearance dialysis to prevent rebound. In this report, we present two cases of neonatal hyperammonemia which were successfully treated using this dialytic strategy.
Case reports
Case 1
The first patient was a 4-day-old, full term male neonate weighing 3.9 kg who presented with 1 day of lethargy and poor feeding. Laboratory work-up revealed a respiratory alkalosis and hyperammonemia (initial ammonia 410 umol/L), raising concern for a UCD. Pharmacologic therapy of intravenous (IV) fluids (multiple normal saline boluses followed by 10 % dextrose, running at 1.5-fold maintenance), sodium phenylacetate, sodium benzoate, and arginine chloride was started; however, ammonia levels continued to rise, reaching 741 umol/L. At this point, a 7 French, 10-cm double lumen temporary dialysis catheter (Medcomp®, Harleysville, PA) was placed in the right internal jugular vein. IV antibiotics were also administered, as the patient was being ruled out for sepsis.
After catheter placement, dialysis initiation was delayed for 10 h secondary to catheter malfunction and clotting, requiring bedside surgical adjustment and tissue plasminogen activator (TPA) instillation. Time from initial ammonia measurement to dialysis initiation, including time for transport to our facility, catheter placement, and catheter adjustment, was approximately 29 h. The dialysis procedure was started with blood circuit priming, following which the patient was started on continuous veno-venous hemodialysis (CVVHD) via the NxStage® System One™ machine (NxStage Medical Inc., Lawrence, MA) with a hemofilter 400, blood flow rate of 30 mL/min, and initial dialysate flow rate of 5,000 mL/h (36,042 mL/h/1.73 m2) [Electronic Supplementary Material (ESM) Table 1]. Prismasate™ dialysate (Gambro® Renal Products, Lakewood, CO) containing 4 mEq/L potassium and 2.5 mEq/L calcium was used. Given the anticipated high clearance rates and attendant risk of hypocalcemia dialyzing against a 0 mEq/L calcium dialysate, heparin anticoagulation, as opposed to regional citrate anticoagulation, was employed.
The ammonia level peaked at 841 umol/L prior to dialysis and was 821 umol/L 1 h prior to dialysis initiation. After 3 h of dialysis, the ammonia level decreased to 135 umol/L, at which point the dialysate flow rate was decreased to 500 mL/h (3, 604 mL/h/1.73 m2). Over the next 24 h, the ammonia level remained at <150 umol/L, and dialysis was discontinued (Fig. 1a). Over the subsequent 8 days, the ammonia level remained at <100 umol/L. The patient tolerated dialysis well; however, he developed asymptomatic hypokalemia (potassium 3.1 mmol/L) and hypophosphatemia (phosphorous 1.9 mg/dL) approximately 3 h into the dialysis treatment. One-time IV potassium phosphate supplementation was administered, and electrolytes normalized.
Fig. 1.
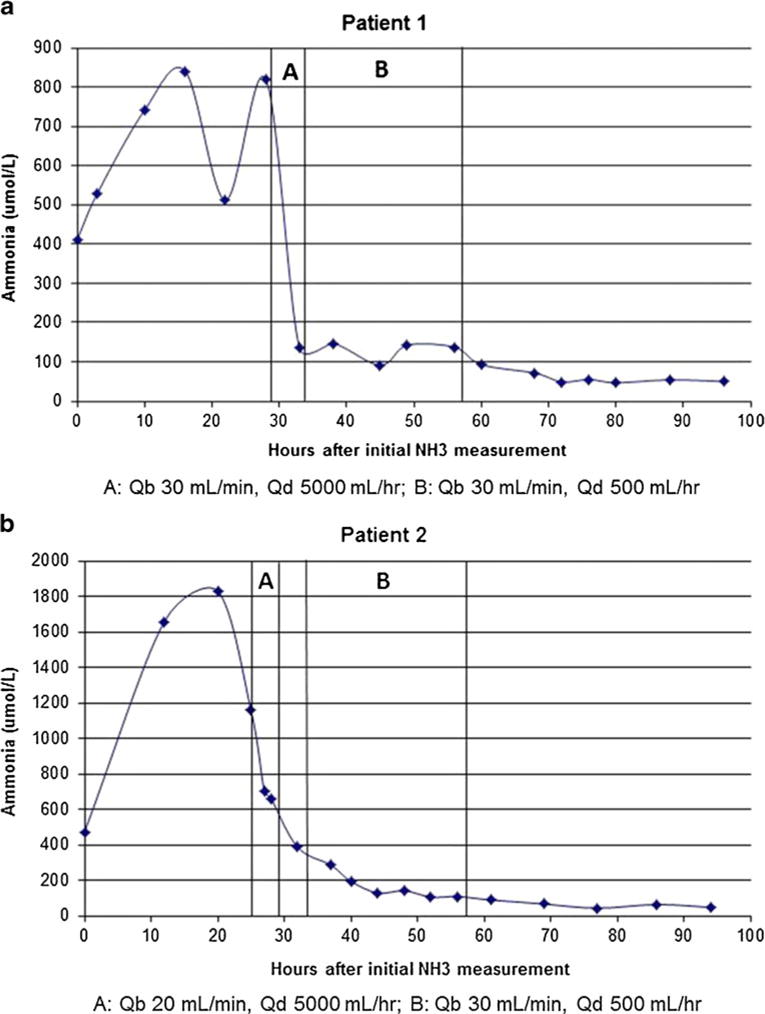
a, b Ammonia levels over time are shown for both patients. Patient 2 was off dialysis for approximately 3 h, represented in b as the time between the second and third bars. Qb Blood flow rate, Qd dialysate flow rate
The patient was diagnosed with citrullinemia via quantitative plasma amino acid testing. A brain magnetic resonance imaging (MRI) scan at 1 week of life revealed diffuse white matter edema and minor periventricular white matter microhemorrhages, findings observed in UCDs. The patient was discharged at 3.5 weeks of age, receiving physical therapy and occupational therapy.
Case 2
The second patient was a 4-day-old, full term male neonate weighing 3.1 kg who developed persistent hypoglycemia over the first 2 days of life in the newborn nursery. On the third day of life, the patient displayed poor feeding. On the fourth day of life, abnormal newborn screening results returned, suggestive of an organic acidemia. Laboratory studies revealed a metabolic acidosis and hyperammonemia (initial ammonia 472 umol/L). A 7 French, 10-cm double lumen temporary dialysis catheter (Medcomp®) was placed in the right internal jugular vein. Pharmacologic therapy of IV fluids (multiple normal saline boluses followed by 10 % dextrose, running at 1.5-fold maintenance), cobalamin, and carnitine was started. Approximately 4 h prior to dialysis initiation, the patient was started on a sodium phenylacetate and sodium benzoate infusion; however, due to vascular access issues, the infusion had to be stopped 1 h later. IV antibiotics were also administered, as the patient was being ruled out for sepsis.
Time from initial ammonia measurement to dialysis initiation, including time for transport to our facility and catheter placement, was approximately 25 h. The dialysis procedure was started with blood circuit priming, following which the patient was started on CVVHD via the NxStage® machine (NxStage Medical Inc.) with a hemofilter 400, blood flow rate of 20 mL/min, and initial dialysate flow rate of 5,000 mL/h (43,250 mL/h/1.73 m2) (ESM Table 1). Prismasate™ dialysate containing 4 mEq/L potassium and 2.5 mEq/L calcium was used. As in the first case, heparin anticoagulation, as opposed to regional citrate anticoagulation, was employed.
The ammonia level peaked at 1,830 umol/L prior to dialysis and was 1,160 umol/L at dialysis initiation. After 3 h of dialysis, the ammonia level had decreased to 661 umol/L; however, the circuit clotted soon thereafter. Dialysis was restarted 3 h later, at which time the ammonia level had decreased to 386 umol/L; therefore, a decreased dialysate flow rate of 500 mL/h (4,325 mL/h/1.73 m2), with a blood flow rate of 30 mL/min, was employed. Over the next 24 h, the ammonia level decreased to 108 umol/L, and dialysis was discontinued (Fig. 1b). Over the subsequent 4 days, the ammonia level remained at <100 umol/L. The patient tolerated dialysis well; however, he developed asymptomatic hypokalemia (potassium 2.9 mmol/L) and hypophosphatemia (phosphorous 2.1 mg/dL) approximately 12 h into dialysis treatment. Supplementation was not given, the electrolytes remained stable, and the levels normalized with dialysis discontinuation.
The patient was diagnosed with methylmalonic acidemia, as suggested by the newborn screening results, and confirmed by plasma amino acid and urine organic acid testing. A brain MRI scan at 1 week of life revealed findings consistent with hypoxic ischemic encephalopathy that likely developed over several days and were thought to be related to the patient’s underlying metabolic disorder. The patient was discharged at 4 weeks of age, receiving physical therapy and occupational therapy.
Discussion
The acute hyperammonemia that occurs in neonates with IEM can result in significant, chronic morbidity and even mortality. Higher peak ammonia levels during hyperammonemic episodes are associated with poorer survival rates [4], and pre-dialysis coma duration is negatively associated with cognitive outcome [8]. Additionally, the rapidity of ammonia clearance has been associated with improved outcomes [6, 7]. As such, aggressive and timely therapy, including the use of dialysis, should be implemented in order to rapidly lower ammonia levels.
Although initial HD may allow for a more rapid decrease in ammonia levels than traditional CRRT, it is accompanied by the risks of intradialytic hemodynamic instability and post-dialytic ammonia rebound. In a retrospective study of 18 children (mean age 56 months) with acute hyperammonemia requiring RRT, 16 of whom had either a UCD or an organic acidemia, McBryde et al. compared outcomes based on dialytic modality (HD vs. CRRT) [9]. These authors demonstrated a trend towards improved survival in patients initially treated with HD (blood flow rate 5–10 mL/kg/min; dialysate flow rate 500 mL/min) and found that the time required for CRRT patients to reach ammonia levels of <200 umol/L was 15-fold longer than that of HD patients; however, the CRRT patients received relatively low dialysate flow rates (2, 000 mL/h/1.73 m2). Also, half of the HD patients required conversion to CRRT for continued metabolic control.
Ideal dialytic management of severe hyperammonemia may consist of initial high-clearance CRRT for rapid reduction of the ammonia levels, followed by short-term, lower clearance CRRT to prevent ammonia rebound. In our two neonatal patients, we used blood flow rates of 20–30 mL/min and initial dialysate flow rates of 5,000 mL/h (approximately 40, 000 mL/h/1.73 m2)—parameters many would consider very high-dose CRRT. This initial dialysate flow rate was selected in order to effect optimal CVVHD ammonia clearance [7], although lower dialysate flow rates have been chosen based on their ability to provide ammonia clearances comparable to those expected with intermittent HD [5]. In both of our patients, over the first 2–3 h of dialysis, the ammonia levels decreased by a rate of 229 umol/L/h. It should be noted, however, that the rate of decrease in ammonia levels is determined not only by the dialysis clearance rate, but also by the degree of catabolism, hydration status, and the effectiveness of pharmacologic therapy. After 3–5 h, the ammonia levels fell to <400 umol/L, at which point we decreased the dialysate flow rates to 500 mL/h (approximately 4,000 mL/h/1.73 m2), closer to those used in standard CRRT prescriptions. When ammonia levels decrease to <400 umol/L, continued very high clearance rates may be unnecessary, and the continued use of very high-dose CRRT may precipitate significant hypokalemia and/or hypophosphatemia. After 24 h of sustained low ammonia levels without rebound, over which time the ammonia levels decreased to <150 umol/L, CRRTwas discontinued. Subsequently, ammonia levels remained at <100 umol/L on dietary and pharmacologic treatment, without the need for further dialysis. Biphasic dialytic treatment strategies for neonatal hyperammonemia have been previously employed. For example, Bunchman et al. reported the case of a hyperammonemic infant, eventually diagnosed with methylmalonic academia, in whom a similar rapid ammonia reduction without rebound was effected using initial HD followed by high-dose CRRT [10].
As opposed to HD or standard-dose CRRT, a recently published case report from Spinale et al. [5] details the use of high-dose CRRT for the treatment of hyperammonemia in two newborns with ornithine transcarbamylase deficiency, a UCD. The authors used the Prisma™ CRRT machine (GAMBRO DASCO S.p.A., Medolla, Italy) with blood flow rates of 30–40 mL/min and dialysate/replacement fluid flow rates of 1,000 mL/h (approximately 8,000 mL/h/1.73 m2). In both patients, CRRT was discontinued after 8–10 h, when the ammonia levels fell to <100 umol/L. The first patient had a peak ammonia rebound of 149 umol/L, and the second patient had a peak ammonia rebound of 402 umol/L. Our two patients remained on RRT for longer periods of time, thereby avoiding ammonia rebound. Therefore, after the initial reduction in ammonia levels, there may be some benefit to continuing CRRT for about 24 h, during which time stable low ammonia levels may be ensured.
Like dialysis, pharmacologic therapy with ammonia-scavenging medications may facilitate ammonia removal; however, pharmacologic therapy may take hours to have an effect. Our second patient was started on sodium phenylacetate and sodium benzoate treatment prior to dialysis initiation, and the ammonia level concurrently decreased from 1,830 to 1,160 umol/L. It is possible that the ammonia levels would have continued to decrease without extracorporeal therapy. However, the rapidity with which they would have done so is unknown, and it is unlikely that continued pharmacologic treatment would have facilitated as prompt a normalization of ammonia levels as dialysis. Furthermore, our first patient was also started on sodium phenylacetate and sodium benzoate treatment prior to dialysis initiation; however, he experienced a concurrent increase in ammonia levels, from 529 to 841 umol/L, demonstrating that dialysis is often required in addition to pharmacologic therapy.
Regardless of the dialytic modality used, HD treatment of hyperammonemia may be associated with possible adverse effects, including hemodynamic instability, electrolyte abnormalities, and catheter malfunction. Both of our patients tolerated dialysis without hypotension, both developed mild hypokalemia and hypophosphatemia, and both patients experienced HD-associated catheter issues. Our first patient experienced primary catheter nonfunction and clotting which delayed dialysis initiation by several hours, while our second patient experienced clotting of the catheter after 3 h of dialysis that required TPA instillation and a dwell. This complication, along with the aforementioned inability to administer sodium phenylacetate and sodium benzoate for longer than 1 h prior to CRRT treatment, likely contributed to the slower decline in ammonia levels seen in the second patient.
In both of our patients, systemic heparin anticoagulation was used instead of regional citrate anticoagulation (RCA). In order to prevent citrate neutralization, many RCA protocols use calcium-free dialysate. However, in our patients, given the high clearance rates, dialyzing against a 0 mEq/L calcium dialysate may have risked hypocalcemia [11, 12]. As such, we chose to use heparin instead of RCA. Whether RCA with calcium-containing dialysate is as effective as RCA with calcium-free dialysate or systemic heparin anticoagulation has been investigated in a few studies. One adult hemodialysis study comparing RCA with calcium-containing dialysate and systemic heparin anticoagulation found more clotting events in the RCA group, but similar urea and creatinine clearances in the two groups [13]. In another study, a small randomized trial in adult HD patients comparing RCA with calcium-containing dialysate and RCA with calcium-free dialysate, more dialyzer clotting and more early HD termination due to dialyzer clotting were observed in the patients on calcium-containing dialysate [14]. In our patients, given the potential risk of hypocalcemia with RCA and calcium-free dialysate, and given the risk of increased clotting with RCA and calcium-containing dialysate, especially with the low blood flow rates we used, we decided that heparin was the most suitable anticoagulation option. Another possible option to help prevent circuit clotting is the administration of pre-filter replacement fluid, which is possible with the NxStage® machine, with or without anticoagulation. However, it has been shown that, in pediatric CRRT patients, circuit survival does not differ among continuous veno-venous hemofiltration, CVVHD, and continuous veno-venous hemodiafiltration [15]. In all three dialytic modalities, though, circuit life is shorter without the use of anticoagulation [15].
We were able to utilize a single dialysis session to accomplish a two-phase treatment strategy, thus avoiding the unnecessary patient risk that accompanies equipment changes. Also, the use of a single machine ensured uninterrupted therapy and limited the use of blood products that are often needed to prime either HD or CRRT circuits in neonatal patients. One potential advantage of using a scale-less volumetric balancing dialysis machine in this scenario is that it obviated the need for staff to constantly empty effluent bags during the high clearance phase. Additionally, since up to 20 L of dialysate can be initially hung, staff intervention was minimized despite the high rate of dialysate use, demonstrating another potential practical advantage.
As opposed to conventional CRRT machines, the scale-less volumetric balancing system does not involve effluent collection or the direct measurement of ultrafiltrate. As such, there may be concern regarding inadvertent over- or under-ultrafiltration, especially in the smallest pediatric patients, which may result in hypotension or relative fluid overload, respectively. The product-specified ultrafiltration accuracy is ±10 %. As even a 10 % error in fluid balance could be clinically significant for neonates, close monitoring of weight and blood pressure is required for all neonatal CRRT patients. However, in most cases of neonatal hyperammonemia, net ultrafiltration volume is set to zero, as the patients often have normal renal function, and hyperammonemia is the only dialysis indication. Furthermore, in hyperammonemia, volume depletion should be avoided, as reduced tissue perfusion may increase catabolism, leading to higher ammonia concentrations. In this report, both of our patients had net ultrafiltration set to zero and had no evidence of intradialytic hypotension or fluid overload.
In summary, these cases provide an example of a biphasic dialytic treatment strategy for neonatal hyperammonemia that effects rapid ammonia reduction without rebound and which may be accomplished during a single dialysis run without equipment changes.
Supplementary Material
Acknowledgments
The authors received no funding or materials for the study from NxStage Medical Inc., and NxStage Medical Inc. did not contribute to or review this manuscript.
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s00467-013-2638-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors report no conflict of interest.
References
- 1.Gropman AL, Summar M, Leonard JV. Neurological implications of urea cycle disorders. J Inherit Metab Dis. 2007;30:865–879. doi: 10.1007/s10545-007-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Msall M, Batshaw ML, Suss R, Brusilow SW, Mellits ED. Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med. 1984;23:1500–1505. doi: 10.1056/NEJM198406073102304. [DOI] [PubMed] [Google Scholar]
- 3.Auron A, Brophy PD. Hyperammonemia in review: pathophysiology, diagnosis, and treatment. Pediatr Nephrol. 2012;27:207–222. doi: 10.1007/s00467-011-1838-5. [DOI] [PubMed] [Google Scholar]
- 4.Enns GM, Berry SA, Berry GT, Rhead WJ, Brusilow SW, Hamosh A. Survival after treatment with phenylacetate and benzoate for urea-cycle disorders. N Engl J Med. 2007;356:2282–2292. doi: 10.1056/NEJMoa066596. [DOI] [PubMed] [Google Scholar]
- 5.Spinale JM, Laskin BL, Sondheimer N, Swartz SJ, Goldstein SL. High-dose continuous renal replacement therapy for neonatal hyperammonemia. Pediatr Nephrol. 2013;28:983–986. doi: 10.1007/s00467-013-2441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbeiter AK, Kranz B, Wingen AM, Bonzel KE, Dohna-Schwake C, Hanssler L, Neudorf U, Hoyer PF, Büscher R. Continuous venovenous haemodialysis (CVVHD) and continuous peritoneal dialysis (CPD) in the acute management of 21 children with inborn errors of metabolism. Nephrol Dial Transplant. 2010;25:1257–1265. doi: 10.1093/ndt/gfp595. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer F, Straube E, Oh J, Mehls O, Mayatepek E. Dialysis in neonates with inborn errors of metabolism. Nephrol Dial Transplant. 1999;14:910–918. doi: 10.1093/ndt/14.4.910. [DOI] [PubMed] [Google Scholar]
- 8.Picca S, Dionisi-Vici C, Abeni D, Pastore A, Rizzo C, Orzalesi M, Sabetta G, Rizzoni G, Bartuli A. Extracorporeal dialysis in neonatal hyperammonemia: modalities and prognostic indicators. Pediatr Nephrol. 2001;16:862–867. doi: 10.1007/s004670100702. [DOI] [PubMed] [Google Scholar]
- 9.McBryde KD, Kershaw DB, Bunchman TE, Maxvold NJ, Mottes TA, Kudelka TL, Brophy PD. Renal replacement therapy in the treatment of confirmed or suspected inborn errors of metabolism. J Pediatr. 2006;148:770–778. doi: 10.1016/j.jpeds.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Bunchman TE, Barletta GM, Winters JW, Gardner JJ, Crumb TL, McBryde KD. Phenylacetate and benzoate clearance in a hyperammonemic infant on sequential hemodialysis and hemofiltration. Pediatr Nephrol. 2007;22:1062–1065. doi: 10.1007/s00467-007-0436-z. [DOI] [PubMed] [Google Scholar]
- 11.Ulozas E, Chebrolu SB, Shanaah A, Daoud TM, Leehey DJ, Ing TS. Symptomatic hypocalcemia due to the inadvertent use of a calcium-free hemodialysate. Artif Organs. 2004;28:229–231. doi: 10.1111/j.1525-1594.2004.47207.x. [DOI] [PubMed] [Google Scholar]
- 12.Toussaint N, Cooney P, Kerr PG. Review of dialysate calcium concentration in hemodialysis. Hemodial Int. 2006;10:326–337. doi: 10.1111/j.1542-4758.2006.00125.x. [DOI] [PubMed] [Google Scholar]
- 13.Evenepoel P, Maes B, Vanwalleghem J, Kuypers D, Messiaen T, Vanrenterghem Y. Regional citrate anticoagulation for hemodialysis using a conventional calcium-containing dialysate. Am J Kidney Dis. 2002;39:315–323. doi: 10.1053/ajkd.2002.30551. [DOI] [PubMed] [Google Scholar]
- 14.Buturovic-Ponikvar J, Cerne S, Gubensek J, Ponikvar R. Regional citrate anticoagulation for hemodialysis: calcium-free vs. calcium containing dialysate—a randomized trial. Int J Artif Organs. 2008;31:418–424. doi: 10.1177/039139880803100507. [DOI] [PubMed] [Google Scholar]
- 15.Brophy PD, Somers MJ, Baum MA, Symons JM, McAfee N, Fortenberry JD, Rogers K, Barnett J, Blowey D, Baker C, Bunchman TE, Goldstein SL. Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT) Nephrol Dial Transplant. 2005;20:1416–1421. doi: 10.1093/ndt/gfh817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


