Abstract
Background
The single-dose live attenuated vaccine CVD 103-HgR protects against experimental Vibrio cholerae infection in cholera-naïve adults for at least 6 months after vaccination. While vaccine-induced vibriocidal seroconversion is associated with protection, vibriocidal titers decline rapidly from their peak 1–2 weeks after vaccination. Although vaccine-induced memory B cells (MBCs) might mediate sustained protection in individuals without detectable circulating antibodies, it is unknown whether oral cholera vaccination induces a MBC response.
Methods
In a study that enrolled North American adults, we measured lipopolysaccharide (LPS)- and cholera toxin (CtxB)-specific MBC responses to PXVX0200 (derived from the CVD 103-HgR strain) and assessed stool volumes following experimental Vibrio cholerae infection. We then evaluated the association between vaccine-induced MBC responses and protection against cholera.
Results
There was a significant increase in % CT-specific IgG, % LPS-specific IgG, and % LPS-specific IgA MBCs which persisted 180 days after vaccination as well as a significant association between vaccine-induced increase in % LPS-specific IgA MBCs and lower post-challenge stool volume (r = −0.56, p < 0.001).
Discussion
Oral cholera vaccination induces antigen-specific MBC responses, and the anamnestic LPS-specific responses may contribute to long-term protection and provide correlates of the duration of vaccine-induced protection.
Keywords: memory B cells, cholera, live vaccine, duration of protection, cholera-naïve, CVD 103-HgR
Background
The oral single-dose live attenuated vaccine CVD 103-HgR provides protection against experimental infection with Vibrio cholerae in cholera-naïve North American adults for at least 6 months after vaccination [1, 2]. However, data on the long-term protection of the CVD 103-HgR vaccine do not exist since no study has assessed protection beyond 6 months post-vaccination. Consequently, clinical studies evaluating the long-term protection provided by CVD 103-HgR or identifying long-lasting immunologic correlates of protection following vaccination would address a key knowledge gap surrounding the properties of this vaccine.
Subsequent to the completion of two successful challenge studies with CVD-103 HgR [1, 2], PaxVax, Inc. acquired a worldwide, exclusive license to the CVD 103-HgR strain in 2009 with the aim of redeveloping the vaccine. The PaxVax research name for the vaccine, prepared from new CVD 103-HgR master and working cell banks, was PXVX0200 (now named Vaxchora®).
In 2013, the results of a challenge study in cholera-naïve volunteers demonstrated that serum vibriocidal response 10 days after vaccination with PXVX0200 was strongly associated with protection for volunteers who were challenged either 10 days or 90 days after vaccination [3, 4]. However, after peaking on Day 10 post-vaccination, vibriocidal titers dropped over 10-fold between Day 10 and Day 90. This longitudinal pattern suggests that the vibriocidal response to vaccine, while useful as a correlate of protection when assessed soon after vaccination, may not be a good measure for assessing the duration of vaccine-derived immunity. Part of the explanation for this limitation may be that vibriocidal seroconversion signals an immune response that mediates long-term protection against V. cholerae at the mucosal surface.
Assessment of anamnestic responses may offer a promising alternative approach for quantifying duration of protection. In particular, circulating memory B cells (MBCs) that are specific for V. cholerae antigens may mediate long-term protective immunity against V. cholerae following either infection or vaccination. It has been demonstrated in endemic Bangladesh that V. cholerae infection induces a long-lasting MBC response [5, 6], and that the presence of LPS-specific IgG MBCs at the time of exposure to cholera is strongly predictive of protection in household contacts of patients with cholera, even among individuals who lack circulating vibriocidal antibodies [7]. Similarly, data from a study in cholera-naïve North American volunteers demonstrate that experimental V. cholerae infection primes a significant anamnestic IgA response to LPS that is detectable 6 months after infection [8].
While the existing data suggest that memory B cell responses may contribute to long-term immunologic memory and protection following infection with V. cholerae, the extent of the MBC response that results from oral cholera vaccination and the contribution of this response to protection against cholera are still unknown. To address this key knowledge gap, we characterized MBC responses following vaccination with the single-dose live attenuated oral cholera vaccine PXVX0200 and compared them to MBC responses following experimental infection in subjects who received placebo at baseline. We then measured the association between MBC responses and protection against challenge administered 90 days after vaccination.
Methods
Description of Study Cohort
This study was conducted in a subset of cholera-naïve subjects who participated in a well-characterized vaccine challenge trial. The study design, efficacy results, and safety data from the challenge trial were first described by Chen et al., who showed that for the outcome of moderate to severe diarrhea, PXVX0200 had a protective efficacy of 90% at 10 days after vaccination and 79% at 90 days after vaccination [3].
In the challenge trial, 197 study volunteers were randomized to receive PXVX0200 or placebo in a 1:1 ratio. Subsets of volunteers were randomly selected to be challenged at either 10 days or 90 days after vaccination: 35 vaccinees and 33 placebo recipients were challenged at Day 10, and 33 vaccinees and 33 placebo recipients were challenged at Day 90. Each challenged participant was administered 1 × 105 CFU of virulent V. cholerae O1 El Tor Inaba strain N16961 – a heterologous biotype but homologous serotype to the classical Inaba strain from which PXVX0200 is derived. Sixty-three additional subjects - 27 vaccine recipients and 36 placebo recipients - were randomized but not challenged.
Since our study is focused upon the role of memory B cells in long-term immunity, one part of the dataset for our analysis is comprised of the 66 subjects who participated in the 90-day challenge. In this cohort, we looked at the association between memory B cell response and protection against moderate/severe diarrhea. We also evaluated MBC responses at Day 180 in 22 of the 27 vaccine recipients who were not challenged with V. cholerae. Finally, we assessed the MBC response 170 days after challenge in 26 of the 33 individuals who received placebo on Day 0 followed by V. cholerae challenge on Day 10.
This trial was approved by Institutional Review Boards at the three enrollment centers (Center for Vaccine Development, University of Maryland School of Medicine, Baltimore, MD; Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; and Vaccine Testing Center, University of Vermont College of Medicine, Burlington, VT). Informed consent was obtained for all trial participants. The registration number of the trial at clinicaltrials.gov is NCT01895855.
Vaccine
Lyophilized PXVX0200 sachets stored at −20°C were reconstituted in a sodium bicarbonate buffer that was made using 100 mL of bottled water and single-dose buffer sachets containing 2.5 g NaHCO3, 1.5 g ascorbic acid, and 0.2 g lactose. At reconstitution, the single-dose PXVX0200 solution contained 5 × 108 CFU. One batch of vaccine, Lot No. P701.550-8WA02, was used in the study. The manufacturer of the vaccine is PaxVax, Inc (San Diego, CA, USA).
Measurement of Memory B Cell Responses
Standard operating protocols for shipping, processing and testing of samples of blood and peripheral blood mononuclear cell (PBMC) were established and followed throughout the clinical trial.
Blood samples for measurements of antigen-specific MBC levels for the 66 subjects in the 90-day challenge cohort were drawn on Day 0 just prior to receipt of vaccine or placebo and on Day 90 prior to challenge. Blood samples for the 22 unchallenged vaccinees and for the 26 placebo subjects in the 10-day challenge cohort were drawn on Days 0 and 180. Blood samples were shipped overnight to PaxVax where peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient separation and cryopreserved in liquid nitrogen until ready for testing.
An enzyme-linked immunospot (ELISPOT) assay was used to measure MBC frequencies and an assay protocol was developed and optimized based on previous studies [9–12]. Cryopreserved PBMC were thawed then stimulated in culture medium containing R848 (InvivoGen VacciGrade, 1 μg/mL) and human recombinant IL-2 (Peprotech, 10 ng/mL) under conditions optimized for proliferation and differentiation of CD19+/CD27+ MBC. PBMC (1 × 106/mL) were stimulated for 5 days at 37 °C in 5% CO2 in upright 25cm2 tissue culture flasks (Corning). Pre- and post-treatment PBMC samples from each subject were batch-tested in the same experiment.
Culture medium for all experiments consisted of RPMI-1640 with HEPES and glutamine (Mediatech) which was further supplemented with 10% fetal bovine serum (Hyclone), 1X MEM non-essential amino acids (Invitrogen), 1 mM sodium pyruvate (Corning), 50 μg/mL gentamicin (Amresco) and 2 mM GlutaMax (Invitrogen).
On the day prior to testing for MBC, 96-well ELISPOT plates (Millipore MIPS4150) were coated overnight at 4 °C with antigen or capture antibody. Coating antigen consisted of LPS from V. cholera, Inaba 569B serotype (University of Maryland Center for Vaccine Development; 50 μg/mL) or cholera toxin B (CtxB) (List Biological Laboratories; 15 μg/mL). Assay wells receiving CtxB were first pre-coated with GM1 ganglioside (Enzo Life Sciences; 3 nM) for 3 hr at room temperature. To detect total IgG- or IgA-secreting MBC, wells were coated with monoclonal anti-human IgG or anti-human IgA capture antibody (Jackson ImmunoResearch #109-005-097 or 109-005-011; 5 μg/mL). Uncoated control wells received phosphate buffered saline (PBS).
Five day-stimulated PBMC were serially diluted and tested at the following concentrations per well based on preliminary titration experiments. For measuring antigen-specific IgA-secreting MBC, 5 × 105 cells were added to LPS, CtxB and PBS-coated wells and 7.81 × 103 and 1.95 × 103 cells were added to anti-IgA-coated wells. For the IgG assay, 2.5 × 105 cells were added to LPS, CtxB and PBS-coated wells. CtxB responses were also tested at 6.25 × 104 cells/well. To measure total IgG-secreting MBC, 488 and 122 cells/well were added. Each test condition was performed in triplicate wells.
PBMC were cultured in ELISPOT plates for 3 hr (IgG assay) or 18–22 hr (IgA assay) at 37 °C, 5% CO2. After culture, wells in the IgG plates were washed with PBS/0.05% Tween-20 (Sigma-Aldrich) and biotin-conjugated anti-human IgG antibody (Jackson ImmunoResearch # 109-065-098, 1:1000) was added and allowed to incubate overnight at 4 °C. For IgA plates, wells were washed and biotin-conjugated anti-human IgA (BD Biosciences #555884; 1:1000) was added and incubated for 1–2 hr at room temperature.
Wells were developed for detection of spot forming cells (SFC) by incubation with Vectastain-Avidin-Peroxidase-Complex solution (Vector Lab # PK6100) for 1 hr at room temperature followed by washing and incubation with TrueBlue peroxidase (KPL #50-78-02) for 5–6 min at room temperature [13]. Plates were then rinsed with tap water and dried. Images of assay wells were captured within 48 hr using the imaging software in a CTL ImmunoSpot S6 Core analyzer (Cellular Technology Ltd) and spots were enumerated by the analyzer counting software. The endpoint data for groups tested at different cell concentrations was selected based on the counts measured in the higher concentration unless counts could not be accurately assessed (e.g. wells with high numbers of overlapping spots or high background color staining). In these circumstances, counts measured at the lower cell number were used for data analysis. Data was expressed as SFC normalized to 5 × 105 PBMC (no-antigen values subtracted for groups tested against antigen) then the frequency of specific MBC was expressed as the percent ratio of antigen-specific spots per 5 × 105 cells measured in CtxB or LPS-coated wells divided by the total IgA or IgG-secreting MBC per 5 × 105 cells.
Statistical Analysis
The primary efficacy endpoint in the challenge trial was moderate or severe diarrhea, defined as ≥3 L total volume of diarrhea post-challenge. This endpoint was chosen because it represents a clinically important manifestation of disease. As noted above, the results of the challenge trial showed that the vaccine was close to 80% effective in preventing moderate to severe diarrhea in the 90-day challenge. However, the statistical power to evaluate immunological correlates of protection is determined by the numbers of both protected and unprotected vaccinees, and since most vaccinees were protected against the challenge, power was limited by the small number of unprotected vaccinees. Thus, to differentiate between outcomes and thereby increase statistical power, the outcome variable that was used for assessments of immunologic correlation with protection in this report was the continuous-valued total post-challenge stool volume rather than the categorical moderate/severe diarrhea. This choice of outcome is also appropriate because memory B cell percentages are continuous variables and are most efficiently analyzed using a continuous endpoint. In addition, since we previously demonstrated that the short-term vibriocidal response to PXVX0200 is tightly associated with outcome in subjects challenged with live cholera [4], we also measured the relationship between memory B cell response and vaccine-induced fold-increase in vibriocidal titer.
The statistical significance of differences between memory B cell percentages at Day 0 and Day 90 or Day 180 was assessed using the Wilcoxon signed rank test. Confidence intervals were constructed nonparametrically using binomial calculations for median changes in memory B cell percentages over time, the Clopper-Pearson method for memory B cell response rates [14], and one-sample t-intervals on log2-transformed data for geometric means of vibriocidal titers and fold-increases in vibriocidal titers. P-values that were used to summarize the relationship between memory B cell response and outcome were calculated using the Wilcoxon rank sum test. Associations between post-vaccination MBC percentages and total post- challenge stool volumes were assessed using Spearman’s nonparametric correlation coefficient. The response cutoff for each type of MBC was defined by identifying the valley to the right of a ratio of one in the smoothed histogram of the fold-increase for the corresponding type. Evaluations of vaccine-induced increases in vibriocidal titer in MBC responders and nonresponders were conducted using two-sample t-tests on log2-transformed titer data.
Results
Both Infection and Vaccination Induce a Memory B Cell Response
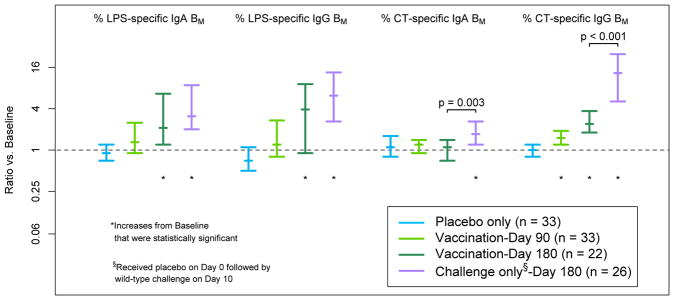
As shown in Table 1 and Figure 1, vaccinees had a significant increase in the percentage of anti-CtxB IgG memory B cells between Day 0 and Day 90 (median ratio of Day 90 to Day 0 = 1.5, or a 50% increase from the pre-vaccination level; p < 0.001; n = 33). In unchallenged vaccinees, significant vaccine-induced increases were observed in the percentages of LPS-specific IgA, LPS-specific IgG, and CtxB-specific IgG memory B cells at Day 180 (p = 0.001, p = 0.004, and p < 0.001, respectively; n = 22). For placebo recipients, no significant memory B cell responses were observed at Day 90 for any antigen or isotype (Table 1; n = 33).
Table 1.
Changes in Percentages of Memory B Cells as a Result of Vaccination or Wild-Type Challenge
| Effect of Placebo | Effect of Vaccination | Effect of Challenge | ||||||
|---|---|---|---|---|---|---|---|---|
| 90 Days After Administration of Placebo* (n = 33) | 90 Days Post-Vaccination * (n = 33) | 180 Days Post-Vaccination* (n = 22) | 170 Days Post-Challenge* (n = 26) | |||||
| Measurement | Median Ratio➉: Day 90/Day 0 (95% CI) | p-value | Median Ratio➉: Day 90/Day 0 (95% CI) | p-value | Median Ratio➉,§: Day 180/Day 0 (95% CI) | p-value | Median Ratio➉,§: Day 180/Day 0 (95% CI) | p-value |
| % LPS-specific IgA BM | 0.9 [0.7, 1.2] | 0.57 | 1.3 [0.9, 2.5] | 0.16 | 2.1 [1.2, 6.6] | 0.001 | 3.1 [2.0, 8.8] | < 0.001 |
| % LPS-specific IgG BM | 0.7 [0.5, 1.1] | 0.07 | 1.2 [0.8, 2.7] | 0.15 | 3.9 [0.9, 9.1] | 0.004 | 6.2 [2.6, 13.5] | < 0.001 |
| % CT-specific IgA BM | 1.1 [0.8, 1.6] | 0.32 | 1.2 [0.9, 1.4] | 0.23 | 1.1 [0.7, 1.4] | 0.74 | 1.7 [1.2, 2.6] | < 0.001 |
| % CT-specific IgG BM | 1.0 [0.8, 1.2] | 0.79 | 1.5 [1.2, 1.9] | < 0.001 | 2.4 [1.8, 3.7] | < 0.001 | 13.2 [5.1, 24.9] | < 0.001 |
Note that each of the four groups in Table 1 is composed of a unique set of subjects.
A ratio of 1.0 indicates no change from the Day 0 percentage of memory B cells.
The duration of post-challenge follow-up through Day 180 was slightly shorter than the post-vaccination duration through Day 180 since challenge occurred on Day 10 and vaccination occurred on Day 0 (170 days vs. 180 days).
Figure 1. Effect of Placebo on Antigen-Specific Memory B Cell Percentages at 90 Days, Effect of Vaccination at 90 and 180 Days, and Effect of V. cholerae Challenge at 180 Days.
Note that for the LPS-specific IgG results on Day 180, the nonparametric 95% confidence interval on the median overlaps one even though the signed-rank test of statistical significance produced a p-value less than 0.05 because the signed-rank test assesses both the direction and magnitude of changes over time and provides greater statistical power.
In contrast to the persistence of antigen-specific memory B cells 6 months after vaccination, vibriocidal activity at 6 months, while significantly above baseline, was less than 5% of the Day 10 peak (Table 2).
Table 2.
Longitudinal Profile of Vibriocidal Immune Response
| Vaccinees Challenged at Day 90 | Unchallenged Vaccinees | |||||
|---|---|---|---|---|---|---|
| Timepoint | n | Serum Vibriocidal GMT (95% CI) | p-value vs. Day 0 | n | Serum Vibriocidal GMT (95% CI) | p-value vs. Day 0 |
| Day 0 | 33 | 36 (25, 52) | Not applicable | 26 | 43 (28, 67) | Not applicable |
| Day 10 | 33 | 3294 (1695, 6399) | < 0.001 | 25 | 3880 (1530, 9842) | < 0.001 |
| Day 28 | 33 | 1363 (734, 2531) | < 0.001 | 24 | 1437 (646, 3194) | < 0.001 |
| Day 90 | 33 | 271 (158, 462) | < 0.001 | Not assessed | ||
| Day 180 | Not assessed | 24 | 155 (82, 294) | < 0.001 | ||
Increases in CtxB-specific IgG and IgA memory B cells at Day 180 were greater in placebo recipients who were infected with V. cholerae than in vaccinees (Figure 1; p < 0.001 and p = 0.003, respectively). While the observed LPS-specific responses to challenge were also greater than the vaccine-induced responses at Day 180, these comparisons were not statistically significant (p = 0.18 and 0.13 for LPS IgG and LPS IgA, respectively).
Association between Memory B Cell Response and Post-Challenge Outcome
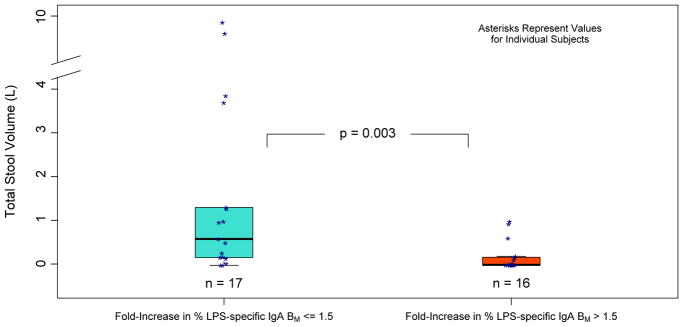
Tables 3a, 3b, 3c provide quantitative summaries of the relationships between total post-challenge stool volumes and post-vaccination, pre-challenge LPS-specific IgA memory B cell response. In the vaccinated group, there was a significant negative association between total post-challenge stool volume and pre-challenge % anti-LPS IgA MBC on Day 90 as well as between stool volume and the ratio (Table 3a; r = −0.39, p = 0.02, and r = −0.56, p < 0.001, respectively). Very similar associations with stool volume were obtained using absolute numbers of LPS-specific IgA MBCs rather than the percentages of IgA-secreting MBCs that were LPS-specific. As shown in Figure 2, an association between categorical anti-LPS IgA response and outcome was also observed, with better post-challenge outcomes for anti-LPS IgA responders to vaccine than for nonresponders (p = 0.003). In the placebo group, there was no significant association between post-challenge stool volumes and the percentage of/increase in % anti-LPS IgA memory B cells, and no significant association with total post-challenge stool volume was found for % anti-LPS IgG, % anti-CtxB IgG, or % anti-CtxB IgA in either the vaccine or the placebo groups.
Table 3a.
Nonparametric Correlation between Vaccine-Induced LPS-Specific IgA Memory B Cell Response at Day 90 and Total Post-Challenge Stool Volume
| Measurement | n | Group | r | p-value |
|---|---|---|---|---|
| Day 90 % LPS-specific IgA BM | 33 | Vaccine | −0.39 | 0.02 |
| % LPS-specific IgA BM: Ratio of Day 90 to Day 0 | 33 | Vaccine | −0.56 | < 0.001 |
| Day 90 % LPS-specific IgA BM | 33 | Placebo | −0.17 | 0.33 |
| % LPS-specific IgA BM: Ratio of Day 90 to Day 0 | 33 | Placebo | 0.02 | 0.92 |
Table 3b.
Total Stool Volume as a Function of % LPS-Specific IgA Memory B Cells on Day 90
| Vaccine | Placebo | |||
|---|---|---|---|---|
| Day 90 % LPS-specific IgA BM | n | Mean (Median) Total Stool Volume (L) |
n | Mean (Median) Total Stool Volume (L) |
| 0.00–0.05 | 11 | 2.4 (0.6) | 11 | 5.6 (5.8) |
| 0.05–0.10 | 8 | 0.7 (0.1) | 6 | 3.6 (3.3) |
| 0.11–0.20 | 6 | 0.5 (0.5) | 11 | 5.9 (3.4) |
| 0.21–1.00 | 8 | 0.2 (0.0) | 5 | 6.4 (7.7) |
Table 3c.
Total Stool Volume as a Function of the change in % LPS-specific IgA Memory B Cells between Day 0 and Day 90
| Vaccine | Placebo | |||
|---|---|---|---|---|
| %LPS-specific IgA BM: Ratio of Day 90 to Day 0 | n | Mean (Median) Total Stool Volume (L) |
n | Mean (Median) Total Stool Volume (L) |
| < 1.50 | 17 | 2.0 (0.6) | 25 | 5.5 (4.6) |
| 1.50–2.99 | 9 | 0.2 (0.0) | 4 | 2.8 (1.2) |
| 3.00–11.0 | 7 | 0.3 (0.0) | 4 | 7.5 (9.4) |
Figure 2. Association between Vaccine-Induced LPS-Specific IgA Memory B Cell Response and Outcome.
The center line of each box represent the median, and the upper and lower edges of the box represent the 75th and 25th percentiles.
Association between Memory B Cell Responses and Vibriocidal Response
As indicated in Table 4a, rates of detectable vaccine-induced anti-LPS IgA and IgG memory B cell responses at Day 90 were 48% and 52%, respectively. Vaccine-induced increases in vibriocidal antibody titer at Day 10 were greater in vaccine recipients with anti-LPS IgA responses at Day 90 and those with anti-LPS IgG responses at Day 90 than in nonresponders. The geometric mean of the fold-increase (GMFI) in vibriocidal antibody titer was equal to 245 for anti-LPS IgA responders vs. 36 in nonresponders (p = 0.01), and the GMFI was 217 vs. 36 in anti-LPS IgG responders and nonresponders, respectively (p = 0.02). As shown in Table 4b, study participants who had multiple LPS-specific memory B cell responses tended to have stronger vibriocidal responses: the GMFI in vibriocidal titer for vaccine recipients with both an anti-LPS IgA response and an anti-LPS IgG response was 380, whereas vaccinees who didn’t have either type of response had a GMFI of 5 (p < 0.001).
Table 4a.
Vaccine-Induced LPS-Specific IgG and IgA Memory B Cell Response Rates at Day 90
| Type of Response at Day 90 | Number of Responders | Number of Vaccinees | Response Rate (95% CI) |
|---|---|---|---|
| LPS-specific IgG BM | 17 | 33 | 52% (34%, 69%) |
| LPS-specific IgA BM | 16 | 33 | 48% (31%, 66%) |
Table 4b.
Association between Vaccine-Induced Memory B Cell Responses at Day 90 and Vibriocidal Response at Day 10
| LPS-specific IgG BM Response |
LPS-specific IgA BM Response |
n | Geometric Mean of Fold-Increase in Vibriocidal Titer at Day 10 (95% CI) |
|---|---|---|---|
| Yes | Yes | 7 | 380 [101, 1434] |
| No | Yes | 9 | 174 [60, 507] |
| Yes | No | 10 | 147 [38, 572] |
| No | No | 7 | 5 [1, 19] |
Vibriocidal response to vaccine increased as the diversity of the memory B cell response increased.
Discussion
Memory B cell responses against LPS and CtxB have been described following infection with V. cholerae [5, 15], and the presence of circulating LPS-specific MBCs is associated with protection against cholera in an endemic area even in individuals without elevated levels of circulating vibriocidal antibodies [7]. Since cholera-specific peripheral blood MBC responses appear to persist a year or longer after infection [5], assessment of these responses may be useful for inferring the duration of protection following cholera vaccination. However, MBC responses to attenuated oral cholera vaccines have not been previously evaluated. It is also unknown whether MBC responses to vaccination contribute to subsequent protection against V. cholerae infection.
In this study, we found that the live attenuated V. cholerae vaccine PXVX0200 did indeed induce MBC responses against both LPS and CtxB, the two dominant V. cholerae antigens [6]. Moreover, increases in circulating CtxB-specific IgG, LPS-specific IgA and LPS-specific IgG MBCs were observed up to 180 days following vaccination, the last time point evaluated in this study.
The magnitude of the LPS IgA MBC response that was observed 90 days after vaccination and just prior to challenge with wild type V. cholerae was robustly and inversely associated with the volume of diarrhea that was observed after the challenge. In contrast, CtxB MBC responses and LPS IgG MBC responses were not associated with post-challenge outcomes. Given our study’s main finding that the vaccine-induced percentage/increase of LPS-specific IgA MBCs is more strongly correlated with outcome than the IgG MBC response, these results are consistent with the model that IgA responses to the O-antigen are the primary drivers of immunity to V. cholerae infection. The hypothesized primacy of the LPS-specific IgA MBC response in protection against V. cholerae is also consistent with another recent study of immune responses to cholera at the single-cell level, which demonstrated that cross-reactive expansion of clonal populations of LPS-specific MBCs following cholera were primarily of the IgA and IgM isotype, and not derived from IgG-expressing cells, though in contrast the responses to CtxB were largely derived from IgG-expressing MBCs [6].
While the association between the percentage/increase in LPS-specific IgA memory B cells at 90 days and total post-challenge stool volume was statistically significant, only about 50% of vaccinees had detectable LPS IgA MBC responses at 90 days (Table 4a; anti-LPS IgA MBC response rate = 48%). Since 88% of vaccinees were protected against the 90-day challenge, LPS IgA response appears to reflect only part of the mechanism of protection. This finding points to the complexity of the immune system and the likelihood that protection is mediated by multiple immunologic variables [16]. Indeed, in this study both LPS-specific IgA and LPS-specific IgG memory B cell responses to vaccine were associated with vibriocidal response, which has previously been shown to be tightly linked with protection in cholera-naïve subjects [4]. Mechanistically, protective immunity is likely to be mediated via a combination of residual persisting antibody and the production of increased levels of antibody by MBCs that are stimulated by memory T cells [17, 18]. Furthermore, there is evidence that the long-term persistence of some types of memory cells might vary substantially from individual to individual [19], which is consistent with the modest LPS-specific IgA MBC response rate that was observed here and with the fact that LPS-specific IgA and LPS-specific IgG responses tended to be detected in different vaccinees.
Cautious interpretation of these data is warranted due to the inherent limitations of the study. As a result of the efficacy of the PXVX0200 vaccine, most vaccinees were well-protected against cholera challenge, and the statistical power to detect correlations between memory B cell responses and post-challenge outcomes was limited by the lack of variation in the outcomes. Also, antigen-specific MBCs are rare and the ELISpot technology is subject to low signal-to-noise ratios when spot counts are low.
In summary, this is the first study of memory B cell responses to a live attenuated oral cholera vaccine, PXVX0200, and it demonstrated robust responses to the two primary V. cholerae antigens, cholera toxin B and lipopolysaccharide. Among these vaccine-induced responses, increases in LPS-specific IgA and IgG memory B cells were strongly correlated with the peak vibriocidal antibody response, and LPS-specific IgA response was associated with diarrheal outcome following challenge, supporting the hypothesis that these cells are mechanistically associated with protection against cholera and/or have the potential to serve as proxies for long-term protection following vaccination.
Acknowledgments
This work was supported, in part, by the National Institute of Allergy and Infectious Diseases, National Institutes of Health grant number U19 AI-082655 to MBS and AI103055 to JBH. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Clinical Trials Registration: NCT01895855
clinicaltrials.gov
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tacket CO, Losonsky G, Nataro JP, Cryz SJ, Edelman R, Kaper JB, Levine MM. Onset and Duration of Protective Immunity in Challenged Volunteers after Vaccination with Live Oral Cholera Vaccine CVD 103-HgR. Journal of Infectious Diseases. 1992;166:837–841. doi: 10.1093/infdis/166.4.837. [DOI] [PubMed] [Google Scholar]
- 2.Tacket CO, Cohen MB, Wasserman SS, et al. Randomized, Double-Blind, Placebo-Controlled, Multicentered Trial of the Efficacy of a Single Dose of Live Oral Cholera Vaccine CVD 103-HgR in Preventing Cholera following Challenge with Vibrio cholerae O1 El Tor Inaba Three Months after Vaccination. Infection and Immunity. 1999;67(12):6341–6345. doi: 10.1128/iai.67.12.6341-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen WH, Cohen MB, Kirkpatrick BD, et al. Single-Dose Live Oral Cholera Vaccine CVD 103-HgR Protects against Human Experimental Infection with Vibrio cholerae O1 El Tor. Clinical Infectious Disease. 2016;62:1329–1335. doi: 10.1093/cid/ciw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haney DJ, Lock MD, Simon JK, Harris JB, Gurwith MG. Antibody-Based Correlates of Protection Against Cholera. Clinical and Vaccine Immunology. 2017;24:e00098–17. doi: 10.1128/CVI.00098-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris AM, Bhuiyan MS, Chowdhury F, et al. Antigen-Specific Memory B-Cell Responses to Vibrio cholerae O1 Infection in Bangladesh. Infection and Immunity. 2009;77:3850–3856. doi: 10.1128/IAI.00369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kauffman RC, Bhuiyan TR, Nakajima R, et al. Single-Cell Analysis of the Plasmablast Response to Vibrio cholerae Demonstrates Expansion of Cross-Reactive Memory B Cells. mBio. 2016;7(6):e02021–16. doi: 10.1128/mBio.02021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SM, Rahman MA, Mohasin M, et al. Memory B Cell Responses to Vibrio cholerae O1 Lipopolysaccharide are Associated with Protection against Infection from Household Contacts of Patients with Cholera in Bangladesh. Clinical and Vaccine Immunology. 2012;19:842–848. doi: 10.1128/CVI.00037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losonsky GA, Yunyongying J, Lim V, Reymann M, Lim YL, Wasserman SS, Levine MM. Factors Influencing Secondary Vibriocidal Immune Responses: Relevance for Understanding Immunity to Cholera. Infection and Immunity. 1996;64(1):10–15. doi: 10.1128/iai.64.1.10-15.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotty S, Aubert RD, Glidewell G, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. Journal of Immunological Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Jahnmatz M, Kesa G, Netterlid E, Buisman AM, Thorstensson R, Ahlborg N. Optimization of a Human IgG B-cell ELISpot Assay for the Analysis of Vaccine-Induced B-cell Responses. Journal of Immunologic Methods. 2013;391:50–59. doi: 10.1016/j.jim.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Jayasekera CR, Harris JB, Bhuiyan S, et al. Cholera Toxin-Specific Memory B Cell Responses are Induced in Patients with Dehydrating Diarrhea Caused by Vibrio cholerae O1. Journal of Infectious Diseases. 2008;198(7):1055–1061. doi: 10.1086/591500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh PN, Friedrich DP, William JA, Smith RJ, Stewart TL, Carter DK, Liao H, McElrath MJ, Frahm N the NIAID HTVTN. Optimization and qualification of a memory B-cell ELISpot for the detection of vaccine-induced memory responses in HIV vaccine trials. Journal of Immunological Methods. 2013;394:84–93. doi: 10.1016/j.jim.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurwith M, Lock M, Taylor EM, Ishioka G, Alexander J, Mayall T, Ervin JE, Greenberg RN, Strout C, Treanor JJ, Webby R, Wright PF. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infectious Diseases. 2013;13:238–50. doi: 10.1016/S1473-3099(12)70345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 15.Alam MM, Riyadh MA, Fatema K, et al. Antigen-Specific Memory B-Cell Responses in Bangladeshi Adults after One- or Two-Dose Oral Killed Cholera Vaccination and Comparison with Responses in Patients with Naturally Acquired Cholera. Clinical and Vaccine Immunology. 2011;18(5):844–850. doi: 10.1128/CVI.00562-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plotkin SA. Complex Correlates of Protection after Vaccination. Clinical Infectious Diseases. 2013;56(10):1458–1465. doi: 10.1093/cid/cit048. [DOI] [PubMed] [Google Scholar]
- 17.Weil AA, Arifuzzaman M, Bhuiyan T, et al. Memory T-Cell Responses to Vibrio cholerae O1 Infection. Infection and Immunity. 2009;77(11):5090–5096. doi: 10.1128/IAI.00793-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhuiyan TR, Lundin SB, Khan AI, et al. Cholera Caused by Vibrio cholerae O1 Induces T-Cell Responses in the Circulation. Infection and Immunity. 2009;77:1888–1893. doi: 10.1128/IAI.01101-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crotty S, Ahmed R. Immunological Memory in Humans. Seminars in Immunology. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]