Ultralight and fire-resistant ceramic nanofibrous aerogels with temperature-invariant superelasticity to 1100°C.
Abstract
Ultralight aerogels that are both highly resilient and compressible have been fabricated from various materials including polymer, carbon, and metal. However, it has remained a great challenge to realize high elasticity in aerogels solely based on ceramic components. We report a scalable strategy to create superelastic lamellar-structured ceramic nanofibrous aerogels (CNFAs) by combining SiO2 nanofibers with aluminoborosilicate matrices. This approach causes the random-deposited SiO2 nanofibers to assemble into elastic ceramic aerogels with tunable densities and desired shapes on a large scale. The resulting CNFAs exhibit the integrated properties of flyweight densities of >0.15 mg cm−3, rapid recovery from 80% strain, zero Poisson’s ratio, and temperature-invariant superelasticity to 1100°C. The integral ceramic nature also provided the CNFAs with robust fire resistance and thermal insulation performance. The successful synthesis of these fascinating materials may provide new insights into the development of ceramics in a lightweight, resilient, and structurally adaptive form.
INTRODUCTION
Ultralow density ceramic aerogels are attractive for their fascinating characteristics of low density and thermal conductivity, chemical and thermal inertness, high porosity, and large surface area, and they have been widely studied for thermal, catalytic, electrical, environmental, and energy applications (1–3). In the ultralight regime below 10 mg cm−3, very few ceramic aerogels currently exist (4–8): silicon carbide foams (>9 mg cm−3), ZnO/silica aerogels (>8 mg cm−3), silica aerogels (>3 mg cm−3), boron nitride aerogels (>1.4 mg cm−3), and boehmite sponges (>1.2 mg cm−3). All of the above aerogels have a similar three-dimensional (3D) pearl necklace–like microstructure: The network consists of ceramic nanoparticles that are connected through narrow interparticle necks (6, 9). This random porous structure leads to some beneficial properties (that is, large surface area and restriction of liquid and gas flow), but generally, the inefficient structure continuity and connection result in poor mechanical properties (10, 11). As with most of the present porous ceramic materials, these ceramic aerogels are usually rigid and brittle, with only a slight elastic deformation before fracture unless they are hybrid with polymers or grown on preexisting carbon networks (12, 13). The superelasticity that has been achieved in aerogels made of polymer or carbon has not been observed in aerogels solely based on ceramic components (14, 15). Because the specific elastic bending strain of ceramic is known to be intrinsically inferior to that of polymer or carbon, previous analyses suggest that it would be quite challenging to achieve superelasticity in porous ceramic networks (3, 16).
The properties of aerogels are generally dictated by the intrinsic properties of the solid components, the density, and the cellular architectures (3, 17). To develop an appropriate structure that can allow the ceramic networks to maintain structural integrity upon large deformation, we examined a variety of existing cellular materials and were particularly impressed by the high mechanical efficiency of nanofibrous networks (11, 18). For instance, natural cellular fibrous structures such as spider webs and the silkworm cocoon have a relatively low amount of solid constituents, similar to that of aerogels, but are structurally robust (19, 20). In these structures, nanofibers are closely connected to form a uniform cell geometry, a structure that is highly helpful in optimizing cellular-specific mechanical strength (17, 21). Our previous work also revealed that intrinsically brittle ceramics including silica, titania, and zirconia could become soft and flexible when hierarchically structured as nanofibrous membranes (22–24). Ceramic nanofibers, in this regard, show great promise as exceptional candidates for constructing mechanically robust ceramic aerogels. Despite their outstanding potential, the major problem associated with ceramic nanofibers is their direct spinning-deposition character; the resulting nanofibers usually assemble into random-deposited fluffy nonwovens rather than into organized aerogels. Most recently, ceramic nanofiber–based sponges have been fabricated by using specially designed cage-like collectors (25–27). However, the principle of these methods remains the direct deposition of nanofibers; thus, most of the products are not true 3D bulk aerogels but rather fluffy cotton-like nanofiber deposits without precise control of the density and shape (28, 29). Moreover, these materials usually lack organized cellular structure and stable cross-linking between nanofibers and so exhibit poor mechanical strength with a weak elastic resilience of <30% strain. The challenge, therefore, is to construct continuous, 3D-structured, and mechanically robust CNFAs capable of forming organized cellular architectures without compromising the properties of the intrinsic ceramic nanofibers.
Herein, we demonstrate a robust method for creating superelastic lamellar-structured ceramic nanofibrous aerogels (CNFAs) by combining flexible SiO2 nanofibers with aluminoborosilicate (AlBSi) matrices. The advantage of the design is that random-deposited SiO2 nanofibers are reconstructed into elastic ceramic aerogels with tunable densities and shapes on a large scale. The resulting CNFAs exhibit the integrated properties of ultralow density (minimum of 0.15 mg cm−3), complete recovery from large deformation, zero Poisson’s ratio, temperature-invariant superelasticity, low thermal conductivity, and fire resistance, all resulting from the synergistic effects of organized nanofibrous architectures and well-bonded ceramic nanofibers.
RESULTS
Fabrication and hierarchical cellular structure
We prepared the CNFAs based on three criteria: (i) the ceramic nanofibers must be flexible and mechanically robust, (ii) the nanofibers must assemble into 3D aerogels with controllable porous structure and shape, and (iii) the nanofibers must cross-link to form elastic and thermally stable networks. The first two requirements are satisfied by using micro/nanotextured structures; we combined the unique properties of electrospun ceramic nanofibers (soft and flexible) with the fibrous freeze-shaping method (isotropic and controllable assembly) to yield 3D ceramic nanofibrous networks. To satisfy the last criterion—the formation of stable cross-linking—our material design is based on nonalkaline AlBSi, which is widely used as high-temperature ceramic matrices in the glass industry and inorganic synthesis (30). The cross-linking activity came from the formation of silicate bonding (X-O-Si) with silica nanofibers when they were calcined in the presence of oxygen (30, 31).
The synthetic pathway mainly involved four components: water, SiO2 nanofibers, polyacrylamide, and AlBSi sol, as illustrated in Fig. 1A. The fabrication process began with the preparation of flexible SiO2 nanofibers using a sol-gel electrospinning method. The resulting nanofibers had an average fiber diameter of 206 nm (fig. S1 and Supplementary Methods). Upon homogenization with polyacrylamide in water, the SiO2 nanofibers became highly entangled with each other and well dispersed because of mutual repulsion, which was caused by the site-specific wrapping of the fiber surface with cationic polyacrylamide (32, 33). The resulting homogenized fiber dispersion could remain stable without gravity sedimentation (figs. S2 and S3 and Supplementary Methods). Subsequently, AlBSi sol was prepared by the mixing and hydration of tetraethyl orthosilicate, aluminum chloride, and boric acid. This mixture was added to the above nanofiber dispersion for further homogenization. After degassing in a vacuum chamber, the dispersion was frozen in a dry ice/acetone bath and freeze-dried into AlBSi gel/polyacrylamide/SiO2 nanofibers composite pre-aerogels (PNFAs) (see details in fig. S4 and Supplementary Methods). The freshly prepared PNFAs were fragile and heat-intolerant due to the lack of effective bonding. To build robust bonding among the fibers, the obtained PNFAs were calcined at 900°C to form cross-linked ceramic nanofibrous networks, endowing the resultant CNFAs with elastic resilience and heat resistance. As a result of the calcination in air, the organic polyacrylamide was decomposed and removed; simultaneously, the in situ sintering of the AlBSi gel in the fiber surface led to the formation of amorphous AlBSi glass ceramics and finally cemented the adjacent silica nanofibers (31, 34). The densities of the CNFAs could be readily regulated by changing the concentrations of the precursor dispersions. Except as noted, all the structure and property investigations were performed by using the CNFAs with a density of 5 mg cm−3. Evidence of the formation of AlBSi ceramics was obtained from the x-ray photoelectron spectroscopy (XPS) analysis (Fig. 1B and a magnified spectrum in fig. S5); the characteristic peaks of Al2p, B1s, Si2p3, and Si2s were assigned to Al- and B-doped silica tetrahedron structures (34, 35). Significantly, the integral ceramic nature of both the silica nanofibers and the AlBSi surface layer allows the CNFAs to withstand even 1100°C of high-temperature flames (butane blowtorch) without any visible damage or deformation (Fig. 1C), demonstrating the robust fire resistance.
Fig. 1. Structure design and cellular architectures of CNFAs.
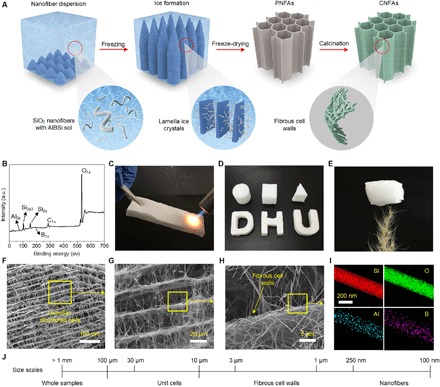
(A) Schematic illustration of the fabrication of CNFAs. (B) XPS spectrum of CNFAs for all elements. a.u., arbitrary unit. (C) A CNFA heated by a butane blowtorch without any damage. (D) An optical image of CNFAs with diverse shapes. (E) An optical image showing a 20-cm3 CNFA (ρ = 0.15 mg cm−3) standing on the tip of a feather. (F to H) Microscopic structure of CNFAs at different magnifications demonstrating the hierarchical nanofibrous cellular architecture. (I) STEM-EDS images of a single nanofiber with corresponding elemental mapping images of Si, O, Al, and B, respectively. (J) Schematic showing the three levels of hierarchy of the relevant structures.
Compared with traditional methods for preparing ceramic aerogels, our synthetic method combined the simplicity of fibrous freeze shaping with the large-scale applicability of electrospun ceramic nanofibers, enabling the quick, easy, and reproducible preparation and wide structural scalability of the products. Preparation can be finished within a day, and the resulting CNFAs can be formed in any shape desired, such as a cylinder, cuboid, triangular prism, or even the premolded shapes of “D,” “H,” and “U” (Fig. 1D). The advantages of the structurally robust CNFAs can be further demonstrated by constructing ultralight and elastic aerogels with a minimum density of 0.15 mg cm−3 (figs. S6 and S7), corresponding to a porosity of 99.993%, which is significantly superior to that of the previously lightest ceramic aerogels (1.2 mg cm−3) (8). Figure 1E showed that a typical 20-cm3 CNFA (0.15 mg cm−3) could freely stand on the tip of a feather, highlighting the ultralight feature. In a marked contrast to the pearl necklace–like structure of traditional ceramic aerogels, the fibrous freeze shaping also concurrently caused the nanofibers to organize into a hierarchical and lamellar cellular structure on both the nanometer and micrometer scales. The scanning electron microscopy (SEM) images in Fig. 1 (F to J) demonstrate that the cellular architecture of CNFAs can be distilled to three levels of hierarchy: unit cells (10 to 30 μm), cell walls (1 to 3 μm), and nanofibers (100 to 250 nm). Ceramic nanofibers in the cell walls are tightly bonded and entangled in a highly packed morphology to maximize strength. Each fibrous cell is then closely connected to form a lamellar-cell geometry, a structure that is helpful to withstand cellular-specific deformation (11, 36). A significant difference between CNFAs and other cellular aerogels is the unique fibrous cell walls that consist of numerous minor pores (100 to 1000 nm) due to the bonding of nanofibers, as shown in fig. S8. The basic principle of forming the organized cellular structure could be attributed to the phase separation induced by the crystallization of water (37, 38), as illustrated in Fig. 1A. When the mixed nanofiber dispersion was quickly frozen, ice formation can lead to the rejection of solid nanofibers from the growing crystal front; the nanofibers were then accumulated among the laminar crystals and locked into a 3D solid network. Simultaneously, the precipitated AlBSi also aggregated on the fiber surface. After freeze drying and calcination, the hierarchical cellular structure was obtained, which was a direct replica of the frozen and solidified nanofiber networks. It should also be noted that the cellular structure of CNFAs exhibited a special micro-orientation and macro-isotropic character, resulting in the isotropic mechanical properties (figs. S9 and S10 and the Supplementary Discussions). The highly cross-linked structure was further supported by the scanning transmission electron microscopy (STEM) observation and the relevant energy-dispersive x-ray spectroscopy (EDS) mapping results. As shown in Fig. 1I, the Al and B elements were almost exclusively covering the Si and O elements, revealing that the AlBSi components were homogenously wrapped on the surface of silica nanofibers. The junctions between the SiO2 nanofibers were also examined by the EDS mapping, which confirmed the robust cross-linking by the AlBSi matrices (fig. S11). Moreover, the EDS samples were prepared by homogenization and sonication for a long time (20 min), whereas the AlBSi layer was still observed to attach to the surface of nanofibers, indicating the robust silicate bonding with nanofibers.
Characterization of superelasticity at room temperature
In dramatic contrast to the stiff and brittle character of traditional ceramic aerogels, the CNFAs exhibited robust mechanical properties, tolerating large compression without cracking (insets in Fig. 2A and movie S1). The stress-strain (σ-ε) curves (Fig. 2A) obtained in the loading process exhibit the three characteristic stages typically found in cellular networks (13, 17): a linear elastic regime for ε < 8%, a subsequent plateau stage for 8% < ε < 65%, and a densification regime of ε > 65% with σ rising sharply. The maximum σ at 80% strain was found to be 10.5 kPa, indicating that the CNFAs can support over 7000 times their own weight without fracture, a property that has rarely been observed in other porous ceramics. The highly elastic CNFAs also exhibited durable cycling performance. Hysteresis curves (Fig. 2B) for 500 loading-unloading cycles at large ε (60%) showed slight plastic deformation (8% at the 100th cycle and 12% at the 500th cycle). No significant decay in strength or stiffness was found for the CNFAs after 500 cyclic compressions; they retained over 70% of the initial Young’s modulus and maximum stress (Fig. 2C), highlighting their structural robustness. Moreover, the CNFAs also exhibited promising resistance to shear and tensile deformations, which achieved robust shear and tensile fracture stresses of 6.79 and 4.17 kPa, respectively (see details in figs. S12 and S13 and the Supplementary Discussions).
Fig. 2. Multicycle compressive properties of the CNFAs.
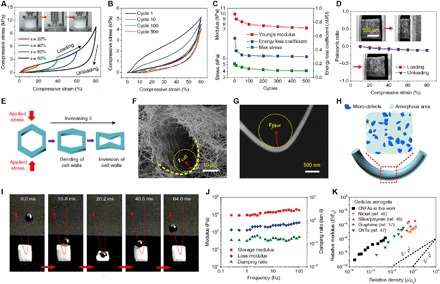
(A) Compressive σ versus ε curves during loading-unloading cycles with increasing ε amplitude. (B) A 500-cycle fatigue test with compressive ε of 60%. (C) Young’s modulus, energy loss coefficient, and maximum stress versus compressive cycles. (D) The Poisson’s ratio of the CNFAs versus ε. Inset: SEM observations of the CNFAs under compression and release, focusing on a small piece (<1 mm). (E) Sketch of the inversion of the nanofibrous cell walls under compression. SEM images showing the curvature radius of (F) a single cellular cell and (G) a single nanofiber. (H) Schematic illustration of the microstructure of a bent silica nanofiber. (I) A set of real-time images showing that CNFAs can rebound a steel ball at high speed. (J) The frequency dependence of the storage modulus, loss modulus, and damping ratio for CNFAs (oscillatory ε of 3%). (K) The relative Young’s modulus of selected cellular aerogels with low densities.
The elasticity observed in the CNFAs was a surprise to us. Nearly all the existing ceramic aerogels and porous ceramics are easy to crack in a brittle and stiff manner upon slight deformation (27, 39, 40). Previous studies also found that when an aerogel made of ceramic nanoparticles is severely compressed, the “neck-limited” connection of particles will cause the serious collapse of networks, preventing elastic recovery (3, 6, 41). Slight elasticity (ε < 25%) has recently been observed in ceramic networks by increasing the structure continuity within lattice and fiber micro-units (25, 27, 29); however, these structures fail to bear large cyclic deformation due to the lack of organized cellular geometry and stable bonding. We also found that CNFAs with a random organized structure or a nonbonding network fabricated from the same precursor all collapsed when subjected to large compression (see fig. S14 and Supplementary Discussions). We thus believe that it is the unique cellular structure and well-bonded nanofibers that have endowed the CNFAs with structurally robustness and high elasticity. To provide insight into the elasticity, we investigated the dependence of the Poisson’s ratio of the CNFAs on applied compression strain up to 80% by in situ SEM observation. As shown in Fig. 2D, the Poisson’s ratios remained near zero (from 0 to −0.1) and showed little strain dependence for both the loading and unloading processes. These slightly negative Poisson’s ratios indicated a cell inversion–dominated deformation behavior similar to that of cellular-structured foams (13, 42). The ultralight CNFAs still maintained a high porosity of 99.1% even at 80% of ε, which means that the fibrous cell walls largely do not come into contact to provide lateral expansion (43). The compressive strain is thus absorbed at a high level by the buckling and inversion of the nanofibrous cells, which suggests that the hierarchical cellular structure is significantly important for elastic recovery, as illustrated in Fig. 2E.
At the top level, the cellular structure of the CNFAs is organized in a lamellar-cell geometry to maximize the elastic modulus and strength. At the meta-scale level, the cell walls consisting of tangled and bonded nanofibers bend freely with a typical curvature radius of 5 to 10 μm (Fig. 2F), and the cell inversion feature has also proven to be highly beneficial for transferring loading strain with minimum cell wall bending (17, 42). At the lowest level, a single silica nanofiber was able to bear a large bending deformation at a curvature radius of less than 0.5 μm without generating any cracks (Fig. 2G). The key factor in the flexible ceramic nanofiber is the amorphous atomic structure of both the silica fiber matrix and the AlBSi cross-linking components (fig. S15). The dispersed defects in the glassy silica tetrahedron networks act as a “lubrication region” that can dissipate external stress and yield large deformations (23, 44), as shown in Fig. 2H. Consider that the allowable bending radius of a single nanofiber is only one-tenth of that of the cell walls, which has enabled a high compliance in the strain direction and the ability of the CNFAs to accommodate a severe elastic bending deformation. Therefore, the efficient combination of organized nanofibrous cellular networks and stable AlBSi cross-linking is the key aspect for realizing high specific elasticity and strength. A detailed discussion for the effect of the hierarchical cellular structure (fiber diameters and lamellar spacing) on the mechanical properties of CNFAs is presented in figs. S16 to S18 and Supplementary Discussions.
The properties of mechanically robust, cellular-structured CNFAs can be further demonstrated by their fast elastic resilience. A movie taken by a high-speed camera shows that a typical CNFA can rebound a steel ball (7.5 g, 70 times heavier than itself) with a fast recovery speed (860 mm s−1) (Fig. 2I and movie S2), revealing an elastc performance that achieved almost 60% of the recovery speed of the commercial polyurethane (PU) elastic foams (fig. S19). Further dynamic compressive tests by dynamic mechanical analysis (DMA) revealed that the storage and loss modulus were almost stable and independent of the angular frequency upon three orders of magnitude from 0.2 to 100 Hz (Fig. 2J). A damping ratio of 0.1 to 0.2 over the entire tested angular frequency range indicated that the elastic response was predominant for the CNFAs, which could be attributed to the strong and viscoelastic nanofibrous networks (10). The effect of the hierarchical cellular nanofibrous architecture on the mechanical properties is also reflected in the elasticity of the CNFAs with a wide range of densities from 0.5 to 10 mg cm−3 (fig. S20). Plotting the relative Young’s modulus (E/Es) of a variety of CNFAs versus the relative density (ρ/ρs) indicated that the modulus scaled as E/Es ~ (ρ/ρs)2 (Fig. 2K), revealing a cellular-governed elastic behavior similar to that of a low-density, open-cell architecture (17, 45). These modulus-density scaling laws correspond well with the observed efficient load transfer to the CNFA struts (presently nanofibrous cell walls), which undergo reversible transverse bending (17). In contrast to this result, most existing inorganic aerogels exhibit a larger E/Es ~ (ρ/ρs) scaling of >2.5 because of the inefficient stress transfer among random structural units (46, 47).
Fire resistance and elasticity at high temperature
The vast majority of cellular aerogels have been made from polymers, carbon, and metals, which exhibit excellent elasticity at room temperature. However, these constituent materials typically cannot withstand high temperatures in air. By contrast, ceramic aerogels are robust heat-resistant materials but have always suffered from poor mechanical properties. As a new alternative, CNFAs combining the unique properties of the cellular nanofibrous structure with a ceramic nature are expected to yield promising temperature-invariant elasticity. As shown in Fig. 3 (A to C), a frequency dependence test (0.1 to 10 Hz) measured by DMA in ambient air showed stable viscoelastic properties (storage modulus, loss modulus, and damping ratio) of the CNFAs over a wide range of temperatures from −100° to 500°C. As far as we know, common DMAs are usually limited to 500°C. To extend the temperature range studied, we first heated the CNFAs in a furnace at temperatures ranging from 100° to 1400°C for 30 min; then, we estimated the work done during the compression and recovery processes (see details in the Supplementary Methods). As shown in Fig. 3D, both the compression and recovery work were nearly constant and independent of the treating temperature to 1100°C. Above that temperature, the compression work exhibited a slight decrease, whereas the recovery work decreased markedly. These results indicated that the CNFAs did maintain their original shape and cellular structure upon the high temperature of 1400°C, and the elastic resilience was also well retained up to 1100°C.
Fig. 3. Mechanical properties of the CNFAs over a wide range of temperature.
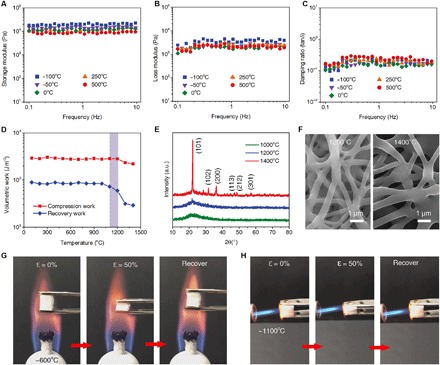
(A to C) Storage modulus, loss modulus, and damping ratio of the CNFAs versus angular frequency (0.1 to 10 Hz) at temperatures from −100° to 500°C, with an oscillatory ε of 3%. (D) Compression and recovery work of the CNFAs after treatment at various temperatures for 30 min. (E) XRD patterns of CNFAs after treatment at 1000°, 1200°, and 1400°C for 30 min. (F) SEM images of CNFAs after treatment at 1200° and 1400°C for 30 min. Compression and recovery process of the CNFAs in the flame of (G) an alcohol lamp and (H) a butane blowtorch.
To gain insight into the thermal stability, the heat-induced structural reorganization of the CNFAs was studied by x-ray diffraction (XRD) analysis. As shown in Fig. 3E, the XRD patterns of CNFAs upon heating at 1000° and 1200°C only exhibited a wide peak around 22°, characterizing the typical amorphous structure of silica. Meanwhile, after heating at 1400°C, the XRD pattern revealed distinct peaks at 23.9° (210), 35.3° (311), 49.6° (421), 54.1° (422), and 62.5° (440), which indicated the crystalline β-quartz structure of silica (6, 44). As mentioned previously, the amorphous character of the ceramic nanofibers is the critical precondition for the bendable properties. The excessive crystallite growth in nanofibers during high-temperature treatment significantly reduces the “lubrication region,” and the external stress can easily become concentrated in the crystalline region, resulting in a catastrophic failure as macrocracks propagate similar to the behavior in typical porous ceramics (44, 48). These results were also confirmed by SEM observation (Fig. 3F). Clear adhesion and fusion were observed among nanofibers in CNFAs treated at 1200°C and especially at 1400°C, which could be attributed to the crystallite growth in silica nanofibers. Moreover, the high-temperature elasticity and fire resistance of the CNFAs were further assessed by in situ compression testing in the flame of an alcohol lamp (~700°C) and a butane blowtorch (~1100°C); the measured temperatures at the position of the CNFAs upon the alcohol lamp and the butane blowtorch were 691° and 1063°C, respectively (fig. S21). As demonstrated in Fig. 3 (G and H) (see also movies S3 and S4), no ignition or structure collapse was observed when the CNFAs were exposed to those high-temperature flames; moreover, they still exhibited good elastic resilience after several compression cycles upon flame burning, highlighting the temperature-invariant superelasticity.
DISCUSSION
Thermal insulation plays a significant role in controlling energy efficiency of both industry and daily life, and aerogels are among the most well-known thermal insulation materials, with significantly low thermal conductivity originating from their high porosity (49, 50). Generally, the thermal conductivity of aerogels is mainly made up of two components: thermal transportation by the gas phase and by the solid phase. The as-prepared CNFAs had both high porosity and tortuous porous channels, which could lead to the promising low gas and solid thermal transportation. As shown in Fig. 4A, the CNFAs with a density of 10 mg cm−3 exhibited a low thermal conductivity (λ) of 0.025 W m−1 K−1, which is quite close to that of air under ambient conditions (0.023 W m−1 K−1). With the density of the CNFAs decreased to 0.5 mg cm−3, the λ increased slightly to 0.032 W m−1 K−1 due to the increase in pore size (50). Combining the extraordinarily ultralight feature, the specific thermal insulation performance per mass of the CNFAs (take the sample of 5 mg cm−3 as an example) was estimated to be one or two orders of magnitude superior to that of commercialized insulation materials (table S1) (48).
Fig. 4. Thermal insulation properties of the CNFAs.
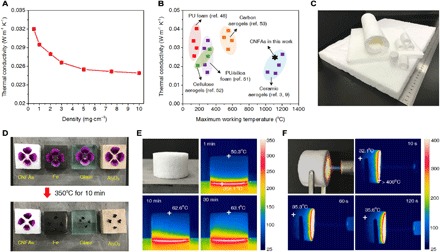
(A) The thermal conductivities of the CNFAs as a function of density. (B) Thermal conductivity versus maximum working temperature for aerogel-like materials. (C) An optical image of the CNFAs on the large scale for thermal insulation applications. (D) Thermal insulation capacity of the CNFAs compared with those of Fe, SiO2, and Al2O3 materials for protecting fresh petals from withering. (E) Optical and infrared images of CNFAs on a 350°C heating stage for 30 min. (F) Optical and infrared images of CNFAs exposed to a butane blowtorch for 120 s.
The CNFAs present a unique combination of low thermal conductivity and temperature-invariant superelasticity, which offer a far superior high-temperature insulation performance to that of current insulating materials. As shown in Fig. 4B, polymeric insulation materials such as PU foams or cellulose aerogels are easily collapsed at temperatures above 200°C, and their ignitable nature also requires the addition of toxic flame retardants (48). It has also been shown that the fire retardancy of organic aerogels can be improved to over 400°C by the addition of inorganic fillers, such as silicate and clays (51, 52). Carbon aerogels derived from thermal or hydrothermal carbonization of polymer aerogels can further increase heat resistance (53). However, structure shrinkage and ignition still occur upon exposure to fire over 600°C. All of these polymeric, hybrid, and carbon aerogels were elastic but were not able to bear high-temperature conditions (>1000°C) or flame ignition. Meanwhile, traditional ceramic aerogels, such as SiO2, Al2O3, and ZrO2, exhibited excellent heat resistance to 1200°C with low λ values even less than 0.2 W m−1 K−1, but the widespread use of these aerogels has been limited by their poor mechanical properties and safety concerns related to dust release (3, 9). Significantly, the as-prepared CNFAs show robust heat resistance and low λ similar to those of common ceramic aerogels while exhibiting superelasticity like that of a polymeric elastomer. Considering that insulating materials can easily encounter squeezing or shaking in their working conditions (48), this performance is very promising from the perspective of practical insulation applications. Moreover, because of the simplicity of the assembly process in our method, CNFA fabrication can be easily scaled up to obtain meter-sized insulating panels, felts, or even prefabricated pipes (Fig. 4C), which could be conveniently used as common insulating materials without additional processing.
As a proof of concept for thermal insulation applications, 15-mm-thick plates made of CNFAs, iron, glass, and Al2O3 ceramics were placed on a 350°C heating stage, and fresh petals were then further loaded on top of each plate. As shown in Fig. 4D, the petals on the CNFAs exhibited only slight wilting after 10 min of heating, whereas the petals loaded on other plates were significantly scorched and carbonized, highlighting the robust insulating performance of the CNFAs. We further monitored the dynamic temperature distribution of the CNFAs upon heating and flame by infrared camera observation. As shown in Fig. 4E, a gradient distribution of temperature from the heating plate through the CNFAs was observed. The top surface of the CNFAs (15 mm thick) maintained a relatively low temperature of about 50°C after being placed on a 350°C heating stage for 1 min, and the temperature increased slightly to 62°C after 10 min and then remained nearly constant with further heating until 30 min. Similarly, the CNFAs (15 mm thick) exhibited promising thermal insulation against a high-temperature butane blowtorch flame, achieving a significantly low temperature of 35°C at the far end of the CNFAs (Fig. 4F). These results indicate that the CNFAs could serve as a robust insulation material with potential broad applications, such as insulation layers combining flyweight density and elasticity for aviation and aerospace industries or scalable heat- and fire-proof materials for modern buildings.
In summary, we have presented that ultralight ceramic-based aerogels can be made superelastic by the combination of hierarchical cellular fibrous architectures and AlBSi-bonded SiO2 nanofibers. With their ultralow density, resilient compressibility, zero Poisson’s ratio, temperature-invariant superelasticity, low thermal conductivity, and fire resistance, we anticipate that these exceptional CNFAs will open broad technological implications in thermal insulation, catalyst supports, adsorbents, flexible electrical devices, and electromagnetic, energy, acoustic, or vibration damping. Moreover, our discovery also provides the possibility to explore the properties and applications of ceramics in a lightweight, resilient, and structurally adaptive form. Furthermore, the basic principles of the preparation and the resilient mechanism of CNFAs were demonstrated; thus, we can expect that, similar to SiO2 nanofibers, a variety of other ceramic nanofibers could be involved in the fabrication of CNFAs, offering ample opportunities to develop many new ceramic-based functional aerogels.
MATERIALS AND METHODS
Fabrication of CNFAs
Soft and flexible SiO2 nanofibers were prepared through a similar sol-gel electrospinning method as we previously reported (see details in fig. S1 and Supplementary Methods) (44). In a typical procedure for the fabrication of CNFAs with a density of 5 mg cm−3, 1.2 g of SiO2 nanofibers and 0.03 g of polyacrylamide were dispersed in 280 ml of water by homogenizing (IKA T21) the mixture for 20 min at 13,000 rpm, yielding uniform nanofiber dispersions. An AlBSi sol (with an Al/Si/B molar ratio of 2:5:1) was prepared by mixing and hydrating 1.52 g of tetraethyl orthosilicate, 0.39 g of aluminum chloride, and 0.09 g of boric acid in 20 ml of water for 4 hours. The AlBSi sol was then added to the nanofiber dispersions with further homogenization for 5 min. After degassing in a vacuum chamber, the obtained dispersions were transferred to the desired mold, frozen in a dry ice/acetone bath, and then freeze-dried for 18 hours to obtain the PNFAs. Next, the PNFAs were calcined at 900°C under flowing air for 30 min to generate the AlBSi ceramic–bonded CNFAs. Other CNFAs with densities in the range from 0.15 to 10 mg cm−3 were prepared by changing the concentrations of the precursor dispersions (with a constant weight ratio of SiO2 nanofibers, polyacrylamide, and AlBSi sol). Except as noted, all the structure and property investigations were performed by using the CNFAs with a density of 5 mg cm−3.
Characterization
The compression experiments were tested using an Instron 3365 universal machine with 100-N load cells at a strain rate of 30 mm min−1 for σ-ɛ tests and of 300 mm min−1 for 500 cyclic fatigue tests. The dynamic and temperature-dependent compressive characteristics were evaluated by a DMA instrument (TA-Q800) equipped with a parallel-plate compression clamp. The DMA was also equipped with a heating and cooling chamber for transitions from −100° to 500°C with flowing air. The storage modulus, loss modulus, and damping ratio as a function of frequency were measured with a prestrain of 5% and an oscillatory strain of 3%. The shear and tensile mechanical properties were performed using a TA-Q800 DMA instrument with the sandwich shear and tensile clamps, respectively.
The weight of the CNFA samples was measured using an ultra-micro balance (MS105DU, Mettler Toledo) with an accuracy of 0.08 mg on the basis of the ISO 845:2006 standard (see details in Supplementary Methods). The microscopic architecture and chemical structure of the CNFAs were characterized by SEM (Hitachi S-4800), STEM (JEM-2100F), EDS (Bruker Quantax 400), XPS (PHI 5000C ESCA), and XRD (Bruker D8 ADVANCE). The fast recovery of a compressed CNFA was recorded by a high-speed EX-FA CASIO camera operated at 1000 frames per second. The thermal conductivity was measured on a Hot Disk instrument (TPS2500S) with the transient-plane source method. The infrared images of the CNFAs were recorded using a Fotric 225 (−10° to 400°C) and an Optris PI 640 (100° to 1500°C) infrared thermal camera.
Supplementary Material
Acknowledgments
Funding: This work is supported by the National Natural Science Foundation of China (nos. 51673037 and 51473030), the Shanghai Committee of Science and Technology (no. 15JC1400500), and the Innovation Program of Shanghai Municipal Education Commission (no. 2017-01-07-00-03-E00024). Author contributions: B.D. and Y.S. designed the research. Y.S. wrote the paper. X.W. and L.D. synthesized and characterized the samples. J.Y. was involved in the analysis of mechanical mechanism. Y.S. and X.W. contributed equally to this work. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/4/eaas8925/DC1
fig. S1. Fabrication of electrospun SiO2 nanofibers.
fig. S2. Homogenization of nanofibers.
fig. S3. Morphology of homogenized nanofibers.
fig. S4. The freezing of nanofiber dispersions.
fig. S5. Magnified XPS spectrum.
fig. S6. The ultralow density of the CNFAs.
fig. S7. Compressibility of the CNFAs with a density of 0.15 mg cm−3.
fig. S8. SEM images of the nanofibrous cell walls.
fig. S9. Micro-orientation and macro-isotropic structure of CNFAs.
fig. S10. Mechanical properties of the CNFAs upon different orientation.
fig. S11. EDS mapping of junctions between nanofibers.
fig. S12. Shear mechanical properties of CNFAs.
fig. S13. Tensile mechanical properties of CNFAs.
fig. S14. Elastic resilience of CNFAs with different structures.
fig. S15. Amorphous character of the CNFAs.
fig. S16. Effect of fiber diameter on the structure of CNFAs.
fig. S17. Effect of fiber diameter on the mechanical properties of CNFAs.
fig. S18. Effect of lamellar spacing on the structure and properties of CNFAs.
fig. S19. Elasticity of a PU foam.
fig. S20. Elasticity of CNFAs with a wide range of densities.
fig. S21. The temperature at the position of the CNFAs upon flames.
table S1. The relevant densities and thermal conductivities of CNFAs and other insulation materials.
Supplementary Methods
Supplementary Discussions
movie S1. Compression and recovery processes of CNFAs.
movie S2. Fast recovery of CNFAs by rebounding a steel ball.
movie S3. Compression testing in the flame of an alcohol lamp.
movie S4. Compression testing in the flame of a butane blowtorch.
REFERENCES AND NOTES
- 1.Meza L. R., Das S., Greer J. R., Strong, lightweight, and recoverable three-dimensional ceramic nanolattices. Science 345, 1322–1326 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Wu H., Chan G., Choi J. W., Ryu I., Yao Y., McDowell M. T., Lee S. W., Jackson A., Yang Y., Hu L., Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 7, 310–315 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Pierre A. C., Pajonk G. M., Chemistry of aerogels and their applications. Chem. Rev. 102, 4243–4265 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Chabi S., Rocha V. G., García-Tuñón E., Ferraro C., Saiz E., Xia Y., Zhu Y., Ultralight, strong, three-dimensional SiC structures. ACS Nano 10, 1871–1876 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Kucheyev S. O., Biener J., Wang Y. M., Baumann T. F., Wu K. J., van Buuren T., Hamza A. V., Satcher J. H. Jr, Elam J. W., Pellin M. J., Atomic layer deposition of ZnO on ultralow-density nanoporous silica aerogel monoliths. Appl. Phys. Lett. 86, 083108 (2005). [Google Scholar]
- 6.Dorcheh A. S., Abbasi M. H., Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 199, 10–26 (2008). [Google Scholar]
- 7.Lei W., Mochalin V. N., Liu D., Qin S., Gogotsi Y., Chen Y., Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization. Nat. Commun. 6, 8849 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayase G., Nonomura K., Hasegawa G., Kanamori K., Nakanishi K., Ultralow-density, transparent, superamphiphobic boehmite nanofiber aerogels and their alumina derivatives. Chem. Mater. 27, 3–5 (2015). [Google Scholar]
- 9.Hüsing N., Schubert U., Aerogels—Airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 37, 23–45 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Schaedler T. A., Jacobsen A. J., Torrents A., Sorensen A. E., Lian J., Greer J. R., Valdevit L., Carter W. B., Ultralight metallic microlattices. Science 334, 962–965 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Si Y., Yu J., Tang X., Ge J., Ding B., Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 5, 5802 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Ziegler C., Wolf A., Liu W., Herrmann A.-K., Gaponik N., Eychmüller A., Modern inorganic aerogels. Angew. Chem. Int. Ed. 56, 13200–13221 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Si Y., Wang X., Yan C., Yang L., Yu J., Ding B., Ultralight biomass-derived carbonaceous nanofibrous aerogels with superelasticity and high pressure-sensitivity. Adv. Mater. 28, 9512–9518 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Lin Z., Zeng Z., Gui X., Tang Z., Zou M., Cao A., Carbon nanotube sponges, aerogels, and hierarchical composites: Synthesis, properties, and energy applications. Adv. Energy Mater. 6, 1600554 (2016). [Google Scholar]
- 15.Jiang S., Agarwal S., Greiner A., Low-density open cellular sponges as functional materials. Angew. Chem. Int. Ed. 56, 15520–15538 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Kanamori K., Nakanishi K., Controlled pore formation in organotrialkoxysilane-derived hybrids: From aerogels to hierarchically porous monoliths. Chem. Soc. Rev. 40, 754–770 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Qiu L., Liu J. Z., Chang S. L. Y., Wu Y., Li D., Biomimetic superelastic graphene-based cellular monoliths. Nat. Commun. 3, 1241 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Jiang S., Duan G., Kuhn U., Mörl M., Altstädt V., Yarin A. L., Greiner A., Spongy gels by a top-down approach from polymer fibrous sponges. Angew. Chem. Int. Ed. 129, 3333–3336 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cranford S. W., Tarakanova A., Pugno N. M., Buehler M. J., Nonlinear material behaviour of spider silk yields robust webs. Nature 482, 72–76 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Shao Z., Vollrath F., Materials: Surprising strength of silkworm silk. Nature 418, 741–741 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Cheung K. C., Gershenfeld N., Reversibly assembled cellular composite materials. Science 341, 1219–1221 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Si Y., Yan C., Hong F., Yu J., Ding B., A general strategy for fabricating flexible magnetic silica nanofibrous membranes with multifunctionality. Chem. Commun. 51, 12521–12524 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Song J., Wang X., Yan J., Yu J., Sun G., Ding B., Soft Zr-doped TiO2 nanofibrous membranes with enhanced photocatalytic activity for water purification. Sci. Rep. 7, 1636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han W., Ding B., Park M., Cui F., Ghouri Z. K., Saud P. S., Kim H.-Y., Facile synthesis of luminescent and amorphous La2O3–ZrO2:Eu3+ nanofibrous membranes with robust softness. Nanoscale 7, 14248–14253 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Mi H.-Y., Jing X., Huang H.-X., Turng L.-S., Instantaneous self-assembly of three-dimensional silica fibers in electrospinning: Insights into fiber deposition behavior. Mater. Lett. 204, 45–48 (2017). [Google Scholar]
- 26.Mi H.-Y., Jing X., Napiwocki B. N., Li Z.-T., Turng L.-S., Huang H.-X., Fabrication of fibrous silica sponges by self-assembly electrospinning and their application in tissue engineering for three-dimensional tissue regeneration. Chem. Eng. J. 331, 652–662 (2018). [Google Scholar]
- 27.Shah H. V., Sandy J. R., Ireland A. J., Su B., Electrospinning of 2D and 3D silica nanofibres from a colloidal solution. Ceram.-Silik 56, 112–116 (2012). [Google Scholar]
- 28.Moradipour P., Dabirian F., Rajabi L., Derakhshan A. A., Fabrication and characterization of new bulky layer mixed metal oxide ceramic nanofibers through two nozzle electrospinning method. Ceram. Int. 42, 13449–13458 (2016). [Google Scholar]
- 29.Sun B., Long Y. Z., Zhang H. D., Li M. M., Duvail J. L., Jiang X. Y., Yin H. L., Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 39, 862–890 (2014). [Google Scholar]
- 30.Wu J., Stebbins J. F., Quench rate and temperature effects on boron coordination in aluminoborosilicate melts. J. Non-Cryst. Solids 356, 2097–2108 (2010). [Google Scholar]
- 31.Wu J., Stebbins J. F., Temperature and modifier cation field strength effects on aluminoborosilicate glass network structure. J. Non-Cryst. Solids 362, 73–81 (2013). [Google Scholar]
- 32.Si Y., Wang L., Wang X., Tang N., Yu J., Ding B., Ultrahigh-water-content, superelastic, and shape-memory nanofiber-assembled hydrogels exhibiting pressure-responsive conductivity. Adv. Mater. 29, 1700339 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Wei M., Zhang G., Wu Q., The surface characteristics of polyacrylamide-coated silicon carbide powder used in the preparation of highly concentrated ceramics suspensions. J. Mater. Sci. 38, 4033–4039 (2003). [Google Scholar]
- 34.Shao G., Lu Y., Wu X., Wu J., Cui S., Jiao J., Shen X., Preparation and thermal shock resistance of high emissivity molybdenum disilicide- aluminoborosilicate glass hybrid coating on fiber reinforced aerogel composite. Appl. Surf. Sci. 416, 805–814 (2017). [Google Scholar]
- 35.Rivera L. O., Bakaev V. A., Banerjee J., Mueller K. T., Pantano C. G., Characterization and reactivity of sodium aluminoborosilicate glass fiber surfaces. Appl. Surf. Sci. 370, 328–334 (2016). [Google Scholar]
- 36.Wang X., Ding B., Sun G., Wang M., Yu J., Electro-spinning/netting: A strategy for the fabrication of three-dimensional polymer nano-fiber/nets. Prog. Mater. Sci. 58, 1173–1243 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao H.-L., Zhu Y.-B., Mao L.-B., Wang F.-C., Luo X.-S., Liu Y.-Y., Lu Y., Pan Z., Ge J., Shen W., Zheng Y.-R., Xu L., Wang L.-J., Xu W.-H., Wu H.-A., Yu S.-H., Super-elastic and fatigue resistant carbon material with lamellar multi-arch microstructure. Nat. Commun. 7, 12920 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deville S., Freeze-casting of porous ceramics: A review of current achievements and issues. Adv. Eng. Mater. 10, 155–169 (2008). [Google Scholar]
- 39.Maleki H., Durães L., Portugal A., Synthesis of mechanically reinforced silica aerogels via surface-initiated reversible addition-fragmentation chain transfer (RAFT) polymerization. J. Mater. Chem. A 3, 1594–1600 (2015). [Google Scholar]
- 40.Fey T., Betke U., Rannabauer S., Scheffler M., Reticulated replica ceramic foams: Processing, functionalization, and characterization. Adv. Eng. Mater. 19, 1700369 (2017). [Google Scholar]
- 41.Zhao S., Malfait W. J., Demilecamps A., Zhang Y., Brunner S., Huber L., Tingaut P., Rigacci A., Budtova T., Koebel M. M., Strong, thermally superinsulating biopolymer–silica aerogel hybrids by cogelation of silicic acid with pectin. Angew. Chem. Int. Ed. 54, 14282–14286 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Wu Y., Yi N., Huang L., Zhang T., Fang S., Chang H., Li N., Oh J., Lee J. A., Kozlov M., Chipara A. C., Terrones H., Xiao P., Long G., Huang Y., Zhang F., Zhang L., Lepró X., Haines C., Lima M. D., Lopez N. P., Rajukumar L. P., Elias A. L., Feng S., Kim S. J., Narayanan N. T., Ajayan P. M., Terrones M., Aliev A., Chu P., Zhang Z., Baughman R. H., Chen Y., Three-dimensionally bonded spongy graphene material with super compressive elasticity and near-zero Poisson’s ratio. Nat. Commun. 6, 6141 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Zhu C., Han T. Y.-J., Duoss E. B., Golobic A. M., Kuntz J. D., Spadaccini C. M., Worsley M. A., Highly compressible 3D periodic graphene aerogel microlattices. Nat. Commun. 6, 6962 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shan H., Wang X., Shi F., Yan J., Yu J., Ding B., Hierarchical porous structured SiO2/SnO2 nanofibrous membrane with superb flexibility for molecular filtration. ACS Appl. Mater. Interfaces 9, 18966–18976 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Zheng X., Lee H., Weisgraber T. H., Shusteff M., Deotte J., Duoss E. B., Kuntz J. D., Biener M. M., Ge Q., Jackson J. A., Kucheyev S. O., Fang N. X., Spadaccini C. M., Ultralight, ultrastiff mechanical metamaterials. Science 344, 1373–1377 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Woignier T., Reynes J., Alaoui A. H., Beurroies I., Phalippou J., Different kinds of structure in aerogels: Relationships with the mechanical properties. J. Non-Cryst. Solids 241, 45–52 (1998). [Google Scholar]
- 47.Worsley M. A., Kucheyev S. O., Satcher J. H. Jr, Hamza A. V., Baumann T. F., Mechanically robust and electrically conductive carbon nanotube foams. Appl. Phys. Lett. 94, 073115 (2009). [Google Scholar]
- 48.L. J. Gibson, M. F. Ashby, Cellular Solids: Structure and Properties (Cambridge Univ. Press, 1997). [Google Scholar]
- 49.Wang H., Zhang X., Wang N., Li Y., Feng X., Huang Y., Zhao C., Liu Z., Fang M., Ou G., Gao H., Li X., Wu H., Ultralight, scalable, and high-temperature–resilient ceramic nanofiber sponges. Sci. Adv. 3, e1603170 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wicklein B., Kocjan A., Salazar-Alvarez G., Carosio F., Camino G., Antonietti M., Bergström L., Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 10, 277–283 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Verdolotti L., Lavorgna M., Lamanna R., Di Maio E., Iannace S., Polyurethane-silica hybrid foam by sol–gel approach: Chemical and functional properties. Polymer 56, 20–28 (2015). [Google Scholar]
- 52.Cai J., Liu S., Feng J., Kimura S., Wada M., Kuga S., Zhang L., Cellulose–silica nanocomposite aerogels by in situ formation of silica in cellulose gel. Angew. Chem. Int. Ed. 51, 2076–2079 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Zuo L., Zhang Y., Zhang L., Miao Y.-E., Fan W., Liu T., Polymer/carbon-based hybrid aerogels: Preparation, properties and applications. Materials 8, 6806–6848 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/4/eaas8925/DC1
fig. S1. Fabrication of electrospun SiO2 nanofibers.
fig. S2. Homogenization of nanofibers.
fig. S3. Morphology of homogenized nanofibers.
fig. S4. The freezing of nanofiber dispersions.
fig. S5. Magnified XPS spectrum.
fig. S6. The ultralow density of the CNFAs.
fig. S7. Compressibility of the CNFAs with a density of 0.15 mg cm−3.
fig. S8. SEM images of the nanofibrous cell walls.
fig. S9. Micro-orientation and macro-isotropic structure of CNFAs.
fig. S10. Mechanical properties of the CNFAs upon different orientation.
fig. S11. EDS mapping of junctions between nanofibers.
fig. S12. Shear mechanical properties of CNFAs.
fig. S13. Tensile mechanical properties of CNFAs.
fig. S14. Elastic resilience of CNFAs with different structures.
fig. S15. Amorphous character of the CNFAs.
fig. S16. Effect of fiber diameter on the structure of CNFAs.
fig. S17. Effect of fiber diameter on the mechanical properties of CNFAs.
fig. S18. Effect of lamellar spacing on the structure and properties of CNFAs.
fig. S19. Elasticity of a PU foam.
fig. S20. Elasticity of CNFAs with a wide range of densities.
fig. S21. The temperature at the position of the CNFAs upon flames.
table S1. The relevant densities and thermal conductivities of CNFAs and other insulation materials.
Supplementary Methods
Supplementary Discussions
movie S1. Compression and recovery processes of CNFAs.
movie S2. Fast recovery of CNFAs by rebounding a steel ball.
movie S3. Compression testing in the flame of an alcohol lamp.
movie S4. Compression testing in the flame of a butane blowtorch.


