Abstract
A number of studies show that environmental stress conditions such as drought, high salt, and air pollutants increase polyamine levels in plant cells. However, little is understood about the physiological function of elevated polyamine levels. We report here that polyamines regulate the voltage-dependent inward K+ channel in the plasma membrane of guard cells and modulate stomatal aperture, a plant “sensor” to environmental changes. All natural polyamines, including spermidine, spermine, cadaverine, and putrescine, strongly inhibited opening and induced closure of stomata. Whole-cell patch-clamp analysis showed that intracellular application of polyamines inhibited the inward K+ current across the plasma membrane of guard cells. Single-channel recording analysis indicated that polyamine regulation of the K+ channel requires unknown cytoplasmic factors. In an effort to identify the target channel at the molecular level, we found that spermidine inhibited the inward K+ current carried by KAT1 channel that was functionally expressed in a plant cell model. These findings suggest that polyamines target KAT1-like inward K+ channels in guard cells and modulate stomatal movements, providing a link between stress conditions, polyamine levels, and stomatal regulation.
Polyamines have been found in all the organisms studied and are required for normal development of both prokaryotes and eukaryotes (Tabor and Tabor, 1984). In higher plants, earlier studies suggest that polyamine levels are critical for a number of developmental processes including cell division, somatic embryogenesis, root growth, floral initiation, and flower and fruit development (Evans and Malmberg, 1989; Galston and Kaur-Sawhney, 1990; Slocum and Flores, 1991, 1995). Recent molecular genetic analyses have shown that altered polyamine levels have profound effects on plant growth and development (Fritze et al., 1995; Kumar et al., 1996; Masgrau et al., 1997; Watson and Malmberg, 1998).
In addition to their function in plant development, polyamines may play a role in stress responses because their levels in plant cells increase under a number of environmental stress conditions (Slocum and Flores, 1991; Galston and Kaur-Sawhney, 1995). K+ deficiency may be the first stress condition that has been shown to increase putrescine levels in plants (Richard and Coleman, 1952; Young and Galston, 1984). Watson and Malmberg (1996) have shown that Arg decarboxylase, an enzyme critical for inducible polyamine synthesis, is activated upon K+ deficiency in Arabidopsis. Osmotic shock, drought, and salt stress also increase polyamine levels (Evans and Malmberg, 1989). Upon drought treatment, polyamines, especially spermidine, accumulate in wheat seedlings to a level severalfold higher than that in control plants (Kubis and Krzywanski, 1989). Spermidine titer returns to the control level when seedlings are watered and recovered from drought. Salt stress increases polyamine levels in several crop plants including rice, mung bean, maize, and sorghum (Friedman et al., 1989; Krishnamurthy and Bhagwat, 1989; Erdei et al., 1996). Air pollution has been correlated with elevated polyamine levels in plants as well. Plants respond to pollutants such as ozone and SO2 by producing high levels of polyamines (Priebe et al., 1978; Kramer et al., 1991; Wellburn and Wellburn, 1996). In all these studies, polyamine concentrations in plant tissues increase from high micromolar (under normal conditions) to millimolar range upon stress treatments. Although a correlation between stress and polyamine levels has been demonstrated in a number of studies using a variety of plant materials, the physiological rationale for stress-induced polyamine accumulation remains unknown.
Plants respond to stress through many mechanisms, stomatal regulation being one of the most studied. A stomatal aperture is defined by two guard cells and is responsible for gas exchange between plants and the atmosphere (Zeiger; 1983; Mansfield et al., 1990). Changes in guard cell turgor that instigate stomatal movements are controlled by a number of ion channels and pumps (Raschke et al., 1988; Hedrich and Schroeder, 1989; Blatt, 1991; Ward et al., 1995). Among the ion channels in guard cells, the inward K+ channel (IKin), outward K+ channel (IKout), and anion channels in the plasma membrane are well characterized by patch-clamp studies (Schroeder et al., 1984, 1987; Hedrich and Schroeder, 1989). Many environmental stress factors regulate stomatal aperture through modulation of ion channel activity in guard cells (for reviews, see MacRobbie, 1997). Some of these stress factors including drought, high salt, and air pollutants are also the factors that elevate polyamine levels. We speculate that elevated polyamine levels may be related to stomatal regulation. We report here that polyamines mimic stress conditions in blocking stomatal opening and inducing stomatal closure. Consistent with this finding, patch-clamp analyses showed that the IKin in guard cells was inhibited by polyamines. In addition, KAT1, a putative guard cell K+ channel cloned from Arabidopsis, was also inhibited by polyamines. These studies have provided a possible mechanism for polyamine function in higher plants.
RESULTS
Spermidine Inhibits Light-Invoked Stomatal Opening and Induces Stomatal Closure
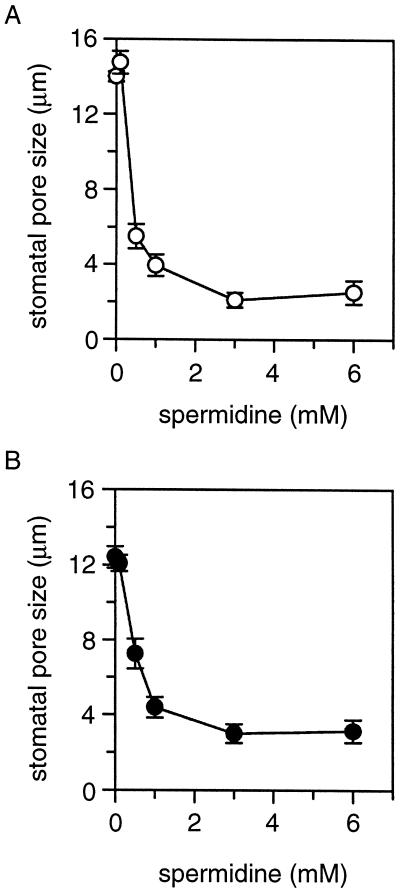
To test the hypothesis that polyamines may regulate stomatal aperture, we performed stomatal opening assays in the presence of spermidine. According to previous studies, polyamine concentrations in plant tissues reach low millimolar range under stress conditions (Slocum and Flores, 1991; Watson and Malmberg, 1996). Therefore, submillimolar and low millimolar concentrations of spermidine were used in the stomatal opening experiment. Prior to light illumination, the stomatal aperture was typically in the range of 3.7 ± 0.6 μm. After a 2-h illumination, stomatal aperture reached 14.0 ± 2.7 μm in the peeled strips incubated in the control solution without spermidine. In the solution with 1 mm spermidine, the light-induced stomatal opening was completely blocked. Significant effect of spermidine on stomatal opening was observed at 0.5 mm or higher concentrations (Fig. 1A).
Figure 1.
Effect of spermidine on stomatal movements. Epidermal peels were obtained from 4-week-old V. faba leaves. Stomatal pore size was measured after 2-h incubation in peel solution containing 0, 0.1, 0.5, 1.0, 3.0, and 6.0 mm spermidine. For stomatal opening assay (A), peels were collected from the plants before light cycle, and the initial stomatal pore size was typically in the range of 3.7 ± 0.6 μm. After 2-h illumination, the pore size reached 14.0 ± 2.7 μm in the control solution without spermidine. For stomatal closure assay (B), peels were collected from the plants 4 h after light cycle, and the initial pore size was 12.4 ± 0.6 μm. The data in both A and B are presented as mean ± se from seven individual experiments.
Stress conditions such as drought and air pollution not only inhibit stomatal opening but also induce closure of fully open stomata. To examine whether spermidine induces stomatal closure, we incubated epidermal strips with open stomata in the peel solution containing various concentrations of spermidine. After a 2-h incubation under light, stomatal aperture was measured. As shown in Figure 1B, when spermidine concentration reached 0.5 mm or higher in the peel solution, the stomatal aperture was significantly reduced during the 2-h incubation. At 3 mm, spermidine reduced the aperture from 12.4 ± 0.6 μm to the size prior to light illumination (3.7 ± 0.5 μm).
All Natural Polyamines Modulate Stomatal Aperture
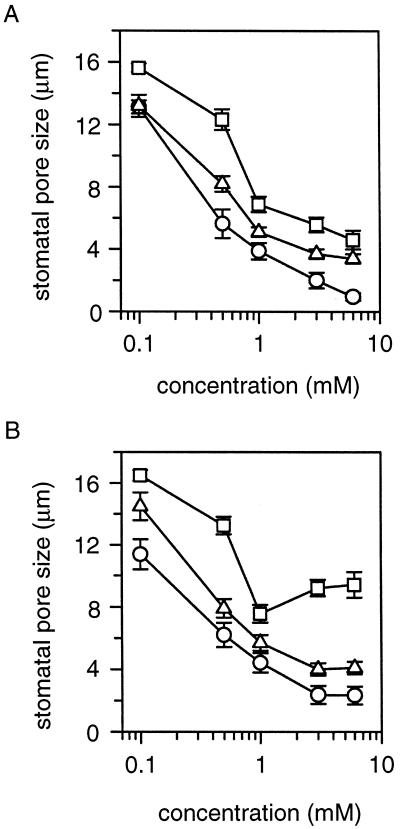
Plants under stress conditions accumulate higher levels of polyamines including putrescine, spermidine, spermine, and cadaverine (Galston and Kaur-Sawhney, 1990). Spermidine regulation of stomatal movements may represent a general function for all polyamines. We tested this notion by including putrescine, spermine, and cadaverine, respectively, in the peel assay. The inhibitory effect of these three polyamines on stomatal opening process is shown in Figure 2A. After a 2-h incubation, all polyamines significantly inhibited stomatal opening. The potency of these four polyamines was reflected by the inhibition of stomatal opening at a given concentration. For example, 1 mm spermidine and spermine completely prevented light-induced stomatal opening, whereas cadaverine and putrescine inhibited this opening by 88% and 63%, respectively. Their effects in inducing stomatal closure are shown in Figure 2B. Although all polyamines significantly reduced the stomatal aperture, spermidine and spermine appeared to be more effective than putrescine at 1 mm (P < 0.05).
Figure 2.
Regulation of stomatal aperture by natural polyamines. At various concentrations, effects on stomatal aperture of three polyamines (spermine, ○; putrescine, □; and cadaverine, ▵) were compared. The procedures of stomatal opening assay (A) and closure assay (B) are as described in Figure 1. All polyamines were used at concentrations of 0.1, 0.5, 1.0, 3.0, and 6.0 mm and presented in common log scale. Seven individual experiments were performed and the data were presented as mean ± se.
To determine the specificity of polyamine effect on stomatal movements, we tested three groups of chemicals in the peel assay. One group, Orn and Arg, represent the precursors of polyamines. The second group, γ-aminobutyric acid, and succinate are metabolic products of polyamine oxidation (Evans and Malmberg, 1989). The third group, butylamine, is a chemical analog of polyamines. None of these compounds (at 1-mm concentration) had significant effect on stomatal aperture (data not shown).
Spermidine Inhibits the IKin in Guard Cells
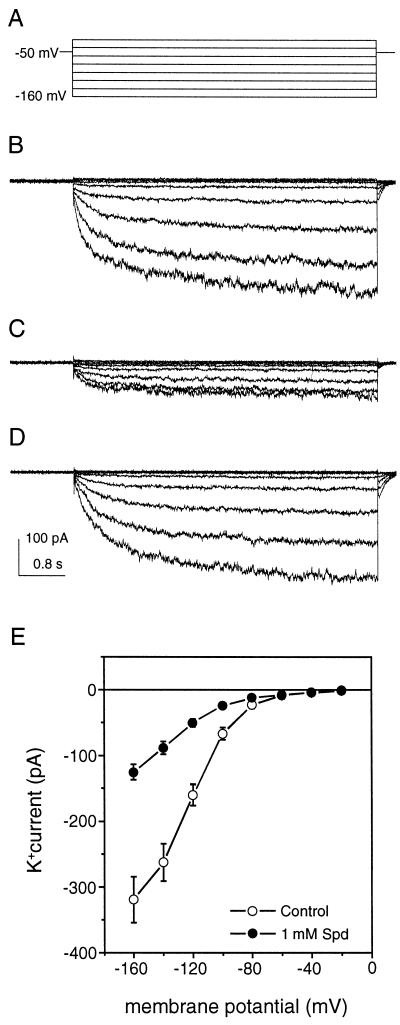
After we observed the inhibitory effect of polyamines on stomatal movements, we examined whether polyamines regulate IKin in guard cells using patch-clamp techniques. Figure 3A shows the voltage protocol used in the whole-cell configuration. The membrane potential was clamped at −50 mV and stepped to values from −160 to −20 mV with 20-mV increment to activate IKin. Under the control condition, a typical time course of IKin was recorded from a guard cell protoplast of Vicia faba (Fig. 3B). When 1 mm spermidine was included in the pipette solution, the magnitude of IKin was reduced immediately following the establishment of the whole-cell configuration (Fig. 3C). If 1 mm spermidine was perfused to the bath solution for a 15-min period, the current was not affected (Fig. 3D). Figure 3E summarizes the current-voltage (I–V) relationship under the control condition and in the presence of 1 mm spermidine in the pipette solution. At −160 mV, 1 mm spermidine reduced the whole-cell current from 325 ± 46 pA/cell to 126 ± 12 pA/cell, a 61% decrease of the control level.
Figure 3.
Effect of spermidine on IKin in guard cells. In V. faba guard cell protoplasts, whole-cell patch-clamp recordings revealed the typical IKin current (B), IKin current with 1 mm spermidine in pipette solution (C), and with 1 mm spermidine in bath solution (D). During the recordings, the holding potential was −50 mV, the currents were recorded at the membrane potentials from −20 to −160 mV with increment of −20 mV (A), and the current and time scales for B, C, and D are indicated by vertical and horizontal bars shown in D. The current amplitudes (mean ± se) from 14 control cells (○) and 18 cells treated with 1 mm spermidine in the pipette solution (●) are presented as current-voltage (I–V) curves in E. All data shown here were collected from whole-cell configuration with 2.5 GΩ or higher seal resistance. Spd, Spermidine.
External perfusion of polyamines did not affect IKin, suggesting that spermidine exerts its specific effect on IKin from the cytoplasmic side of the plasma membrane. In the peel assay, extracellular application of polyamines regulated stomatal aperture possibly due to polyamine uptake by plant cells during the 2-h incubation (Caffaro et al., 1993). In the patch-clamp recording, extracellular perfusion was conducted up to 15 min that may not be long enough to allow sufficient polyamine transport. For this reason, we have also tried to incubate the protoplasts with spermidine for 2 h before patch-clamp recording. However, at 1-mm concentration, spermidine severely prevented seal formation, making the patch-clamp recording technically unfeasible.
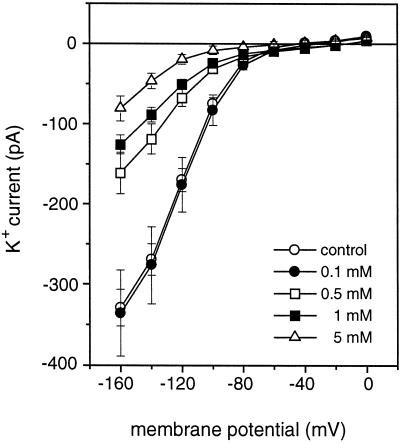
When we included 0, 0.1, 0.5, 1, and 5 mm spermidine in the pipette solution, a dose-dependent inhibition of IKin was observed. As shown in Figure 4, 0.5 mm or higher concentrations of spermidine had a significant inhibitory effect on IKin. At 5 mm, spermidine reduced the current by 74% of the control level at −160 mV.
Figure 4.
Dose response of IKin to spermidine. The responses of IKin to different concentrations of spermidine in the pipette solution are shown as I–V curves (control, n = 14; 0.1 mm, n = 9; 0.5 mm, n = 12; 1 mm, n = 18; and 5 mm, n = 7). The same voltage protocol was used as in Figure 3. The data were presented as mean ± se.
We have also tried to test spermidine on IKout and the anion channel activity across the plasma membrane in guard cells. But no effect was observed on these channels (data not shown), which may indicate a channel type-specific modulation by spermidine in guard cells.
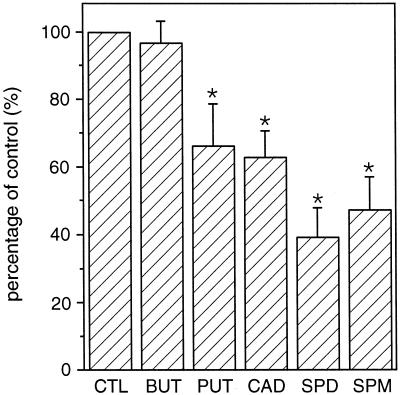
Effect of Four Natural Polyamines on IKin
As shown earlier, all natural polyamines regulate stomatal aperture. To test whether all polyamines inhibit IKin, we included 1 mm putrescine, cadaverine, spermidine, and spermine, respectively, in the pipette solution. The results in Figure 5 compare the effects of four polyamines and butylamine on IKin at −160 mV. All these polyamines inhibited IKin except butylamine. In addition to butylamine, several related compounds such as Orn and γ-aminobutyric acid that did not affect stomatal aperture, had no effect on IKin in guard cells (data not shown).
Figure 5.
Effects of natural polyamines on IKin. At −160 mV, the whole-cell current amplitudes in the presence of 1 mm tested compounds are shown as percentage of the control. CTL, control, n = 14; BUT, butylamine, n = 9; PUT, putrescine, n = 8; CAD, cadaverine, n = 9; SPD, spermidine, n = 18; SPM, spermine, n = 11. Asterisks indicate significant differences as compared with the control (P < 0.05).
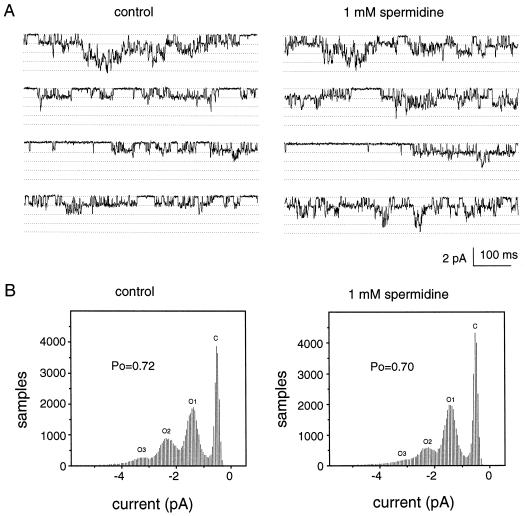
Spermidine Does Not Affect the Single-Channel Current of IKin
To further investigate the mechanism of polyamine inhibition of IKin in guard cells, we performed single-channel analyses of IKin in both inside-out and outside-out configurations. One major type of single-channel current for IKin was identified in both inside-out and outside-out patches excised from the plasma membrane of guard cells as described before (Liu and Luan, 1998). This current was present in 65 of 70 membrane patches excised from different cells. In both configurations, the single-channel current was activated at −80 mV and more negative membrane potentials. The channel conductance was 13.12 ± 0.54 pS (n = 6) in inside-out patches and 16.84 ± 1.73 pS (n = 20) in outside-out patches under the given conditions.
To test whether polyamines modulate IKin by a membrane-delimited mechanism, we perfused the membrane patches with 1 mm spermidine in bath solution. Under the outside-out configuration, bath perfusion applied spermidine to the extracellular face of the plasma membrane and had no effect on the single-channel current, consistent with the study under the whole-cell configuration (Fig. 3). Under the inside-out configuration, bath perfusion supplied spermidine to the intracellular face of the plasma membrane. The single-channel activity of the inside-out patch was also unaffected by spermidine (Fig. 6A). Figure 6B summarizes the analysis of opening probability (Po) for a representative patch. The data from six patches in inside-out configuration showed no significant difference in the Po values between spermidine and control groups (data not shown). This finding suggests that cytoplasmic components play a role in polyamine regulation of IKin.
Figure 6.
Effect of spermidine on single-channel current of IKin in guard cells. The inside-out patch was obtained from V. faba guard cell protoplasts, and single-channel current of Ikin was recorded in the control bath solution for 30 s. Then the bath chamber was perfused with the solution containing 1 mm spermidine. The single-channel recording was performed again for 30 s after perfusion for 5 min. The current traces from a representative patch are shown in A before (control) and after perfusion of 1 mm spermidine. During the recording, the membrane potential was held at −100 mV. The dotted lines indicate the closing state and different opening levels. In this patch, there are at least 4 channels opening to hyperpolarized membrane potential. The analysis of Po for this patch is shown in B. Six patches have been studied upon spermidine application. The average single-channel conductance is 13.83 ± 0.43 pS, which is not significantly different from the control (13.12 ± 0.54 pS, P > 0.05).
Polyamine Regulation of KAT1 Channel in a Plant Cell Model
To provide further molecular basis for polyamine regulation of IKin in higher plants, it is important to identify the channel molecules that are regulated by polyamines. Several IKin have been cloned and characterized from higher plants (Anderson et al., 1992; Sentenac et al., 1992; Cao et al., 1995; Mueller-Roeber et al., 1995). One of these channel genes, KAT1, is preferentially expressed in guard cells (Nakamura et al., 1995), suggesting that KAT1 channel activity may constitute, at least part of, inward K+ current detected in a guard cell. We decided to examine whether the KAT1 channel, like IKin in guard cells, is regulated by spermidine.
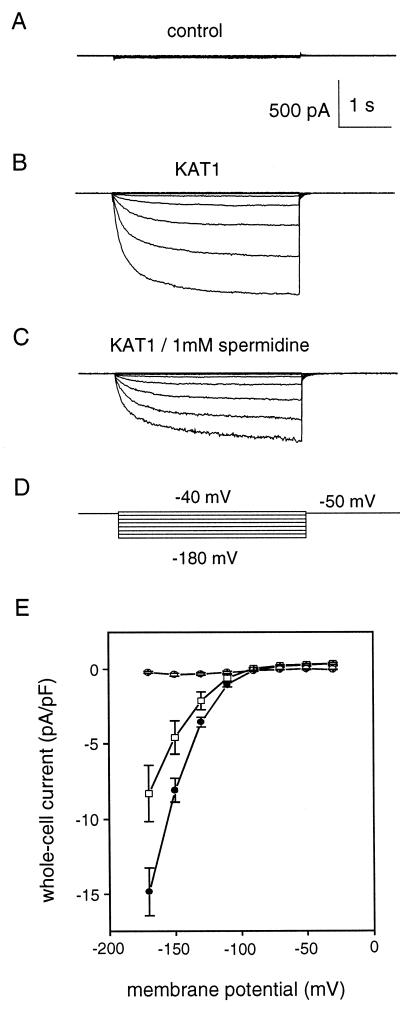
In a recent study (Bei and Luan, 1998), we found that tobacco mesophyll cells did not produce endogenous inward K+ current. Ectopic expression of KAT1 in these cells generated a large inwardly rectifying current with similar properties as compared with IKin in guard cells. This has established a model system for further study of KAT1 regulation in plant cells. Using this model, we tested whether spermidine regulates KAT1 channel in mesophyll cells. As shown in Figure 7A, control cells do not produce inward K+ currents. A KAT1-expressing cell generated large inward current (Fig. 7B). In the presence of 1 mm spermidine in the pipette solution, the magnitude of KAT1 current declined to 62% of the control (Fig. 7C). The curves (I–V) in Figure 7E summarized the current magnitudes in control and KAT1-expressing cells in the presence or absence of spermidine.
Figure 7.
Effect of spermidine on KAT1 channel activity in a plant cell model. Whole-cell patch-clamp recording was performed on the tobacco mesophyll cells over-expressed with KAT1 gene (for details, see “Materials and Methods”). IKin was recorded in the cells from vector-only transformed plant (A), KAT1-expressing plant (B), and KAT1-expressing plant with 1 mm spermidine in the pipette solution during the recording (C). The current shown here was elicited at the membrane potential from −40 to −180 mV, whereas the holding potential was kept at −50 mV (D). E, I–V curve summarizing the currents from the control cells (○), KAT1-expressing cells (●), and KAT1-expressing cells with 1 mm spermidine (□). The data presented as mean ± se were collected from more than 10 individual cells in each group. The currents were normalized to pA per pF membrane capacitance.
Consistent with the regulation pattern in guard cells, spermidine applied to the bath solution did not have any significant effect on KAT1 current. In addition, single-channel current of KAT1 channel in an excised membrane patch was not affected by polyamines (data not shown). Together with previous analyses of KAT1 channel expressed in plant cells (Bei and Luan, 1998), these findings support the hypothesis that KAT1-like channel activity is part of the inward K+ current in guard cells. Identification of KAT1 channel as a target for polyamines provides a model for dissecting further the molecular basis of polyamine action in higher plants.
Free Polyamine Levels and Their Responses to Drought in V. faba Leaves
Polyamines inhibit IKin and KAT1 currents, consistent with their effects on stomatal movement. However, the effective concentrations of polyamines (millimolar range) in this study are much higher than those of typical plant hormones (micromolar range). Although many studies have shown that free polyamine levels in plants accumulate to millimolar levels in response to environmental stresses (Galston and Kaur-Sawhney, 1995), no evidence has been provided that the same is true in 4-week-old V. faba leaves that we used as plant materials for the stomatal assays and patch-clamp studies.
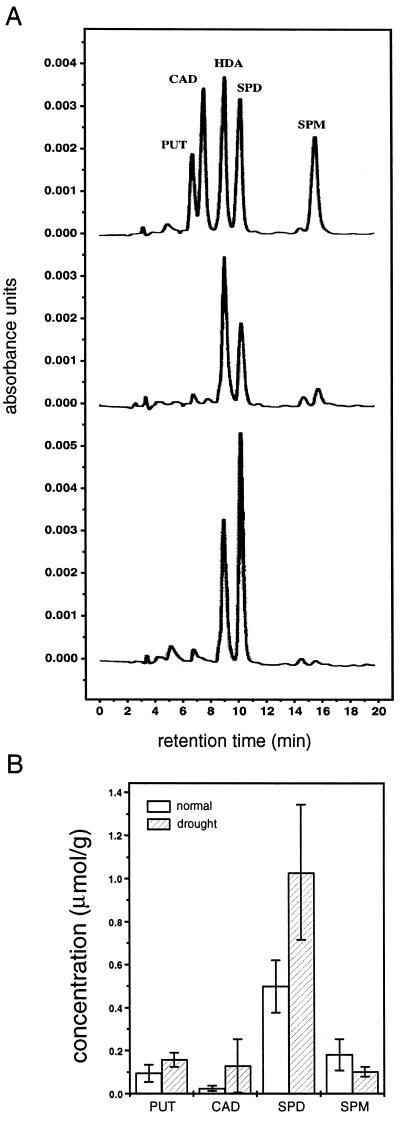
To determine whether millimolar levels of polyamines are in the physiological range, we measured free polyamine levels in V. faba leaves and their response to drought conditions. As shown in Figure 8A, four natural polyamines including putrescine, cadaverine, spermidine, and spermine were detected in the plant extract, whereas hexanediamine was used as an internal standard. The concentrations of polyamines were calculated and illustrated in Figure 8B. Of four assayed analogs, spermidine is present at the highest level. The concentration is 0.5 ± 0.12 μmol per gram fresh weight, which can be approximately converted to 0.5 ± 0.12 mm, assuming that polyamines distributed evenly in plant cells. Drought treatment doubled spermidine concentration to 1.03 ± 0.31 μmol/g (mm), and also increased the levels of putrescine and cadaverine but not spermine. These results are consistent with findings in other plant species and suggest that millimolar concentrations of polyamines mimic the polyamine levels under stress conditions such as drought.
Figure 8.
HPLC analysis of polyamines in V. faba leaves. The standard HPLC procedure was used to measure polyamine levels in the extracts from 3- to 4-week-old V. faba leaves. Four analogs of natural polyamines were assayed, and an unnatural polyamine, hexanediamine, was added as an internal standard. The recording traces in A show the peaks of polyamine standards (upper trace), extracts of normal plant (middle trace), and extracts of drought-treated plant (lower trace). The calculated polyamine concentrations from three individual experiments were presented as mean ± se in micromolars per gram fresh weight and plotted in B. PUT, Putrescine; CAD, cadaverine; HDA, hexanediamine; SPD, spermidine; SPM, spermine.
DISCUSSION
Polyamines in Stress Responses
Environmental stresses often increase tissue levels of plant hormones that in turn elicit further cellular responses. A typical instance is abscisic acid (ABA) response to water stress. Water stress increases ABA level to hundreds of nanomole or less, which is sufficient to close stomata (Davies et al., 1990). Polyamines also close stomata by inhibition of IKin. However, the effective concentrations for polyamines in all studies including patch-clamp analyses presented here are much higher than those for conventional plant hormones. This is a major concern regarding whether polyamines should be considered as a group of plant hormones (Evans and Malmberg, 1989).
It is conceivable that the high “threshold” of effective concentration for polyamines may simply reflect their high background (or basal) levels under normal conditions. If the basal levels are in the high micromolar range (Galston and Kaur-Sawhney, 1995), only higher levels (millimolar) of polyamines would elicit an effect that is absent under normal conditions. Indeed, in many different plants, basal polyamine levels range from tens to hundreds of micromole, and all stress conditions examined so far increase the polyamine levels to submillimolar and millimolar range (Galston and Kaur-Sawhney, 1995). Concerning the physiological relevance of these high concentrations of polyamines on stomatal movement and IKin, we measured polyamine levels in the same plant material, V. faba leaves. The basal level of total polyamines is as high as 0.5 mm. Spermidine constitutes a major proportion of total polyamines, and drought treatment increases spermidine level to 1.0 mm (Fig. 8B). These findings are consistent with the data in stomatal aperture assay and patch-clamp. The high basal level of polyamines suggests that polyamines may not function as conventional plant hormones to regulate plant response to stress. The fact that polyamines inhibit stomatal opening at the concentration that can be induced under stress conditions support the idea that polyamines serve as stress “messengers” for plants to respond to the encountered stresses.
Polyamines are cations. The electrostatic charges in their molecules are associated with anions, which is considered as part of polyamine functions (Schuber, 1989). Many metabolic products and precursors of polyamines have electrostatic charges. Because polyamines are readily metabolized in the cell, the effect observed in stomatal aperture assay might be a consequence of polyamine metabolism during the lengthy assay process (Evans and Malmberg, 1989). To address the specificity of polyamine function, we included polyamine precursors, oxidative products, and chemical analogs (either charged or uncharged) as negative controls in both stomatal aperture assays and patch-clamp experiments. Our studies strongly suggest the presence of a specific mechanism for polyamine regulation of the IKin in plant cells. Several results support this conclusion: (a) Polyamines exert their effect rapidly (2–5 min) on IKin with a dose-dependent manner, suggesting a direct regulation by polyamines but not by their metabolites; (b) Polyamines function specifically upon delivery into cytosol, indicating an intracellular location of polyamine target(s); (c) Spermine has more positive charges than spermidine but did not show stronger effect, suggesting that the polyamine effect is not simply a “charge effect”; (d) Spermidine does not affect the single-channel activity of IKin in either inside-out or outside-out patch under bath perfusion, suggesting that the K+ channel is not regulated in a membrane-delimited manner. This finding excludes the possibility that polyamines exert their non-specific effect by binding to the membrane lipids or proteins; and (e) A cloned K+ channel molecule, KAT1, was regulated by spermidine in a similar fashion. Together, these results suggest that polyamines regulate IKin in guard cells through a specific intracellular pathway.
Ion Channel Regulation as a Mechanism for Polyamine Action in Organisms Ranging from Bacteria and Plants to Animals
Both electrophysiological and molecular studies demonstrate that polyamines modulate a number of ion channels in animal systems (for reviews, see Johnson, 1996). In higher plants, a recent study shows that polyamines block the fast-activating vacuolar cation channel. The channel activities are regulated at both whole-cell and single-channel level, indicating a direct blockage (Brüggemann et al., 1998). This is consistent with the mechanism found in both animal and bacterial cells where polyamines modulate ion channels by direct binding to the channel protein or membrane component (Delavega and Delcour, 1995; Johnson, 1996). However, polyamine inhibition of IKin reported here appears to be mediated by a cytoplasmic pathway, which is different from the direct blockage previously reported in other systems. This non-membrane delimited mechanism has been shown with both native K+ channels in guard cells and KAT1 channel expressed in tobacco mesophyll cells.
As to the molecular mechanism for polyamine regulation of ion channels in plant cells, evidence has been reported on specific polyamine-binding proteins in cytoplasmic fractions from plant cells (Apelbaum et al., 1988; Mehta et al., 1991). In addition, polyamines have been shown to regulate a protein kinase and a Tyr phosphatase activity in both animal and plant cells (Keuhn et al., 1979; Datta et al., 1987; Gupta et al., 1998). More work will be required to delineate the molecular details involved in polyamine regulation of ion channels in guard cells and other cell types. Toward this goal, we have established a model system for further study of KAT1 regulation by polyamines. Future studies will allow identification of KAT1 structural element that is responsible for polyamine regulation. However, identification of polyamine “receptor” molecules will provide a more direct link between polyamines and ion channels.
As an important player in stomatal regulation, the IKin is an indirect target of polyamine action. A number of studies have shown that IKin-inhibiting processes or factors often inhibit stomatal opening (Assmann, 1993). Such factors include ABA (Schwartz et al., 1994), high Ca2+ levels (Schroeder and Hagiwara, 1989; Gilroy et al., 1990), and polyamines (this study). Both ABA and polyamines also induce stomatal closure. Whereas ABA has been shown to activate anion channels and IKout that elicit turgor loss in guard cells leading to stomatal closure (MacRobbie, 1997; Pei et al., 1997), polyamines did not affect the IKout or anion channel. There must be other polyamine targets in addition to IKin in guard cells that account for induction of stomatal closure. We speculate that polyamines may serve as “chemical messengers” for plants to respond to various stress signals. Inhibition of IKin, together with other unidentified polyamine-induced cellular processes, modulates stomatal aperture, which serves as one of the mechanisms for protecting plants from further stress damage.
MATERIALS AND METHODS
Chemicals
Spermidine, spermine, cadaverine, putrescine, hexanediamine, and butylamine were purchased from Sigma (St. Louis).
Plant Materials and Peel Assays
Vicia faba plants were grown in a plant growth chamber with a 10-h light/14-h dark cycle. The light intensity was set at 180 μmol m−2 s−1. The temperature during day and night was 20°C and 18°C, respectively. Epidermal peels were obtained from leaves of 4-week-old seedlings. For the “stomatal opening” assay, leaves were picked in the early morning before the light cycle started. Epidermal peels were prepared under dim green light and then transferred in the peel solution (50 mm KCl and 10 mm MES [2-(N-morpholino)ethanesulfonic acid], pH 5.7) containing various concentrations of polyamines. Because polyamines are alkaline, the pH of all solutions was always readjusted to the desired values with HCl after addition of polyamines. After 2-h incubation under light (200 μmol m−2 s−1), stomatal aperture was examined under the microscope equipped with a CCD camera. The images of stomata in the epidermal peels were captured and stored in the computer. The maximum width of each stomatal pore was measured, and data were collected from 100 stomata for each treatment. For the “stomatal closure” assay, leaves were picked 4 h after the light cycle went on. The peeled strips were prepared and incubated in control and polyamine-containing solutions for 2 h under light before being examined under the microscope. A total of 100 stomata were examined for each treatment unless otherwise stated. All data in this study are presented as mean ± se, and Student's t test was used to determine the significant difference between each two groups.
Protoplast Preparation and Patch-Clamp Procedures
Guard cell protoplasts were isolated using leaves from 3-week-old seedlings according to the procedure described by Kruse et al. (1989). The whole-cell patch-clamp procedure we used has been described previously (Liu and Luan, 1998). Currents from isolated guard cell protoplasts were recorded with an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA) connected to a Dell Optiplex GL+5100 computer system via DigiData-1200 interface (Axon Instruments). Recording pipettes were made from borosilicate glass capillaries (Kimax-51, Kimble Glass, Vineland, NJ) by using a vertical two-stage pipette puller (PB-7, Narishige, Tokyo) and fire-polished by a microforge (MF90, Narishige) before use. The pipette solution contained 100 mm K-Glu, 2 mm EGTA, 2 mm MgCl2, 2 mm MgATP, and 10 mm HEPES (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid), pH 7.2. The osmolality was adjusted to 500 mosmol with d-sorbitol. Protoplasts were bathed in a solution consisting of 10 mm K-Glu, 1 mm CaCl2, 4 mm MgCl2, and 10 mm MES, pH 5.7. The bath solution osmolality was adjusted to 450 mosmol with d-sorbitol. Voltage-clamp steps and data acquisitions were performed using pClamp 6.0 software (Axon Instruments). Whole-cell currents were low-pass filtered at 1 kHz during each measurement. Liquid junction potentials were corrected in all the experiments according to Neher (1992).
The same equipment assembly was used for single-channel recording. To obtain a membrane patch in the outside-out configuration, protoplasts were bathed in the solution containing 100 K-Glu, 1 mm CaCl2, 4 mm MgCl2, and 10 mm MES, pH 5.7. To obtain an inside-out patch, protoplasts were bathed in a sealing solution containing 100 mm K-Glu, 5 mm CaCl2, 4 mm MgCl2, and 10 mm MES (pH 5.7). In both cases, osmolality was adjusted to 450 mosmol with d-sorbitol. After the inside-out configuration was set up, the membrane patch was perfused with the solution used as pipette solution in whole-cell experiments. Recording pipettes were coated with Sylgard 184 (Dow Corning, Midland, MI) under the microforge microscope. Under the outside-out configuration, the pipette was filled with the same pipette solution as for whole-cell recording. For the inside-out patch, the pipette was filled with the bath solution used for whole-cell recording. Data acquisition was conducted during the subsequent 30 s under holding potentials of −80, −100, −120, −140, and −160 mV, respectively. Data were filtered at 1 kHz, digitized at 4 kHz, and stored on computer disc. The pClamp 6.0 software was used for analysis of single-channel currents.
Transgenic Tobacco Plants and KAT1 Channel Analyses
Procedures for generating transgenic plants and selection of transgenic lines for patch-clamp studies were previously described in detail by Bei and Luan (1998). The F1 seeds of tobacco (Nicotiana tabacum cv SR-1) transformed by vector (as control) or KAT1 transgene were surface sterilized and plated on Petri dishes containing Murashige and Skoog medium solidified by 0.8% (v/v) agar. Three-week-old seedlings were transferred to the soil and grown in a growth chamber for an additional 5 weeks before use in patch-clamp experiments. Conditions in the growth chamber are set at 24°C/22°C (day/night) and 14-h light/10-h dark cycle. Tobacco mesophyll cell protoplasts were isolated as described previously (Li and Assmann, 1993; Bei and Luan, 1998). Whole-cell patch-clamp recordings were performed on isolated tobacco mesophyll protoplasts with an Axopatch 200A patch-clamp amplifier connected to a Gateway 2000 P4D computer system via DigiData-1200 interface (Axon Instruments). Recording pipettes were made as described above. The pipette solution included 100 mm K-Glu, 2 mm EGTA, 2 mm MgCl2, 2 mm Mg-ATP, and 10 mm HEPES, pH 7.2. The osmolality was adjusted to 720 mosmol with d-mannitol. Protoplasts were bathed in a solution consisting of 10 mm K-Glu, 2 mm CaCl2, 4 mm MgCl2, and 10 mm MES, pH 5.7. The osmolality was adjusted to 630 mosmol with d-mannitol. Voltage-clamp steps and data aquisitions were performed using pClamp 6.0 software. Whole-cell currents were low-pass filtered at 1 kHz during measurements. Liquid junction potentials were corrected in all the experiments according to Neher (1992).
Measurement of Polyamine Levels by HPLC
For polyamine extraction and HPLC analysis, benzoylation method was performed as described previously (Flores and Galston, 1982; Smith and Davies, 1987) with some modifications. In brief, 1 g of fresh tissue was homogenized in 10 mL of cold 0.2 n per-chloride acid containing 1 μmol of hexanediamine as an internal standard. The samples are incubated on ice for 40 min, and then centrifuged at 4°C for 20 min. Aliquots of 0.5 mL of supernatant were added to 1 mL of 2 n NaOH with 10 μL of benzoyl chloride. The mixtures were incubated at room temperature for 20 min, and the reaction was terminated by the addition of 2 mL of saturated NaCl. Benzoylamines were extracted with 2 mL of diethyl ether. After centrifugation, the ether layer was collected and dried under nitrogen gas. The residues were redissolved in 120 μL of methanol. Standards were treated in a similar way with 1 μmol of putrescine, cadaverine, hexanediamine, spermidine, and spermine in the reaction mixture. HPLC analysis was performed with a programmable Kratos dual-pump liquid chromatograph with a detector 773. The solvent system consisted of methanol and water, run at 65% (v/v) methanol at a flow rate of 0.7 mL/min. Five microliters of benzoylated extracts was eluted at room temperature through a 4.6 × 250 mm, 5-μm particle size reverse-phase (C18) column (Varian, Walnut Creek, CA) and detected at 254 mm. The peak areas were recorded on a pen recorder and calculated by a computer with NIH Image software (National Institutes of Health, Bethesda, MD).
ACKNOWLEDGMENTS
We thank Drs. Lewis Feldman and Anny Jiang for the HPLC experiment and Drs. Sydney Kustu and Dalai Yan for critical reading of this manuscript.
Footnotes
This work was supported by the U.S. Department of Agriculture-National Research Initiative Competitive Grants Program (grant no. 97–35100–4190 to S.L.).
LITERATURE CITED
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A, Canellakis ZN, Applewhite PB, Kaur-Sawhney R, Galston AW. Binding of spermidine to a unique protein in thin-layer tobacco tissue culture. Plant Physiol. 1988;88:996–998. doi: 10.1104/pp.88.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Bei QX, Luan S. Functional expression and characterization of an inward K+ channel gene in a plant cell model. Plant J. 1998;13:857–865. doi: 10.1046/j.1365-313x.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- Blatt MR. Ion channel gating in plants: physiological implications and integration for stomatal function. J Membr Biol. 1991;124:95–112. doi: 10.1007/BF01870455. [DOI] [PubMed] [Google Scholar]
- Brüggemann UI, Pottosin II, Schönknecht G. Cytoplasmic polyamines block the fast-activating vacuolar cation channel. Plant J. 1998;16:101–105. [Google Scholar]
- Caffaro S, Scaramagli S, Antognoni F, Bagni N. Polyamine content and translocation in soybean plants. J Plant Physiol. 1993;141:563–568. [Google Scholar]
- Cao Y, Ward JM, Kelly WB, Ichida AM, Gaber RF, Anderson JA, Uozumi N, Schroeder JI, Crawford NM. Multiple genes, tissue specificity, and expression-dependent modulation contribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol. 1995;109:1093–1106. doi: 10.1104/pp.109.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N, Schell MJ, Roux SJ. Spermidine stimulation of nuclear NII kinase from pea plumules and its role in the phosphorylation of nuclear polypeptide. Plant Physiol. 1987;84:1397–1401. doi: 10.1104/pp.84.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Mansfield TA, Hetherington AM. Sensing of soil water status and the regulation of plant growth and development. Plant Cell Environ. 1990;13:709–720. [Google Scholar]
- Delavega AL, Delcour AH. Cadaverine induces closing of Escherichia coli porins. EMBO J. 1995;14:6058–6065. doi: 10.1002/j.1460-2075.1995.tb00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdei L, Szegletes Z, Barabas K, Pestenacz A. Responses in polyamine titer under osmotic and salt stress in sorghum and maize seedlings. J Plant Physiol. 1996;147:599–603. [Google Scholar]
- Evans PT, Malmberg RL. Do polyamines have roles in plant development? Annu Rev Plant Physiol Plant Mol Biol. 1989;40:235–269. [Google Scholar]
- Flores HE, Galston AW. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982;69:701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R, Altman A, Levin N. The effect of salt stress on polyamine biosynthesis and content in mung bean plants and in halophytes. Physiol Plant. 1989;76:295–302. [Google Scholar]
- Fritze K, Czaja I, Walden R. T-DNA tagging of genes influence polyamine metabolism: isolation of mutant plant lines and rescue of DNA promoting growth in the presence of a polyamine biosynthetic inhibitor. Plant J. 1995;7:261–271. [Google Scholar]
- Galston AW, Kaur-Sawhney R. Polyamines in plant physiology. Plant Physiol. 1990;94:406–410. doi: 10.1104/pp.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galston AW, Kaur-Sawhney R. Polyamines as endogenous growth regulators. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry, and Molecular Biology. Norwell, MA: Kluwer Academic Publishers; 1995. pp. 158–178. [Google Scholar]
- Gilroy S, Read ND, Trewavas AJ. Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature. 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Gupta R, Huang Y, Kieber J, Luan S. Identification of a dual-specificity protein phosphatase that inactivate a map kinase from Arabidopsis. Plant J. 1998;16:581–589. doi: 10.1046/j.1365-313x.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- Hedrich R, Schroeder JI. The physiology of ion channels and electrogenic pumps in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:539–569. [Google Scholar]
- Johnson TD. Modulation of channel function by polyamines. Trends Biochem Sci. 1996;17:22–27. doi: 10.1016/0165-6147(96)81566-5. [DOI] [PubMed] [Google Scholar]
- Keuhn GD, Affolter H-U, Atmar VJ, Seebeck T, Gubler U, Braun R. Polyamine-mediated phosphorylation of a nucleolar protein from Physarum polycephalum that stimulates rRNA synthesis. Proc Natl Acad Sci USA. 1979;76:2541–2545. doi: 10.1073/pnas.76.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer GF, Lee EH, Rowland RA, Mulchi CL. Effects of elevated carbon dioxide concentration on the polyamine levels of field-grown soybean at three ozone regimes. Environ Pollut. 1991;73:137–152. doi: 10.1016/0269-7491(91)90019-s. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy R, Bhagwat KA. Polyamines as modulators of salt tolerance in rice cultivars. Plant Physiol. 1989;91:500–504. doi: 10.1104/pp.91.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse T, Tallman G, Zeiger E. Isolation of guard cell protoplasts from mechanically prepared epidermis of Vicia faba leaves. Plant Physiol. 1989;90:1382–1386. doi: 10.1104/pp.90.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubis J, Krzywanski Z. The dynamics of polyamine accumulation in spring wheat leaves during increasing water stress. Acta Physiol Plant. 1989;11:157–164. [Google Scholar]
- Kumar A, Taylor MA, Arif SAM, Davies HV. Potato plants expressing antisense and sense S-adenosylmethio-nine decarboxylase (SAMDC) transgenes show altered levels of polyamines and ethylene: antisense plants display abnormal phenotypes. Plant J. 1996;9:147–158. [Google Scholar]
- Li W, Assmann SM. Characterization of a G-protein-regulated outward K+ current in mesophyll cells of Vicia faba L. Proc Natl Acad Sci USA. 1993;90:262–266. doi: 10.1073/pnas.90.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Luan S. Voltage-dependent K+ channels as targets for osmosensing in guard cells. Plant Cell. 1998;10:1957–1970. doi: 10.1105/tpc.10.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EAC. Signaling in guard cells and regulation of ion channel activity. J Exp Bot. 1997;48:515–528. doi: 10.1093/jxb/48.Special_Issue.515. [DOI] [PubMed] [Google Scholar]
- Mansfield TA, Hetherington AM, Atkinson CJ. Some current aspects of stomatal physiology. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:55–75. [Google Scholar]
- Masgrau C, Altabella T, Farras R, Flores D, Thompson AJ, Besford RT, Tiburcio AF. Inducible overexpression of oat arginine decarboxylase in transgenic tobacco plants. Plant J. 1997;11:465–473. doi: 10.1046/j.1365-313x.1997.11030465.x. [DOI] [PubMed] [Google Scholar]
- Mehta AM, Saftner RA, Schaeffer GW, Mattoo AK. Translational modification of an 18-kilodalton polypeptide by spermidine in rice cell suspension cultures. Plant Physiol. 1991;95:1294–1297. doi: 10.1104/pp.95.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Roeber B, Ellenberg J, Provart N, Willmitzer L, Busch H, Becker D, Dietrich P, Hoth S, Hedrich R. Cloning and electrophysiological analysis of KST1, an inward rectifying K+ channel expressed in potato guard cells. EMBO J. 1995;14:2409–2416. doi: 10.1002/j.1460-2075.1995.tb07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura RL, McKendree WL, Jr, Hirsch RE, Sedbrook JC, Gaber RF, Sussman MR. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch-clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe A, Klein F, Jager HJ. Role of polyamines in SO2-polluted pea plants. J Exp Bot. 1978;29:1045–1050. [Google Scholar]
- Raschke K, Hedrich R, Reckmann U, Schroeder JI. Exploring biophysical and biochemical components of the osmotic motor that drives stomatal movements. Bot Acta. 1988;101:283–294. [Google Scholar]
- Richard FJ, Coleman RG. Occurrence of putrescine in potassium-deficient barley. Nature. 1952;170:460. doi: 10.1038/170460a0. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. Cytoplasmic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- Schroeder JI, Hedrich R, Fernandez JM. Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature. 1984;312:361–362. [Google Scholar]
- Schroeder JI, Raschke K, Neher E. Voltage dependence of potassium channels in guard-cell protoplasts. Proc Natl Acad Sci USA. 1987;84:4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuber F. Influence of polyamines on membrane functions. Biochem J. 1989;260:1–10. doi: 10.1042/bj2600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Wu W-H, Tucker EB, Assmann SM. Inhibition of inward K+ channels and stomatal response by abscisic acids: an intracellular locus of phytohormone action. Proc Natl Acad Sci USA. 1994;91:4019–4023. doi: 10.1073/pnas.91.9.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, Gaymard F, Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Slocum RD, Flores HE. Biochemistry and Physiology of Polyamines in Plants. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Smith TA, Davies PJ. Monitoring polyamines in plant tissues by high performance liquid chromatography. In: Linkens HF, Jackson JF, editors. High Performance Liquid Chromatography in Plant Sciences (Modern Methods of Plant Analysis. New Series) Vol. 5. Berlin: Springer-Verlag; 1987. pp. 209–227. [Google Scholar]
- Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Ward JM, Pei ZM, Schroeder JI. Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell. 1995;7:833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MB, Malmberg RL. Regulation of Arabidopsis thaliana (L.) heynh arginine decarboxylase by potassium deficiency stress. Plant Physiol. 1996;111:1077–1083. doi: 10.1104/pp.111.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MB, Malmberg RL. Arginine decarboxylase (polyamine synthesis) mutants of Arabidopsis thaliana exhibit altered root growth. Plant J. 1998;13:231–239. doi: 10.1046/j.1365-313x.1998.00027.x. [DOI] [PubMed] [Google Scholar]
- Wellburn FAM, Wellburn AR. Variable patterns of antioxidant protection but similar ethene emission differences in several ozone-sensitive and ozone-tolerant plant selections. Plant Cell Environ. 1996;19:754–760. [Google Scholar]
- Young ND, Galston AW. Physiological control of arginine decarboxylase activity in potassium deficient oat shoots. Plant Physiol. 1984;76:331–335. doi: 10.1104/pp.76.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E. The biology of stomatal guard cells. Annu Rev Plant Physiol. 1983;34:441–475. [Google Scholar]