Abstract
The ability of the aorta to buffer blood flow and provide diastolic perfusion (Windkessel function) is a determinant of cardiovascular health. We have reported cardiac dysfunction indicating downstream vascular abnormalities in young adult baboons who were intrauterine growth restricted (IUGR) at birth as a result of moderate maternal nutrient reduction. Using 3T MRI, we examined IUGR offspring (8 male, 8 female; 5.7 yr; human equivalent 25 yr) and age-matched controls (8 male, 8 female; 5.6 yr) to quantify distal descending aortic cross-section (AC) and distensibility (AD). ANOVA showed decreased IUGR AC/Body Surface Area (0.9 ± 0.05 cm2/m2vs. 1.2 ± 0.06 cm2/m2, M ± SEM, p<0.005) and AD (1.7 ± 0.2 vs. 4.0 ± 0.5 10−3/mmHg, p<0.005) without sex difference or group-sex interaction, suggesting intrinsic vascular pathology and impaired development persisting in adulthood. Future studies should evaluate potential consequences of these changes on coronary perfusion, afterload, and blood pressure.
Keywords: Aorta, maternal nutrient restriction, intrauterine growth restriction, developmental programing, baboons
Introduction
The developmental origins of health and disease hypothesis postulates that reduced fetal growth programs offspring susceptibility to chronic later life conditions, including heart disease, stroke, and hypertension. The Harvard Nurses’ Health Study of 121,700 nurses showed that birth weight is negatively correlated with the incidence of coronary heart disease, stroke, and total cardiovascular disease after adjusting for both age and BMI.1 The Health Professionals Follow-up Study in 22,846 men concluded low birth weight men were at higher risk for hypertension and diabetes, even after correcting for parental history and obesity.2 We have reported cardiac dysfunction characteristic of increased afterload and vascular abnormalities in young adult intrauterine growth restricted (IUGR) male and female baboons without blood pressure changes.3
It is well recognized that the aorta is not a passive conduit. Aortic wall stretching and resultant excess flow accommodation in systole allows it to act as a blood reservoir. Its subsequent elastic recoil generates forward blood flow for continued diastolic perfusion of vital organs, termed the Windkessel function. The aorta thus significantly modulates left ventricular function, coronary blood flow, and systemic arterial function. The aortic Windkessel function hinges upon its systolic buffering capacity and diastolic elastic potential, both strongly correlated with aortic size and distensibility.4 Furthermore, aortic distensibility affects baroreceptor function5 and is proposed as a marker of hypertensive risk. We hypothesize that IUGR reduces aortic cross-section (AC) and aortic distensibility (AD).
Materials and Methods
Ethical Approval
All procedures were approved by the Texas Biomedical Research Institute Institutional Animal Care and Use Committee (IACUC) and conducted in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Animal Model
Baboons (Papio hamadryas) were group housed and maintained with full social interaction and physical activity with access to individual feeding when required. Healthy non-pregnant female baboons of similar age, morphology (weight, body length, abdominal circumference, etc.), and phenotype to ensure homogeneity of the mothers in both groups were randomly assigned either to an ad lib monkey chow diet (controls; CTL) during pregnancy and lactation or a globally reduced diet regimen consisting of 70% of feed eaten by CTL ad lib fed mothers (maternal nutrient reduction; MNR) from 0.16 gestation (G) to end of lactation. Diet was Purina 5038 (Purina LabDiets, St Louis, MO) containing 13% calories from fat, 18% calories from protein, 69% calories from carbohydrates, mineral and vitamin additives, and a metabolizable energy content of 3.22 kcal/g. Offspring of MNR mothers were intrauterine growth restricted (IUGR) at term.6 CTL and IUGR offspring were weaned to monkey chow at 9 months and moved to juvenile cages. Baseline data are shown in Table 1.
Table 1. Measurement Data.
Measurements (mean ± SEM).
| CTL | IUGR | ANOVA | |||
|---|---|---|---|---|---|
| Baseline Data | M (N = 8) | F (N = 8) | M (N = 8) | F (N = 8) | |
| Age | 5.4 ± 0.5 | 5.7 ± 0.5 | 5.9 ± 0.4 | 5.5 ± 0.5 | NS |
| Current Weight (kg) | 19.5 ± 2.4 | 13.9 ± 0.7 | 21.6 ± 1.5 | 13.4 ± 0.4 | M > F*** |
| Body Surface Area (m2) | 0.55 ± 0.05 | 0.44 ± 0.01 | 0.60 ± 0.03 | 0.44 ± 0.01 | M > F*** |
| Birth Weight (kg) | 0.93 ± 0.05 | 0.89 ± 0.04 | 0.82 ± 0.03 | 0.74 ± 0.05 | CTL > IUGR** |
| Heart Rate | 111 ± 4.2 | 135 ± 4.6 | 112 ± 4.6 | 131 ± 4.2 | F > M*** |
| Blood Pressure (mmHg) | M (N = 8) | F (N = 8) | M (N = 8) | F (N = 7)# | |
| Systolic | 118 ± 5.7 | 116 ± 9.5 | 113 ± 6.7 | 119 ± 4.2 | NS |
| Diastolic | 68 ± 7.8 | 71 ± 9.2 | 63 ± 8.1 | 66 ± 4.9 | NS |
| Mean Arterial | 85 ± 7.1 | 86 ± 9.2 | 80 ± 7.8 | 84 ± 3.8 | NS |
| Pulse Pressure | 50 ± 4.2 | 52 ± 1.8 | 49 ± 3.5 | 53 ± 4.2 | NS |
NS: not significant;
: p < 0.01;
: p < 0.005.
The blood pressure measurements could not be obtained in 1 female IUGR baboon.
Blood Pressure Measurement and Calculation
Blood pressure measurements were acquired with the Omron HBP-1300 professional sphygmomanometer in the left upper arm, using a small (17-22 cm) or a medium (22-32 cm) cuff as appropriate. Measurements were obtained upon intramuscular ketamine hydrochloride sedation induction (10-12 mg/kg) with the baboons in the supine position. Six measurements were obtained and averaged. Pulse pressure is defined as the difference between systolic and diastolic blood pressure. Mean arterial pressure was approximated by standard formula using systolic and diastolic blood pressures, MAP = 1/3 SBP + 2/3 DBP.
MRI
MRI was performed on two groups of baboons: IUGR (8 male, 8 female, age = 5.7 ± 1.3 yr; mean ± SD) and age-matched CTL baboons (8 male, 8 female, age = 5.6 ± 1.3 yr). To account for potential diurnal and prandial effects on vascular regulation, the studies were conducted during the same time of the day, in the morning (9 am-11 am) after overnight fast. Anesthesia was induced with ketamine hydrochloride (10-12 mg/kg, i.m.) and maintained with isoflurane (0.8-1.0%, inh). Oral intubation was then performed followed by mechanical ventilation and physiologic monitoring.3
All studies were performed on a 3.0 Tesla MR scanner (TIM Trio, Siemens Healthcare, Malvern, PA) with a six-channel anterior phased-array torso coil and corresponding posterior coil elements, resulting in an aggregate of 12 channels of data. Before each imaging session, a standard quality control phantom was scanned to ensure geometric accuracy, spatial resolution, and contrast were within acceptable limits. Breath-held, EKG-gated, steady-state free precession (SSFP) imaging sequence was used to acquire high temporal resolution cine images of the lower thorax with EKG gating (TR/TE 3.0/1.5 ms, 25 cardiac phases, matrix 144×192, FOV 188×250 mm2). Segmentation of a single heart beat into 25 phases results in approximately 20 ms frames. A stack of 20-24 contiguous short-axis slices (2.5 mm thickness, no gap) was acquired at the level of the heart.
Image Processing and Analysis
The CMR42 image analysis package (Circle Cardiovascular, Calgary, AB) was used for data analysis. To account for angulation of the aorta, axial slice images of the aorta were obtained and processed with 3D reconstruction via the CMR42 multi-planar formatting module to ensure the axial slices are oriented perpendicular to the vascular long axis. The distal descending aortas at the level of the heart, above the diaphragm, were examined. Measurements of the aorta cross-sectional area were taken at the end-systolic and end-diastolic phases from the same axial slice level and averaged for further analysis. Additionally, distensibility was calculated using the formula
where ΔΡ is approximated by using the left brachial pulse pressure. Duplicate measurements were made and averaged for use in the final analyses.
Data were analyzed using GraphPad Prism 7. Grubbs’ test (extreme Studentized deviate) was used to evaluate for statistical outliers. Normality of distribution was assessed by the d’Agostino-Pearson test. Two-way ANOVA analysis was used to evaluate the null hypotheses that there were no differences between the factors group and sex and no significant interactions. Correlation analysis between AC and birth weight was performed prior to and following normalization to body surface area (BSA).
Data are presented as mean ± standard error of the mean (SEM). In bar graphs, number of animals used in final analysis is indicated as N. Asterisks denote significance, p < 0.05 for all tests.
Normalization
The cross-section measurements were evaluated with reference to BSA, estimated using weight based models previously described for baboons.7 For females:
and in males,
where weight is measured in kg, and the calculated BSA has unit of m2.
Results
Blood Pressure
Blood pressure data are shown in Table 1. There was no difference detected in systolic blood pressure, diastolic blood pressure, mean arterial blood pressure, or pulse pressure between the groups or the sexes. No group-sex interaction was detected.
Aortic Cross-section
Prior to normalization to BSA, ANOVA revealed no group (p = 0.07) or sex (p = 0.14) difference, and no group-sex interaction (p = 0.38) in AC. After normalization to BSA, group difference (p = 0.004) was seen without sex difference (p = 0.13) or group-sex interaction (p = 0.42) with lower AC/BSA in IUGR (1.2 ± 0.06 cm2/m2 vs. 0.9 ± 0.05 cm2/m2, M ± SEM, Figure 1 A).
Figure 1. Decreased Aortic Cross-section and Distensibility with IUGR.
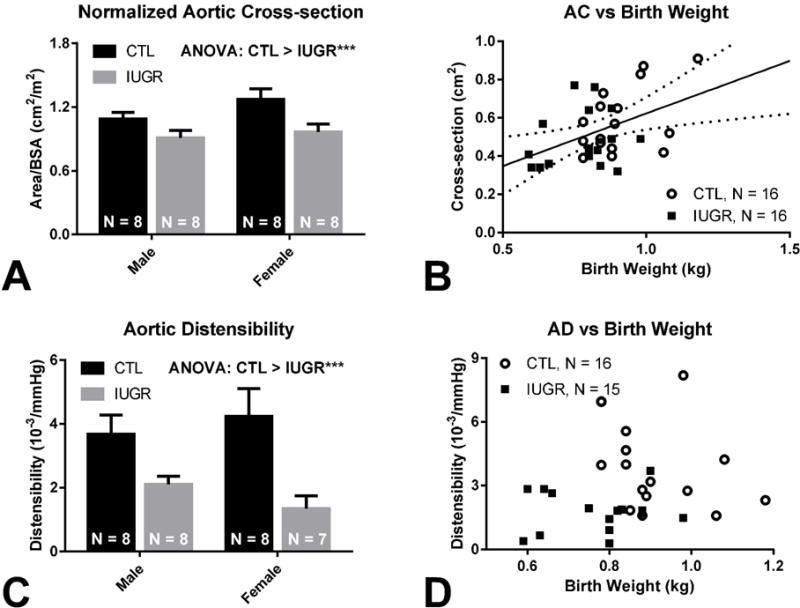
(A) Lower AC/BSA is noted in CTL vs. IUGR (1.2 ± 0.06 cm2/m2 vs. 0.9 ± 0.05 cm2/m2, p < 0.005). No between sex difference or group-sex interaction is detected by ANOVA. (B) AC correlated positively with birth weight (AC = 0.55 · BW + 0.07, p = 0.01, r = 0.44). Solid regression line is seen with dotted 95% confidence bands. (C) Decreased AD is seen in CTL vs. IUGR (4.0 ± 0.5 vs. 1.7 ± 0.2 10−3/mmHg, p < 0.005). No between sex difference or group-sex interaction is detected by ANOVA. (D) No significant correlation is found between AD and birth weight, but group stratification is observed.
Linear regression revealed a positive correlation between AC and birth weight (AC = 0.55 · BW + 0.07, p = 0.01, R = 0.44, Figure 1 B). The within-group correlation was not different between groups (p = 0.39). After normalization to BSA, the overall correlation was not significant (p = 0.13).
Aortic Distensibility
ANOVA revealed group as a significant factor in AD (p = 0.0008) without sex difference (p = 0.87) or group-sex interaction (p = 0.27), with lower AD in IUGR (4.0 ± 0.5 vs. 1.7 ± 0.2 10−3/mmHg, Figure 1 C).
Linear regression between AD and birth weight of the IUGR and CTL animals revealed no significant correlation combined (p = 0.41, Figure 1 D) or individually (pCTL = 0.20, pIUGR = 0.83).
Discussion
The developmental programming hypothesis states that gene-environment interactions and epigenetic changes during perinatal development have long-term impacts upon the life-course health trajectory of the offspring.8 We examined aortic structure in a cohort of baboons in which we have established left ventricular deficits in IUGR compared with contemporaneous controls.3 We uncovered persistent distal thoracic aorta abnormalities in young adult IUGR males and females-decreased lumen size and functional aorta impairment indicated by decreased distensibility.
The clinical literature on the relationship between aortic elasticity and prenatal growth reports mixed findings. Increased aortic stiffness has been noted in very low birth weight premature infants,9 small-for-gestational-age newborns,10 and IUGR children.11 A few studies detected a similar trend of increase in aortic stiffness that did not reach significance.12,13 Some studies failed to demonstrate any correlation.14 We suspect the disparate findings in the literature arose from the use of birth weight as a direct marker of intrauterine growth. Birth weight distribution in healthy neonates indicates low birth weight can result from a normal pregnancy. In our model, overlap of birth weight suggests birth weight alone is an insufficient measure of prenatal health. In placental-insufficiency induced IUGR fetal sheep, aortic stiffness was increased with altered collagen-to-elastin ratio,15 signifying the detected viscoelastic changes of poor prenatal growth have biomechanical basis at the molecular level. We hypothesize from the lack of correlation between birth weight and AD in this study that the presence of a prenatal insult, not the extent of IUGR or birth weight per se, determines abnormal matrix deposition and the resultant aortic mechanical property. Disruption of elastin content was previously noted in hypoxia induced IUGR fetal sheep16 and uterine artery ligation induced IUGR fetal and adult guinea pigs.17 An early study on the rat aorta revealed elastin deposition is largely limited to the prenatal and early post-natal period, and the concentration of elastin decreases thereafter.18 Our findings are the first to indicate that functional changes in a developmentally programmed primate model of IUGR persist into adulthood. In the context of the Windkessel function, decreased aortic distensibility with IUGR is important because the elastic recoil of the aorta provides the bulk of the contractile force of diastolic perfusion of the systemic circulation, including the coronary arteries.
IUGR resulted in diminished aortic cross-section. The role of IUGR in reducing aortic size has been noted in small-for-gestational-age children.12,13 Species differences may exist as IUGR induced by hypoxia in chick embryos19 and hyperthermia in fetal sheep20 reduce aortic cross-section, but aortic size is unaffected by maternal nutrient restriction or hypoxia induced IUGR in rats.21,22 Our results suggest in a primate model that IUGR, induced by 30% reduced maternal nutrition, decreases offspring aortic cross-section. Concurrent decrease in vessel size and distensibility is important because reduction in size further diminishes aortic buffering capacity. With aortic narrowing, flow velocity and luminal pressure increase if flow volume is maintained, potentially leading to self-propagating flow-mediated arterial wall stiffening.23
One of the major questions related to developmental programming is the extent to which programmed outcomes persist through life. Human aorta stiffness increases with age, with one human study reporting that age-related aortic stiffness increase proceeds in a sex dependent manner.24 In future studies we propose to determine whether these programming effects in the aorta increase, decrease, or remain unchanged with aging across the life-course. Thus, we could not perform a necropsy to obtain tissues to test the molecular and cellular mechanisms involved. While studies in other models have provided a potential mechanism for our observed changes, it remains to be established whether comparable cellular processes occur in primates given the differences between monotocous precodal species including primates and polytocous altricial rodents. Understanding species similarities and differences aids evaluation of key mechanisms. On a few occasions, we have assessed blood pressure in IUGR and control animals and found no group differences in systolic, diastolic, and pulse pressures.3 It is unusual for animals with decreased distensibility to exhibit no change in pulse pressure, a known consequence of impaired aortic function.4,23 Although fetal exposure to high doses of steroids leads to hypertension in later life, we have shown that hypertension is not exhibited in exposed sheep by 5 months of age.25 We note that in a large human systemic review by Huxley et al. concluded that negative correlation between birth weight and systolic blood pressure, although present, is both subtle (2 mmHg/kg birth weight) and attenuated in adolescence,26 whereas other animal models have reported much more drastic increases in arterial blood pressure.27-29 We hypothesize that as with many other responses to developmental challenges, compensatory processes occur to normalize anticipated blood pressure increases and that additional stressors, such as aging, are needed for abnormalities to emerge in our animals, which are in early young adulthood (human equivalent 20-25 years). Additionally, we note that the extent of our nutrient reduction is both milder compared to some other global nutrient reduction models (30% vs 50%) and continues into lactation, where catch-up growth may be blunted. Combined, these factors may result in smaller degree of blood pressure that is currently below our detection threshold. Alternatively, it is possible that the lack of difference may originate from the use of anesthesia and supine positioning of the animals during the measurements. Finally, we acknowledge that distensibility is only one facet of “arterial stiffness” and, as measured in this study, reflects more on the aorta’s elastic property rather than compliance, which carries a different mechanical significance.30 We further note that our AD measurements are calculated based on using brachial pulse pressure instead of more invasively obtained and more accurate aortic pulse pressure, meant to examine the differences in physiology between IUGR and control animals instead of providing highly accurate measures of aortic distensibility. Differences in measurement methodologies used in published studies likely contribute to the divergent results seen regarding arterial stiffness.
In summary, we report decreased aortic distensibility and size secondary to IUGR, suggesting IUGR causes long-term impairment of aortic Windkessel function. Reduction in normalized aortic cross-section indicates that elements of poor structural growth persist into adulthood. Importantly, decreased distensibility similar to that observed with advanced aging is seen, suggesting elevated afterload, blood pressure dysregulation, and altered flow require study. Disruption of aortic function by IUGR is important because coronary perfusion occurs predominantly during diastole and heightened incidence of ischemic heart disease has been repeatedly associated with IUGR. Together, our results suggest IUGR-related vascular changes contribute to the known cardiac dysfunction outcomes from poor prenatal growth and warrant further study.
Acknowledgments
The authors thank Dr. Robert Lanford and the Southwest National Primate Center Staff for their ongoing technical support of our baboon research program described in this article. The authors also acknowledge the technical support of Steven Rios and Sam Vega, as well as the administrative support of Karen Moore.
Financial Support
This work was supported by the National Institutes of Health (5P01HD021350 to P.W.N., 5R24OD011183 to P.W.N., 5K25DK089012 to G.D.C., 1R25EB016631 to A.H.K., and OD P51OD011133 from the Office of Research Infrastructure Programs/Office of the Director); EU FP 7/HEALTH/GA No.: 279281: BrainAge-Impact of Prenatal Stress on BRAINAGEing.
Footnotes
Conflicts of interest
The authors have no potential conflict of interest, financial or otherwise, to disclose.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the Association for Assessment and Accreditation of Laboratory Animal Care International and has been approved by the Texas Biomedical Research Institute Institutional Animal Care and Use Committee.
References
- 1.Rich-Edwards JW, Kleinman K, Michels KB, et al. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. BMJ. 2005;330(7500):1115. doi: 10.1136/bmj.38434.629630.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 3.Kuo AH, Li C, Li J, Huber HF, Nathanielsz PW, Clarke GD. Cardiac remodelling in a baboon model of intrauterine growth restriction mimics accelerated ageing. J Physiol. 2016;595(4):1093–1110. doi: 10.1113/JP272908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belz GG. Elastic properties and windkessel function of the human aorta. Cardiovasc Drugs Ther. 1995;9(1):73–83. doi: 10.1007/BF00877747. [DOI] [PubMed] [Google Scholar]
- 5.Klassen SA, Chirico D, Dempster KS, Shoemaker JK, O’Leary DD. Role of aortic arch vascular mechanics in cardiovagal baroreflex sensitivity. Am J Physiol Regul Integr Comp Physiol. 2016;311(1):R24–32. doi: 10.1152/ajpregu.00491.2015. [DOI] [PubMed] [Google Scholar]
- 6.Li C, McDonald TJ, Wu G, Nijland MJ, Nathanielsz PW. Intrauterine growth restriction alters term fetal baboon hypothalamic appetitive peptide balance. J Endocrinol. 2013;217(3):275–282. doi: 10.1530/JOE-13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VandeBerg JL, Williams-Blangero S, Tardif SD. The baboon in biomedical research. Springer Science & Business Media; 2009. [Google Scholar]
- 8.Sun C, Burgner DP, Ponsonby A, et al. Effects of early-life environment and epigenetics on cardiovascular disease risk in children: Highlighting the role of twin studies. Pediatr Res. 2013;73(4–2):523–530. doi: 10.1038/pr.2013.6. [DOI] [PubMed] [Google Scholar]
- 9.Tauzin L, Rossi P, Giusano B, et al. Characteristics of arterial stiffness in very low birth weight premature infants. Pediatr Res. 2006;60(5):592–596. doi: 10.1203/01.pdr.0000242264.68586.28. [DOI] [PubMed] [Google Scholar]
- 10.Mori A, Uchida N, Inomo A, Izumi S. Stiffness of systemic arteries in appropriate- and small-for-gestational-age newborn infants. Pediatrics. 2006;118(3):1035–1041. doi: 10.1542/peds.2006-0386. [DOI] [PubMed] [Google Scholar]
- 11.Cheung YF, Wong KY, Lam BC, Tsoi NS. Relation of arterial stiffness with gestational age and birth weight. Arch Dis Child. 2004;89(3):217–221. doi: 10.1136/adc.2003.025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley D, Stale H, Marsal K. Aortic vessel wall characteristics and blood pressure in children with intrauterine growth retardation and abnormal foetal aortic blood flow. Acta Paediatrica. 1997;86(3):299–305. doi: 10.1111/j.1651-2227.1997.tb08894.x. [DOI] [PubMed] [Google Scholar]
- 13.Brodszki J, Lanne T, Marsal K, Ley D. Impaired vascular growth in late adolescence after intrauterine growth restriction. Circulation. 2005;111(20):2623–2628. doi: 10.1161/CIRCULATIONAHA.104.490326. [DOI] [PubMed] [Google Scholar]
- 14.Schack-Nielsen L, Mølgaard C, Larsen D, Martyn C, Michaelsen KF. Arterial stiffness in 10-year-old children: Current and early determinants. Br J Nutr. 2005;94(06):1004–1011. doi: 10.1079/bjn20051518. [DOI] [PubMed] [Google Scholar]
- 15.Dodson RB, Rozance PJ, Hunter KS, Ferguson VL. Increased stiffness of the abdominal aorta with intrauterine growth restriction in the near-term fetal sheep. ASME 2012 Summer Bioengineering Conference. 2012:1251–1252. [Google Scholar]
- 16.Thompson JA, Richardson BS, Gagnon R, Regnault TR. Chronic intrauterine hypoxia interferes with aortic development in the late gestation ovine fetus. J Physiol (Lond) 2011;589(13):3319–3332. doi: 10.1113/jphysiol.2011.210625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson JA, Gros R, Richardson BS, Piorkowska K, Regnault TR. Central stiffening in adulthood linked to aberrant aortic remodeling under suboptimal intrauterine conditions. Am J Physiol Regul Integr Comp Physiol. 2011;301(6):R1731–7. doi: 10.1152/ajpregu.00274.2011. [DOI] [PubMed] [Google Scholar]
- 18.Looker T, Berry CL. The growth and development of the rat aorta. II. changes in nucleic acid and scleroprotein content. J Anat. 1972;113(Pt 1):17–34. [PMC free article] [PubMed] [Google Scholar]
- 19.Rouwet EV, Tintu AN, Schellings MW, et al. Hypoxia induces aortic hypertrophic growth, left ventricular dysfunction, and sympathetic hyperinnervation of peripheral arteries in the chick embryo. Circulation. 2002;105(23):2791–2796. doi: 10.1161/01.cir.0000017497.47084.06. [DOI] [PubMed] [Google Scholar]
- 20.Dodson RB, Rozance PJ, Petrash CC, Hunter KS, Ferguson VL. Thoracic and abdominal aortas stiffen through unique extracellular matrix changes in intrauterine growth restricted fetal sheep. Am J Physiol Heart Circ Physiol. 2014;306(3):H429–37. doi: 10.1152/ajpheart.00472.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozaki T, Nishina H, Hanson M, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol (Lond) 2001;530(1):141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res. 2009;81(4):713–722. doi: 10.1093/cvr/cvn341. [DOI] [PubMed] [Google Scholar]
- 23.Franklin SS, Gustin W, 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. the framingham heart study. Circulation. 1997;96(1):308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 24.Nethononda RM, Lewandowski AJ, Stewart R, et al. Gender specific patterns of age-related decline in aortic stiffness: A cardiovascular magnetic resonance study including normal ranges. J Cardiovasc Magn Reson. 2015;17(1):20. doi: 10.1186/s12968-015-0126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molnar J, Howe DC, Nijland MJ, Nathanielsz PW. Prenatal dexamethasone leads to both endothelial dysfunction and vasodilatory compensation in sheep. J Physiol (Lond) 2003;547(1):61–66. doi: 10.1113/jphysiol.2002.032565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: A systematic review of the literature. J Hypertens. 2000;18(7):815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: Hypertension and decreased nephron number in offspring at 9 months of age. J Physiol (Lond) 2005;565(1):137–147. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponzio BF, Carvalho MHC, Fortes ZB, do Carmo Franco M. Implications of maternal nutrient restriction in transgenerational programming of hypertension and endothelial dysfunction across F1-F3 offspring. Life Sci. 2012;90(15):571–577. doi: 10.1016/j.lfs.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Kawamura M, Itoh H, Yura S, et al. Undernutrition in utero augments systolic blood pressure and cardiac remodeling in adult mouse offspring: Possible involvement of local cardiac angiotensin system in developmental origins of cardiovascular disease. Endocrinology. 2007;148(3):1218–1225. doi: 10.1210/en.2006-0706. [DOI] [PubMed] [Google Scholar]
- 30.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: Current understanding and future directions. J Am Coll Cardiol. 2011;57(14):1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]


