Abstract
AIM
To compare the effects of the four most commonly used preservation solutions on the outcome of liver transplantations.
METHODS
A systematic literature search was performed using MEDLINE, Scopus, EMBASE and the Cochrane Library databases up to January 31st, 2017. The inclusion criteria were comparative, randomized controlled trials (RCTs) for deceased donor liver (DDL) allografts with adult and pediatric donors using the gold standard University of Wisconsin (UW) solution or histidine-tryptophan-ketoglutarate (HTK), Celsior (CS) and Institut Georges Lopez (IGL-1) solutions. Fifteen RCTs (1830 livers) were included; the primary outcomes were primary non-function (PNF) and one-year post-transplant graft survival (OGS-1).
RESULTS
All trials were homogenous with respect to donor and recipient characteristics. There was no statistical difference in the incidence of PNF with the use of UW, HTK, CS and IGL-1 (RR = 0.02, 95%CI: 0.01-0.03, P = 0.356). Comparing OGS-1 also failed to reveal any difference between UW, HTK, CS and IGL-1 (RR = 0.80, 95%CI: 0.80-0.80, P = 0.369). Two trials demonstrated higher PNF levels for UW in comparison with the HTK group, and individual studies described higher rates of biliary complications where HTK and CS were used compared to the UW and IGL-1 solutions. However, the meta-analysis of the data did not prove a statistically significant difference: the UW, CS, HTK and IGL-1 solutions were associated with nearly equivalent outcomes.
CONCLUSION
Alternative solutions for UW yield the same degree of safety and effectiveness for the preservation of DDLs, but further well-designed clinical trials are warranted.
Keywords: Liver transplantation, Preservation solution, Primary non-function, One-year post-transplant graft survival, Systematic review, Meta-analysis
Core tip: The University of Wisconsin (UW) solution is the gold standard for static cold storage in liver transplantation. Numerous clinical trials have investigated the potential benefit of the most frequently used alternative solutions, histidine-tryptophan-ketoglutarate, Celsior and Institut Georges Lopez, but their results have been variable. This meta-analysis has reviewed the current evidence and found no significant differences in risk of transplant outcomes: primary non-function (RR = 0.02, 95%CI: 0.01-0.03, P = 0.36) and one-year post-transplant graft survival (RR = 0.80, 95%CI: 0.80-0.80, P = 0.37) between UW and the other examined solutions.
INTRODUCTION
Organ transplantation is inevitably associated with ischemia-reperfusion (IR) injury; several methods have thus been formulated to reduce IR-related morbidity and to maintain the viability of tissues[1,2]. The introduction of the University of Wisconsin (UW) solution in 1987 has led to significant clinical progress and increased cold ischemic tolerance and has become the most widely used, gold standard preservation solution for liver transplantation[3]. Nevertheless, in spite of the clinical success, it has many potential shortcomings (Table 1). UW is an intracellular colloid solution with high potassium and low sodium concentration that inhibits activity of Na-K-adenosine triphosphatase and the resultant depletion of adenosine triphosphate stores. However, its low sodium content promotes the accumulation of calcium during ischemia, resulting in calcium-dependent endothelial dysfunction in renal glomeruli and in bile ducts during reperfusion[4,5]. Additionally, the high potassium increases the risk for hyperkalemia-induced cardiac arrest, requiring liver flushing before reperfusion. Moreover, low temperature storage in the container bag may result in the formation of adenosine crystals[6]. Therefore, the use of UW has been intensively challenged, and alternative solutions with potentially more benefits were developed. Among them, histidine-tryptophan-ketoglutarate (HTK), Celsior (CS) and Institut George Lopez (IGL-1) are the most commonly used preservation solutions in transplantation centers[7].
Table 1.
Ingredients in the investigated preservation solutions
| UW | HTK | CS | IGL-1 | |
| HES | 0.25 | - | - | - |
| PEG-35 | - | - | - | 0.03 |
| Na+ | 27 | 15 | 100 | 120 |
| K+ | 125 | 10 | 15 | 25 |
Concentrations are expressed in mmol/L. HES: Hydroxyethyl starch; PEG-35: Polyethylene glycol 35 kDa.
A number of prospective trials have investigated the effects of these preservation solutions on liver transplant outcomes over many years with variable results. HTK, also known as Bretschneider’s solution, is mostly used in European liver transplantation centers, especially in Germany. It has very low viscosity, which is based on a histidine buffer system with two additional substrates (tryptophan and ketoglutarate). A lower index of viscosity allows faster cooling and, theoretically, an improved washout of blood cells from the graft[8]. UW was first compared to HTK in a randomized fashion in liver transplantation in 1994, and these solutions were found to have similar outcomes in terms of initial non-function of the graft and 30-mo patient survival[9]. However, more recent studies with a larger liver transplant population from Europe and North America have provided different conclusions[10,11].
CS has initially been applied in heart transplantation and then for kidney and liver transplantation as well, with the idea of providing preservation for all organs with a single solution[12]. The use of CS is based on similar principles to those of UW and HTK, but certain aspects are different. CS and HTK are categorized as extracellular preservation fluids; however, their buffering systems and substrates, which provide high-energy phosphates, are different. With its high sodium (above 70 mmol/L) and low potassium content, CS is specifically designed to limit calcium overload (Table 1). It contains reduced glutathione concentration together with the addition of mannitol and histidine to prevent reactive oxygen species-induced oxidative injury. Like HTK, CS is devoid of colloids, therefore resulting in decreased viscosity and improved perfusability, it is thus unnecessary to the liver prior to reperfusion[13]. Due to its characteristically low viscosity, high sodium, low potassium and antioxidant properties, CS is considered particularly suitable for preserving liver grafts.
There are promising preliminary reports on the recently introduced Institut Georges Lopez (IGL-1) solution, also known as the UW-polyethylene glycol (PEG) solution. IGL-1 combines a cationic inversion (lower concentration of potassium) and replacement of hydroxyethyl starch with PEG. These properties could improve hepatic microcirculatory changes, thereby decreasing IR- injury[14].
The aim of our study was to provide a systematic review of this topic. The goal was to update current knowledge and compare data evidence on the effectiveness of the most frequently used preservation solutions. The primary endpoint of the study was primary non-function (PNF) of the graft after liver transplantation. PNF is the most common cause of early graft loss, and it has been shown that the organ preservation method is an independent predictive factor of PNF[15]. The secondary endpoint was one-year post-transplant graft survival (OGS-1), this being an appropriate period to evaluate the effect of the preservation solutions according to an expert consensus opinion[16]. Other outcomes, such as primary dysfunction (PDF), early retransplantation rate (RT), post-transplant death within 30 d (POD) and one-year post-transplant patient survival (OPS-1) were also evaluated together with donors and recipient characteristics.
It should be added that three previous systematic reviews and two registry data analyses have explored this topic, each with limitations[10,16-19]. In 2015, Adam et al[10] analyzed the efficacy of the four most commonly used preservation solutions based on the European Liver Transplant Registry (ELTR) database. The largest and most comprehensive study in recent times was performed by analyzing outcomes of 42869 (first) liver transplantations, including living and deceased donors, as well as partial liver graft transplantations. Although the study population in this registry data analysis was relatively large, non-selective groups of donors were included[10,18,20]. Two systematic reviews lacked sufficient sample sizes and therefore were underpowered to identify clinically relevant differences in important outcomes, such as PNF of the graft.[16,17] A systematic review by O’Callaghan et al[19] chose 16 RCTs for analysis; however, it included unpublished data and conference abstracts as well. Since then, new prospective trials have also been published, especially with the IGL-1 solution[8,21].
Therefore, the aim of this systematic review was to evaluate, compare and update the evidence obtained in randomized controlled trials (RCTs) on the efficacy of the four most frequently used preservation solutions for static cold storage of deceased donor liver (DDL) allografts.
MATERIALS AND METHODS
This study was conducted in accordance with the PRISMA (Preferred Reporting Items in Systematic Reviews and Meta-Analysis) statement[22]. The review protocol was registered with the National Institute for Health Research PROSPERO system on January 12th, 2017, and can be found online (Registration No. CRD42017054908)[23].
Literature search
A systematic literature search was performed using EMBASE/MEDLINE, PubMed, Scopus and Cochrane. Database searches were conducted with MeSH keywords, combined with various terms for organ transplantation and organ preservation solutions (Figure 1). No language limitation was applied. The end date for the literature search was January 31st, 2017.
Figure 1.
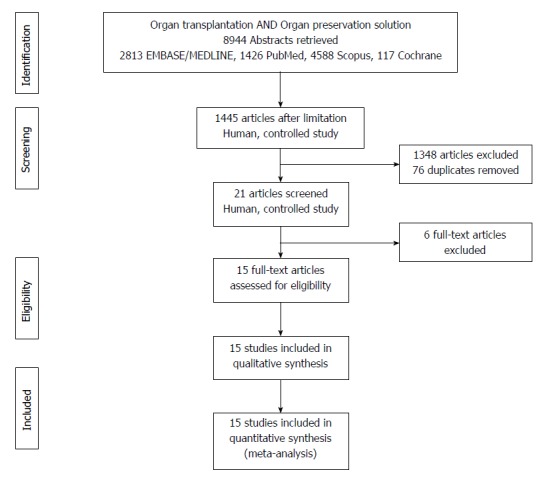
PRISMA flowchart of search strategy with inclusions and exclusions.
Inclusion criteria
Inclusion criteria specified any RCT comparing two or more preservation solutions for the static cold storage of DDLs, from both adult and pediatric donors. Living donor transplantation, multiple organ transplantation, retransplantation, nonhuman and uncontrolled studies were excluded. Abstracts for inclusion were independently reviewed by two authors, and disagreements were resolved by discussion with a third author (Figure 1).
Outcomes
The primary outcome was PNF of the liver grafts. PNF is a life-threatening condition after transplantation that leads to death or to the need for retransplantation within seven days of transplantation. It is characterized by hepatic cytolysis, elevated fasting transaminase levels, diminishing or absent bile production, coalgulation deficit related to severely impaired liver function, high lactate levels, hypoglycemia, respiratory failure requiring ventilatory support, circulatory failure requiring catecholamines, and the onset of renal and multi-organ failure[15].
The secondary outcome was OGS-1, since the one-year post-transplant time point was considered by an expert consensus opinion as most suitable to evaluate the effect of the preservation solutions[16].
Data extraction
Demographic, quality and outcome data were extracted independently into Microsoft Excel by two authors. Data were collected from all articles describing the studies; in the case of discrepancies, the article with the largest number of patients was used. Any questions in data extraction were settled by discussion with a third author.
Statistical analysis
The statistical analysis for this study was conducted by Péter Mátrai, Institute of Bioanalysis, University of Pécs, H-7624 Pécs, Hungary. Risk ratios (RR) from individual studies were pooled statistically by random effect model using the DerSimonian-Laird estimator and were displayed on forest plots. As RR allows for the comparison of two samples, the Celsior and HTK solutions were compared to UW. Summary RRs were calculated with 95% confidence intervals (CI) and p values to test if summary RR = 1 can be rejected. P < 0.05 was defined as a significant difference between solutions. In the analysis of outcomes for PNF and PDF, we used a computational correction recommended in the Cochrane Handbook and proposed by Sweeting et al[24]. to overcome the difficulty of dividing by 0. Statistical heterogeneity was tested using the I2 statistic and the chi-square test to obtain probability-values; P < 0.05 was defined to indicate significant heterogeneity. All statistical calculations were performed using Stata 11 SE (Stata Corp) and Comprehensive Meta-analysis Software (Version 3, Biostat, Englewood). We sought signs of a small study effect with the funnel plot. To identify potential sources of heterogeneity, we defined a priori subgroup analyses with the model of end-stage liver disease (MELD) score and cold ischemia time (CIT). All other outcomes related to the solutions were also investigated by subgroup analysis.
RESULTS
Demographic and clinical characteristics of donors and recipients were homogenous in all trials (Supplementary Tables 1 2 and 3).
MELD score
The MELD score incorporates parameters of recipients (such as abnormal coagulation, creatinine and serum bilirubin levels and the etiology of cirrhosis) and serves as a predictor of mortality after liver transplantation[25]. MELD scores were reported in five studies (Supplementary Table 2). Subgroup analysis showed no significant difference in MELD score between the four solutions (RR = 18.6, 95%CI: 15.7-21.5, P = 0.379) (Supplementary Figure 1A).
CIT
CIT (time interval from the clamping of the donor aorta to the anastomosis of the organ to the recipient’s vascular system or organs disposal) was reported in five studies (Supplementary Table 3). Subgroup analysis showed no significant difference in risk of CIT between the four solutions (RR = 484.7, 95%CI: 445.4-524.0, P = 0.1) (Supplementary Figure 1B).
PNF
PNF rates were reported in 15 studies (Table 2)[8,9,12,13,21,26-35]. In four studies, PNF was defined as patient death or retransplantation in the first week. In eleven studies, PNF was undefined. Overall rates of PNF were very low (range 0-13.7%). Our meta-analysis showed no significant difference in risk of PNF between the UW and CS solutions (z = 0.41, P = 0.680) and between UW and HTK (z = 1.07, P = 0.284) (Figure 2A). We found only one RCT that dealt with IGL-1, which was not sufficient for a meta-analysis to compare IGL-1 with the UW solution. We performed a subgroup analysis to compare the four solutions in the context of PNF. There was no significant difference between solutions (RR = 0.02, 95%CI: 0.01-0.03, P = 0.356) (Figure 2B). We found no evidence of a small study effect using the funnel plot analysis of the meta-analyses for the primary outcome (P = 0.846) (Figure 2C).
Table 2.
Primary non-function rate in included studies
| Study |
Solution 1 |
Solution 2 |
RR | P value | ||||||
| N | n | % | N | n | % | |||||
| Cavallari et al[13], 2003 | UW | 90 | 1 | 1.100 | CS | 83 | 0 | 0.000 | 2.77 | 0.53 |
| Lopez-Andujar et al[26], 2009 | UW | 104 | 2 | 1.900 | CS | 92 | 2 | 2.200 | 0.88 | 0.90 |
| García-Gil et al[33], 2006 | UW | 40 | 0 | 0.000 | CS | 40 | 0 | 0.000 | 1.00 | 1.00 |
| Nardo et al[27], 2001 | UW | 60 | 2 | 3.333 | CS | 53 | 0 | 0.000 | 4.43 | 0.33 |
| Duca et al[28], 2010 | UW | 51 | 0 | 0.000 | CS | 51 | 0 | 0.000 | 1.00 | 1.00 |
| García-Gil et al[35], 2011 | UW | 51 | 4 | 11.100 | CS | 51 | 4 | 11.100 | 1.00 | 1.00 |
| Lama et al[29], 2002 | UW | 10 | 0 | 0.000 | CS | 10 | 0 | 0.000 | 1.00 | 1.00 |
| Rayya et al[30], 2008 | UW | 68 | 1 | 1.471 | HTK | 69 | 1 | 1.449 | 1.01 | 0.99 |
| Meine et al[32], 2006 | UW | 65 | 2 | 3.070 | HTK | 37 | 1 | 3.030 | 1.14 | 0.91 |
| Erhard et al[9], 1994 | UW | 30 | 2 | 6.660 | HTK | 30 | 0 | 0.000 | 5.00 | 0.29 |
| Mangus et al[31], 2008 | UW | 98 | 5 | 5.102 | HTK | 111 | 3 | 2.703 | 1.89 | 0.38 |
| Dondéro et al[37], 2010 | UW | 92 | 4 | 4.350 | IGL-1 | 48 | 1 | 2.080 | 2.09 | 0.51 |
| Meine et al[8], 2015 | HTK | 65 | 2 | 3.100 | IGL-1 | 113 | 3 | 2.700 | 1.16 | 0.87 |
| Wiederkehr et al[21], 2014 | HTK | 125 | 1 | 0.700 | IGL-1 | 53 | 0 | 0.000 | 1.29 | 0.88 |
| Nardo et al[12], 2005 | HTK | 20 | 1 | 5.000 | CS | 20 | 0 | 0.000 | 3.00 | 0.49 |
Studies are grouped by preservation solutions. PNF: Primary non-function; N: Indicates number in group; n: Number of PNF; RR: Relative risk; UW: University of Wisconsin solution; HTK: Histidine-tryptophan-ketoglutarate solution; CS: Celsior solution; IGL-1: Institut Georges Lopez solution.
Figure 2.
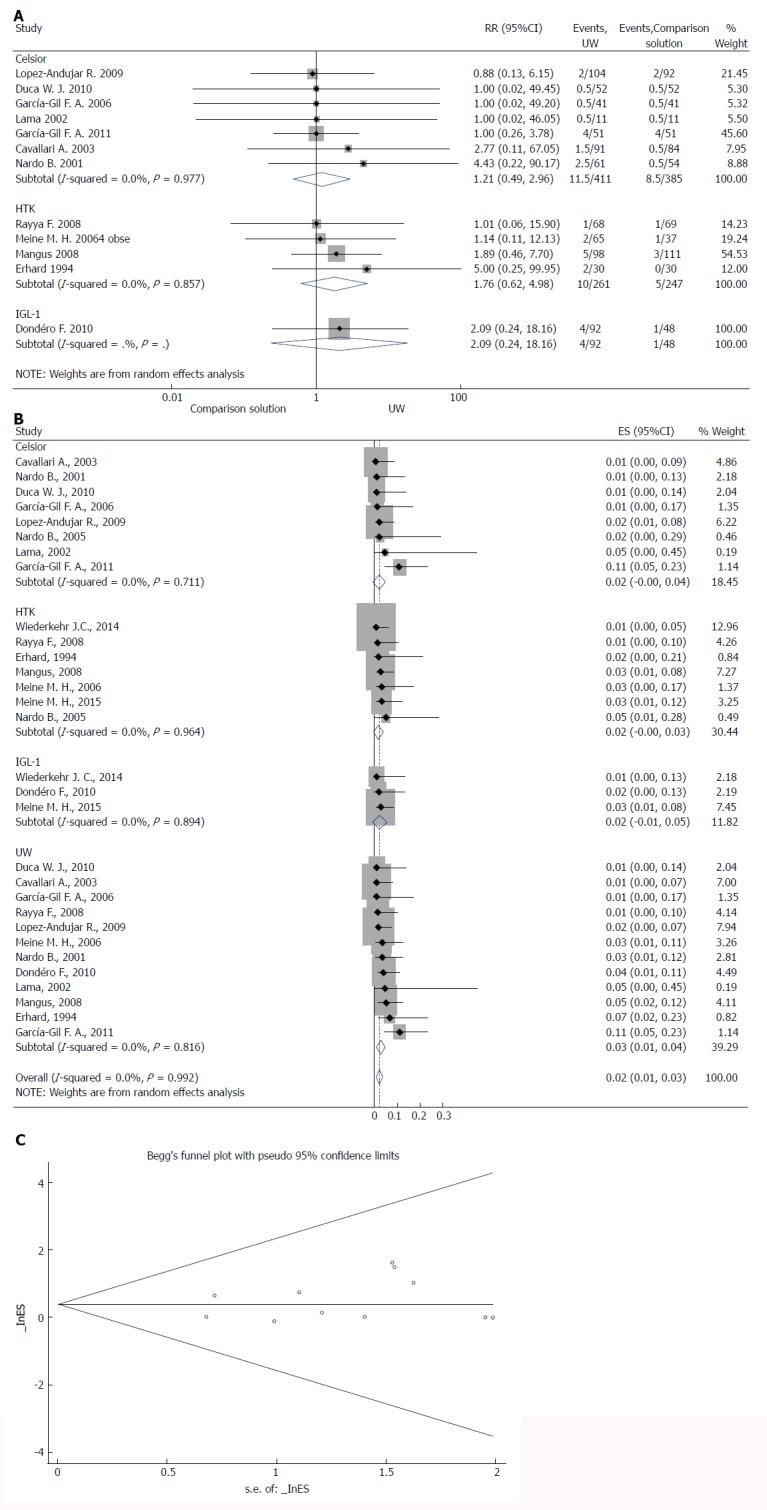
Effects of preservation solutions on primary non-function. A: Meta-analysis of the relative risk (-RR-) of PNF comparing studies using different preservation solutions: UW vs Celsior and UW vs HTK; B: Forest plot for subgroup analysis of PNF; and C: Funnel plot for PNF in studies. Squares represent individual study effects, with the size of the box relating to the weight of the study in the meta-analysis. Each diamond represents a summary effect from meta-analysis. Horizontal bars represent 95% CIs. There is no evidence of a small study effect in the test or the formal plot. PNF: Primary non-function; RCTs: Randomized controlled trials; ES: Effect size; UW: University of Wisconsin solution; HTK: Histidine-tryptophan-ketoglutarate solution; CS: Celsior solution; IGL-1: Institut Georges Lopez solution.
OGS-1
OGS-1 was reported in eleven studies (Table 3). No study was individually powered for small differences in graft survival, and no study reported a difference related to the preservation fluid used. Meta-analysis of the data showed no significant difference in the risk of OGS-1 between the UW and CS solutions (z = 0.30, P = 0.763) (Figure 3A) or between the UW and HTK solutions (z = 0.01, P = 0.991) (Figure 3A). We also performed a subgroup analysis to compare all four solutions, including IGL-1. There was no significant difference between the solutions (RR = 0.80, 95%CI: 0.80-0.80, P = 0.369) (Figure 3B). We found no evidence of a small study effect using the funnel plot analysis from either of the meta-analyses for the OGS-1 (P = 0.397) (Figure 3C).
Table 3.
One-year post-transplant graft survival rate in included studies
| Study |
Solution 1 |
Solution 2 |
RR | P value | ||||||
| N | n | % | N | n | % | |||||
| Cavallari et al[13], 2003 | UW | 90 | 75 | 83.0 | CS | 83 | 71 | 85.0 | 0.97 | 0.69 |
| Lopez-Andujar et al[26], 2009 | UW | 104 | 83 | 80.0 | CS | 92 | 75 | 81.0 | 0.98 | 0.76 |
| García-Gil et al[33], 2006 | UW | 40 | 26 | 66.1 | CS | 40 | 31 | 78.0 | 0.84 | 0.22 |
| Nardo et al[27], 2001 | UW | 60 | 54 | 90.0 | CS | 53 | 48 | 90.6 | 0.99 | 0.92 |
| Duca et al[28], 2010 | UW | 51 | 31 | 60.6 | CS | 51 | 37 | 73.5 | 0.84 | 0.21 |
| Rayya et al[30], 2008 | UW | 68 | 53 | 78.0 | HTK | 69 | 49 | 71.0 | 1.01 | 0.35 |
| Meine et al[32], 2006 | UW | 65 | 61 | 94.0 | HTK | 37 | 35 | 94.0 | 0.99 | 0.88 |
| Mangus et al[31], 2008 | UW | 98 | 82 | 84.0 | HTK | 111 | 95 | 86.0 | 0.98 | 0.70 |
| Dondéro et al[37], 2010 | UW | 92 | 73 | 79.1 | IGL-1 | 48 | 19 | 39.8 | 2.00 | 0.00 |
| Meine et al[8], 2015 | HTK | 65 | 54 | 83.0 | IGL-1 | 113 | 96 | 85.0 | 0.98 | 0.74 |
| Nardo et al[12], 2005 | HTK | 20 | 15 | 75.0 | CS | 20 | 18 | 90.0 | 0.83 | 0.22 |
Studies are grouped by preservation solutions. OGS-1: One-year post-transplant graft survival; N: Number in group; n: Number of OGS-1; RR: Relative risk; UW: University of Wisconsin solution; HTK: Histidine-tryptophan-ketoglutarate solution; CS: Celsior solution; IGL-1: Institut Georges Lopez solution.
Figure 3.
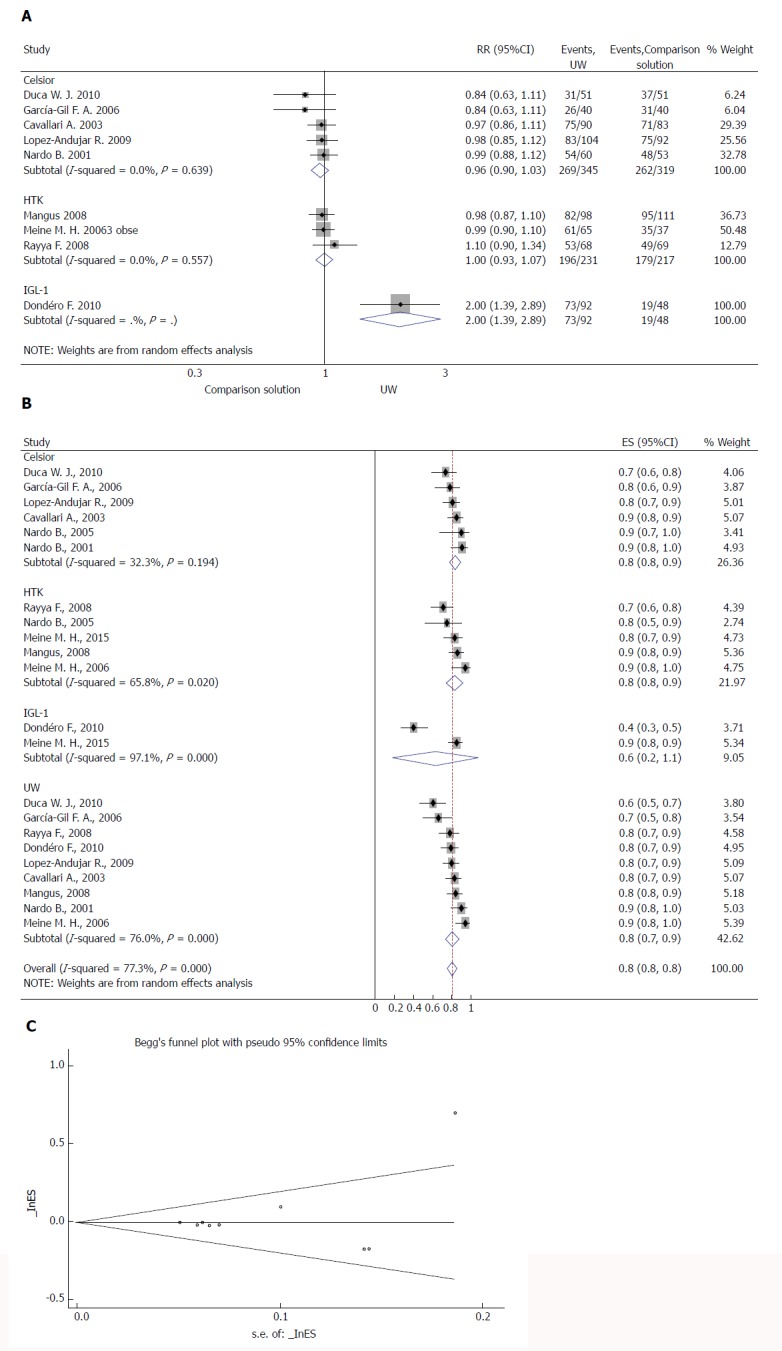
Effects of preservation solutions on one-year post-transplant graft survival. A: Meta-analysis of the relative risk (-RR-) of OGS-1 comparing studies using different preservation solutions: UW vs Celsior and UW vs HTK; B: Forest plot for subgroup analysis of OGS-1; and C: Funnel plot for OGS-1 in studies. Squares represent individual study effects, with the size of the box relating to the weight of the study in the meta-analysis. Each diamond represents a summary effect from meta-analysis. Horizontal bars represent 95% CIs. There is no evidence of a small study effect in the test or the formal plot. OGS-1: One-year post-transplant graft survival; RR: Relative risk; RCTs: Randomized controlled trials; ES: Effect size; UW: University of Wisconsin solution; HTK: Histidine-tryptophan-ketoglutarate solution; CS: Celsior solution; IGL-1: Institut Georges Lopez solution.
PDF rates were reported in six studies: five of them compared UW with CS, and one compared UW with HTK (Supplementary Table 4). Overall rates of PDF were very low (range 0-15.5%). The difference in PDF rate was found higher with the use of UW solutions in one study[32]. However, the subgroup analysis showed no increased risk of PDF in the UW group (RR = 0.1, 95%CI: 0.0-0.1, P = 0.582) (Supplementary Figure 2).
Early RT
Early RT was reported in seven studies and ranged from 0.9% to 20% (Supplementary Table 4). None of the studies found a significant difference in early RT between groups; however, they were underpowered to detect such a low incidence outcome. Similarly, subgroup analysis showed no increased risk of early RT in the UW group (RR = 0.0, 95%CI: 0.0-0.1, P = 0.698) (Supplementary Figure 3).
POD
POD rates were reported in seven studies (Supplementary Table 4). Overall rates of POD were very low (range 1.7%-14.4%). The difference in POD rate was higher with the use of the CS solution compared with the UW solution in two studies[12,33]; however, subgroup analysis showed no increased risk (Supplementary Figure 4). In contrast, there was a significant difference when UW was compared to HTK or IGL-1 (RR = 0.07, 95%CI: 0.04-0.09, P < 0.01).
OPS-1
OPS-1 rates were reported in ten studies (Supplementary Table 4). No study was individually powered for small differences in graft survival, and no study reported a difference related to the preservation fluid used. Subgroup analysis showed no significant difference in risk of OPS-1 between the four solutions (RR = 0.9, 95%CI: 0.8-0.9, P = 0.786) (Supplementary Figure 5).
DISCUSSION
This study reviews the current evidence and updates knowledge on four frequently used preservation solutions for static cold storage of DDLs for transplantation. The treatment groups were homogenous in terms of donor and recipient characteristics; the prediction of primary and secondary outcomes (i.e., PNF and OGS-1) was thus likely independent of individual risk variables, patient selection or the overall severity of the disease at liver transplantation. More importantly, the analysis of outcome parameters (i.e., PNF and OGS-1) provided good evidence that UW is not outperformed by CS, HTK and IGL-1 solutions in maintaining organ function and viability of liver grafts in cold storage.
PNF mainly depends on the organ preservation method[15]. It occurs in 2%-6% of transplants and is unrelated to any direct surgical, immunologic or other complications[34]. Our meta-analysis included eleven trials that evaluated the effectiveness of the UW solution as compared to either the CS or HTK solution. In accordance with the literature, the overall rates of PNF were very low, except in one trial (13%)[35]. When analyzing the single studies, we found two trials with a higher incidence of PNF in the UW group than in the HTK group[9,36], but the difference did not reach statistical significance upon meta-analysis. It should be added that a recent analysis of the ELTR database demonstrated that use of HTK represented an individual risk factor for the development of PNF when compared to the UW solution[10]. The contradictory conclusions can be explained with the selection bias of the database analysis[20]. In either case, we found no difference between UW and the other solutions with regard to the risk of PNF. As regards IGL-1 and HTK, two prospective randomized clinical studies with 356 patients reported identical results[8,21]. Similar outcome was detected in a single-center study with 140 patients that compared IGL-1 and UW solutions[37]. This was confirmed in the current study, since IGL-1 showed a similar PNF risk to that of UW and HTK in our subgroup analyses.
In our study, OGS-1 was the secondary endpoint. Graft survival rates were evaluated one, three and five years after liver transplantation in single studies. The one-year term was chosen as an appropriate period to evaluate the effect of the preservation solutions because other factors could have a greater impact on this outcome parameter after this time. A retrospective analysis of the ELTR database demonstrated that HTK preservation was independently associated with higher mortality than UW, CS and IGL-1 in a multivariate analysis[10]. Another analysis of a large national registry database (United Network for Organ Sharing, UNOS) has also demonstrated differences in graft survival rate between the HTK and UW solutions[18]. However, important risk factors among donors were not considered in the ELTR analysis[20], and selected groups of transplant patients were not homogenous in the other analysis: HTK was utilized in allografts with more favorable recipient traits, as well as shorter CIT and less local and national export[18]. In accordance with the findings from numerous clinical trials, the meta-analyses and subgroup analyses in this study did not show a significant difference in risk of OGS-1 between UW and any of the examined solutions. Similarly, there was no evidence for a difference between IGL-1 and UW solutions and between IGL-1 and HTK in the subgroup analyses.
Apart from the preservation methods used to protect the organ from IR injury, the final outcome of transplantation can also be linked to factors such as donor age, general condition and CIT[38]. A recent UNOS study showed a more pronounced risk for graft loss with longer CIT and donors over 70 years[18]. In our study, subgroup analysis showed that the included trials did not vary significantly and that the mean CITs were beyond the critical 12 h[39]. Several experimental studies demonstrated that the use of the UW solution allows for longer CITs with better graft preservation; however, it remains to be determined whether any of the alternative solutions is better than UW when CIT is prolonged over 12 h.
Recipient morbidity and MELD scores are also important contributing factors to the outcome of liver transplantation. Recipient parameters are incorporated into the MELD score, which indicates the state of health of the recipient; the MELD score-based organ allocation algorithm could thus significantly influence the graft survival rate[40]. In the present study, there was no significant difference between the preservation solutions in the context of the MELD score and other recipient characteristics.
In recent times, the crisis in organ supply has made it necessary to extend the scope of potential donors by using extended criteria donors (ECD). Although there is no precise definition of ECD, frequently cited characteristics are donor age, steatosis, donation after cardiac death (DCD), donors with increased risk of disease transmission and transplantation after prolonged CIT, as well as the use of partial grafts (split grafts and living donor liver transplantation)[41]. Unfortunately, higher rates of graft failure were documented in this class of extended allograft; in addition, very little data is available on the influence of preservation solutions on their post-transplant outcomes[42]. A single-center study by Mangus et al[30] failed to find statistically significant differences in overall graft survival when they compared UW to HTK in ECD transplantations. However, they suggested that HTK may be protective against biliary complications. In contrast, in 2009, the UNOS database analysis reported that HTK was associated with an increased risk of graft loss and early graft loss[18]. More recently, Adam et al[10] compared the four most frequently used preservation solutions and concluded that HTK is an independent risk factor for graft loss after ECD liver transplantations. The remaining three solutions, UW, CE and IGL-1, provided similar results in post-transplant outcomes after ECD transplantations. In the special condition of using a partially deceased donor liver graft, IGL-1 offered the best graft outcome[10]. In another study, it was suggested that IGL-1 was superior to other solutions for preserving fatty livers by protecting against PNF and early allograft dysfunction[43]. However, a prospective randomized study could not show any significant improvement in the subgroup of patients receiving IGL-1-preserved grafts[36]. In living donor liver transplantations, risk-adjusted analyses of single- and double-center studies consistently reported that UW and HTK were equally effective and safe for cold preservation[44-47]. There is currently no evidence-based recommendation on the optimal preservation solution in ECD liver transplantations because the number and quality of RCTs are not sufficient. However, based on the above data, differences in the indications of various preservation solutions are expected.
This study has some limitations. There are so far only three small RCTs that compare IGL-1 with UW or IGL-1 with HTK. Therefore, we could not run a meta-analysis to compare IGL-1 with any of the solutions. In order to compare the risk of the four solutions for PNF, we had to perform a subgroup analysis. In addition, surgery time and hemoderivative transfusions due to recipient coagulation problems are often not cited in the literature as predictors of poor outcome[36]. This factor was not considered in the selected trials. Moreover, different trials presented some differences as regards the operative procedure. Furthermore, the included RCTs were homogenous with regard to donor and recipient parameters. On the one hand, this provided the possibility to exclude selection bias, but, on the other hand, the effects of preservation solutions in the case of longer CIT and involvement of expanded criteria donors could not be evaluated.
In conclusion, elucidation of the role of preservation solutions in the outcome of liver transplantation is complicated by the intrinsic complexity of the clinical procedure, which is made up of many different, but interacting phases. This review evaluated the best available evidence from comparisons of the four most frequently used preservation fluids in DDL transplantation. A direct meta-analysis comparison was made and the sample size of included trials was large enough to estimate the risk of low-incidence outcomes such as PNF or OGS-1 correctly. Based on our results, there is good evidence that the UW, CS, HTK and IGL-1 solutions are associated with nearly equivalent outcomes. Additional studies on larger patient populations including marginal donors, longer cold ischemia time, multi-organ transplantations and economic aspects are needed to evaluate the superiority of any alternative solution over UW.
ARTICLE HIGHLIGHTS
Research background
The introduction of the University of Wisconsin (UW) solution for static cold storage of liver grafts was a breakthrough and has remained the conventional method of organ preservation. However, many alternative preservation solutions exist, and each is thought to offer an advantage over UW solution.
Research motivation
At present, 98% of liver transplantations use the UW, histidine-tryptophan-ketoglutarate (HTK), Celsior (CS) or Institute Georges Lopez (IGL-1) solution for the cold preservation of grafts. Previously, prospective trials have investigated their effects on liver transplant outcomes, but with contradictory results. Furthermore, no systematic review reports the effect of IGL-1, which was first used by 2003, as compared to other solutions.
Research objectives
To provide an update and to compare the latest findings from clinical trials on the effects of the four most frequently used preservation solutions on liver transplant outcomes.
Research methods
A systematic review and meta-analysis were conducted on randomized controlled trials of deceased donor liver transplantations using UW and either HTK, CS or IGL-1 for cold storage of allografts. Primary and secondary outcomes were primary non-function (PNF) and one-year post-transplant graft survival (OGS-1).
Research results
In spite of differences found in individual studies, a meta-analysis of PNF and OGS-1 showed no statistical difference between groups. Subgroup analysis showed no increased risk for other outcomes, such as primary dysfunction, early retransplantation rate, post-transplantation death and one-year post-transplant patient survival.
Research conclusions
This meta-analysis provided evidence that UW and alternative solutions are associated with almost the same transplant outcome. Further studies are needed with extended criteria donors to ascertain the superiority of any alternative solution over UW.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Hungary
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Supported by grants from the National Research Development and Innovation Office, NKFI K120232; Hungarian Science Research Fund, No. GINOP 2.3.2-15-2016-00015 and No. EFOP-3.6.2-16-2017-00006; and New National Excellence Program of the Ministry of Human Capacities, No. UNKP-17-4.
Conflict-of-interest statement: None of the authors has any conflict of interests related to this manuscript.
Data sharing statement: No additional data are available.
Peer-review started: January 31, 2018
First decision: February 24, 2018
Article in press: April 9, 2018
P- Reviewer: Bramhall S, Chiu KW, Inoue K, Rosello-Catafau J, Tsoulfas G S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
Contributor Information
Ágnes Lilla Szilágyi, Institute of Surgical Research, University of Szeged, Szeged H-6720, Hungary.
Péter Mátrai, Institute of Bioanalysis, University of Pécs, Pécs H-7624, Hungary.
Péter Hegyi, Institute for Translational Medicine and First Department of Medicine, University of Pécs, Pécs H-7624, Hungary; MTA-SZTE Translational Gastroenterology Research Group, Szeged H-6720, Hungary; János Szentágothai Research Center, University of Pécs, Pécs H-7624, Hungary.
Eszter Tuboly, Institute of Surgical Research, University of Szeged, Szeged H-6720, Hungary.
Daniella Pécz, Institute of Surgical Research, University of Szeged, Szeged H-6720, Hungary.
András Garami, Institute for Translational Medicine and First Department of Medicine, University of Pécs, Pécs H-7624, Hungary.
Margit Solymár, Institute for Translational Medicine and First Department of Medicine, University of Pécs, Pécs H-7624, Hungary.
Erika Pétervári, Institute for Translational Medicine and First Department of Medicine, University of Pécs, Pécs H-7624, Hungary.
Márta Balaskó, Institute for Translational Medicine and First Department of Medicine, University of Pécs, Pécs H-7624, Hungary.
Gábor Veres, 1st Department of Paediatrics, University of Semmelweis, Budapest H-1085, Hungary.
László Czopf, Department of Cardiology, 1st Department of Medicine, University of Pécs, Pécs H-7624, Hungary.
Bastian Wobbe, Department of Cardiology, 1st Department of Medicine, University of Pécs, Pécs H-7624, Hungary.
Dorottya Szabó, Department of Cardiology, 1st Department of Medicine, University of Pécs, Pécs H-7624, Hungary.
Juliane Wagner, Department of Cardiology, 1st Department of Medicine, University of Pécs, Pécs H-7624, Hungary.
Petra Hartmann, Institute of Surgical Research, University of Szeged, Szeged H-6720, Hungary. hartmann.petra@med.u-szeged.hu.
References
- 1.Jia JJ, Li JH, Jiang L, Lin BY, Wang L, Su R, Zhou L, Zheng SS. Liver protection strategies in liver transplantation. Hepatobiliary Pancreat Dis Int. 2015;14:34–42. doi: 10.1016/s1499-3872(15)60332-0. [DOI] [PubMed] [Google Scholar]
- 2.Brisson H, Arbelot C, Monsel A, Parisot C, Girard M, Savier E, Vezinet C, Lu Q, Vaillant JC, Golmard JL, et al. Impact of graft preservation solutions for liver transplantation on early cytokine release and postoperative organ dysfunctions. A pilot study. Clin Res Hepatol Gastroenterol. 2017;41:564–574. doi: 10.1016/j.clinre.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Stewart ZA. UW solution: still the “gold standard” for liver transplantation. Am J Transplant. 2015;15:295–296. doi: 10.1111/ajt.13062. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Wahlstrom HE, Moore SB, Wiesner RH, Krom RA. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49–53. doi: 10.1002/hep.1840160110. [DOI] [PubMed] [Google Scholar]
- 5.Cutrin JC, Cantino D, Biasi F, Chiarpotto E, Salizzoni M, Andorno E, Massano G, Lanfranco G, Rizzetto M, Boveris A, et al. Reperfusion damage to the bile canaliculi in transplanted human liver. Hepatology. 1996;24:1053–1057. doi: 10.1002/hep.510240512. [DOI] [PubMed] [Google Scholar]
- 6.Tullius SG, Filatenkow A, Horch D, Mehlitz T, Reutzel-Selke A, Pratschke J, Steinmüller T, Lun A, Al-Abadi H, Neuhaus P. Accumulation of crystal deposits in abdominal organs following perfusion with defrosted University of Wisconsin solutions. Am J Transplant. 2002;2:627–630. doi: 10.1034/j.1600-6143.2002.20707.x. [DOI] [PubMed] [Google Scholar]
- 7.Fridell JA, Rogers J, Stratta RJ. The pancreas allograft donor: current status, controversies, and challenges for the future. Clin Transplant. 2010;24:433–449. doi: 10.1111/j.1399-0012.2010.01253.x. [DOI] [PubMed] [Google Scholar]
- 8.Meine MH, Leipnitz I, Zanotelli ML, Schlindwein ES, Kiss G, Martini J, de Medeiros Fleck A Jr, Mucenic M, de Mello Brandão A, Marroni CA, Craco Cantisani GP. Comparison Between IGL-1 and HTK Preservation Solutions in Deceased Donor Liver Transplantation. Transplant Proc. 2015;47:888–893. doi: 10.1016/j.transproceed.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Erhard J, Lange R, Scherer R, Kox WJ, Bretschneider HJ, Gebhard MM, Eigler FW. Comparison of histidine-tryptophan-ketoglutarate (HTK) solution versus University of Wisconsin (UW) solution for organ preservation in human liver transplantation. A prospective, randomized study. Transpl Int. 1994;7:177–181. doi: 10.1007/BF00327084. [DOI] [PubMed] [Google Scholar]
- 10.Adam R, Delvart V, Karam V, Ducerf C, Navarro F, Letoublon C, Belghiti J, Pezet D, Castaing D, Le Treut YP, Gugenheim J, Bachellier P, Pirenne J, Muiesan P; ELTR contributing centres, the European Liver, Intestine Transplant Association (ELITA) Compared efficacy of preservation solutions in liver transplantation: a long-term graft outcome study from the European Liver Transplant Registry. Am J Transplant. 2015;15:395–406. doi: 10.1111/ajt.13060. [DOI] [PubMed] [Google Scholar]
- 11.Fridell JA, Agarwal A, Milgrom ML, Goggins WC, Murdock P, Pescovitz MD. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution for organ preservation in clinical pancreas transplantation. Transplantation. 2004;77:1304–1306. doi: 10.1097/01.tp.0000122222.93740.b2. [DOI] [PubMed] [Google Scholar]
- 12.Nardo B, Bertelli R, Montalti R, Beltempo P, Puviani L, Pacilè V, Cavallari A. Preliminary results of a clinical randomized study comparing Celsior and HTK solutions in liver preservation for transplantation. Transplant Proc. 2005;37:320–322. doi: 10.1016/j.transproceed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Cavallari A, Cillo U, Nardo B, Filipponi F, Gringeri E, Montalti R, Vistoli F, D’amico F, Faenza A, Mosca F, et al. A multicenter pilot prospective study comparing Celsior and University of Wisconsin preserving solutions for use in liver transplantation. Liver Transpl. 2003;9:814–821. doi: 10.1053/jlts.2003.50161. [DOI] [PubMed] [Google Scholar]
- 14.Tabka D, Bejaoui M, Javellaud J, Roselló-Catafau J, Achard JM, Abdennebi HB. Effects of Institut Georges Lopez-1 and Celsior preservation solutions on liver graft injury. World J Gastroenterol. 2015;21:4159–4168. doi: 10.3748/wjg.v21.i14.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ploeg RJ, D’Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Lema Zuluaga GL, Serna Agudelo RE, Zuleta Tobón JJ. Preservation solutions for liver transplantation in adults: celsior versus custodiol: a systematic review and meta-analysis with an indirect comparison of randomized trials. Transplant Proc. 2013;45:25–32. doi: 10.1016/j.transproceed.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Feng L, Zhao N, Yao X, Sun X, Du L, Diao X, Li S, Li Y. Histidine-tryptophan-ketoglutarate solution vs. University of Wisconsin solution for liver transplantation: a systematic review. Liver Transpl. 2007;13:1125–1136. doi: 10.1002/lt.21208. [DOI] [PubMed] [Google Scholar]
- 18.Stewart ZA, Cameron AM, Singer AL, Montgomery RA, Segev DL. Histidine-Tryptophan-Ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am J Transplant. 2009;9:286–293. doi: 10.1111/j.1600-6143.2008.02478.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Callaghan JM, Morgan RD, Knight SR, Morris PJ. The effect of preservation solutions for storage of liver allografts on transplant outcomes: a systematic review and meta-analysis. Ann Surg. 2014;260:46–55. doi: 10.1097/SLA.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 20.Nashan B, Spetzler V, Schemmer P, Kirste G, Rahmel A. Regarding “Compared Efficacy of Preservation Solutions in Liver Transplantation: A Long-Term Graft Outcome Study From the European Liver Transplant Registry”. Am J Transplant. 2015;15:3272–3273. doi: 10.1111/ajt.13513. [DOI] [PubMed] [Google Scholar]
- 21.Wiederkehr JC, Igreja MR, Nogara MS, Goncalves N, Montemezzo GP, Wiederkehr HA, Wassen MP, Nobrega HA, Zenatti KB, Mori LY, et al. Use of IGL-1 preservation solution in liver transplantation. Transplant Proc. 2014;46:1809–1811. doi: 10.1016/j.transproceed.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PMC free article] [PubMed] [Google Scholar]
- 23.The University of York Centre for Reviews and Dissemination. PROSPERO Register of ongoing systematic reviews. Available from: https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017054908 [Google Scholar]
- 24.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 25.Huo TI, Lee SD, Lin HC. Selecting an optimal prognostic system for liver cirrhosis: the model for end-stage liver disease and beyond. Liver Int. 2008;28:606–613. doi: 10.1111/j.1478-3231.2008.01727.x. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Andujar R, Deusa S, Montalvá E, San Juan F, Moya A, Pareja E, DeJuan M, Berenguer M, Prieto M, Mir J. Comparative prospective study of two liver graft preservation solutions: University of Wisconsin and Celsior. Liver Transpl. 2009;15:1709–1717. doi: 10.1002/lt.21945. [DOI] [PubMed] [Google Scholar]
- 27.Nardo B, Catena F, Cavallari G, Montalti R, Di Naro A, Faenza A, Cavallari A. Randomized clinical study comparing UW and Celsior solution in liver preservation for transplantation: preliminary results. Transplant Proc. 2001;33:870–872. doi: 10.1016/s0041-1345(00)02357-5. [DOI] [PubMed] [Google Scholar]
- 28.Duca WJ, da Silva RF, Arroyo PC Jr, Sgnolf A, Cabral CM, Ayres DC, Felício HC, da Silva RD. Liver transplantation using University of Wisconsin or Celsior preserving solutions in the portal vein and Euro-Collins in the aorta. Transplant Proc. 2010;42:429–434. doi: 10.1016/j.transproceed.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 29.Lama C, Rafecas A, Figueras J, Torras J, Ramos E, Fabregat J, Busquets J, Garcia-Barrasa A, Jaurrieta E. Comparative study of Celsior and Belzer solutions for hepatic graft preservation: preliminary results. Transplant Proc. 2002;34:54–55. doi: 10.1016/s0041-1345(01)02664-1. [DOI] [PubMed] [Google Scholar]
- 30.Rayya F, Harms J, Martin AP, Bartels M, Hauss J, Fangmann J. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in adult liver transplantation. Transplant Proc. 2008;40:891–894. doi: 10.1016/j.transproceed.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 31.Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK, Tector AJ. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transpl. 2008;14:365–373. doi: 10.1002/lt.21372. [DOI] [PubMed] [Google Scholar]
- 32.Meine MH, Zanotelli ML, Neumann J, Kiss G, de Jesus Grezzana T, Leipnitz I, Schlindwein ES, Fleck A Jr, Gleisner AL, de Mello Brandão A, Marroni CA, Cantisani GP. Randomized clinical assay for hepatic grafts preservation with University of Wisconsin or histidine-tryptophan-ketoglutarate solutions in liver transplantation. Transplant Proc. 2006;38:1872–1875. doi: 10.1016/j.transproceed.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 33.García-Gil FA, Arenas J, Güemes A, Esteban E, Tomé-Zelaya E, Lamata F, Sousa R, Jiménez A, Barrao ME, Serrano MT. Preservation of the liver graft with Celsior solution. Transplant Proc. 2006;38:2385–2388. doi: 10.1016/j.transproceed.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Uemura T, Randall HB, Sanchez EQ, Ikegami T, Narasimhan G, McKenna GJ, Chinnakotla S, Levy MF, Goldstein RM, Klintmalm GB. Liver retransplantation for primary nonfunction: analysis of a 20-year single-center experience. Liver Transpl. 2007;13:227–233. doi: 10.1002/lt.20992. [DOI] [PubMed] [Google Scholar]
- 35.García-Gil FA, Serrano MT, Fuentes-Broto L, Arenas J, García JJ, Güemes A, Bernal V, Campillo A, Sostres C, Araiz JJ, et al. Celsior versus University of Wisconsin preserving solutions for liver transplantation: postreperfusion syndrome and outcome of a 5-year prospective randomized controlled study. World J Surg. 2011;35:1598–1607. doi: 10.1007/s00268-011-1078-7. [DOI] [PubMed] [Google Scholar]
- 36.Mangus RS, Kinsella SB, Nobari MM, Fridell JA, Vianna RM, Ward ES, Nobari R, Tector AJ. Predictors of blood product use in orthotopic liver transplantation using the piggyback hepatectomy technique. Transplant Proc. 2007;39:3207–3213. doi: 10.1016/j.transproceed.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 37.Dondéro F, Paugam-Burtz C, Danjou F, Stocco J, Durand F, Belghiti J. A randomized study comparing IGL-1 to the University of Wisconsin preservation solution in liver transplantation. Ann Transplant. 2010;15:7–14. [PubMed] [Google Scholar]
- 38.Stahl JE, Kreke JE, Malek FA, Schaefer AJ, Vacanti J. Consequences of cold-ischemia time on primary nonfunction and patient and graft survival in liver transplantation: a meta-analysis. PLoS One. 2008;3:e2468. doi: 10.1371/journal.pone.0002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belle SH, Beringer KC, Detre KM. An update on liver transplantation in the United States: recipient characteristics and outcome. Clin Transpl. 1995:19–33. [PubMed] [Google Scholar]
- 40.Weismüller TJ, Fikatas P, Schmidt J, Barreiros AP, Otto G, Beckebaum S, Paul A, Scherer MN, Schmidt HH, Schlitt HJ, et al. Multicentric evaluation of model for end-stage liver disease-based allocation and survival after liver transplantation in Germany--limitations of the ‘sickest first’-concept. Transpl Int. 2011;24:91–99. doi: 10.1111/j.1432-2277.2010.01161.x. [DOI] [PubMed] [Google Scholar]
- 41.Vodkin I, Kuo A. Extended Criteria Donors in Liver Transplantation. Clin Liver Dis. 2017;21:289–301. doi: 10.1016/j.cld.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Nemes B, Gámán G, Polak WG, Gelley F, Hara T, Ono S, Baimakhanov Z, Piros L, Eguchi S. Extended-criteria donors in liver transplantation Part II: reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev Gastroenterol Hepatol. 2016;10:841–859. doi: 10.1586/17474124.2016.1149062. [DOI] [PubMed] [Google Scholar]
- 43.Zaoualí MA, Mosbah IB, Abdennebi HB, Calvo M, Boncompagni E, Boillot O, Peralta C, Roselló-Catafau J. New insights into fatty liver preservation using Institute Georges Lopez preservation solution. Transplant Proc. 2010;42:159–161. doi: 10.1016/j.transproceed.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 44.Kaltenborn A, Gwiasda J, Amelung V, Krauth C, Lehner F, Braun F, Klempnauer J, Reichert B, Schrem H. Comparable outcome of liver transplantation with histidine-tryptophan-ketoglutarate vs. University of Wisconsin preservation solution: a retrospective observational double-center trial. BMC Gastroenterol. 2014;14:169. doi: 10.1186/1471-230X-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moray G, Sevmis S, Karakayali FY, Gorur SK, Haberal M. Comparison of histidine-tryptophan-ketoglutarate and University of Wisconsin in living-donor liver transplantation. Transplant Proc. 2006;38:3572–3575. doi: 10.1016/j.transproceed.2006.10.174. [DOI] [PubMed] [Google Scholar]
- 46.Jain A, Mohanka R, Orloff M, Abt P, Kashyap R, Cullen J, Lansing K, Bozorgzadeh A. University of Wisconsin versus histidine-tryptophan-ketoglutarate for tissue preservation in live-donor liver transplantation. Exp Clin Transplant. 2006;4:451–457. [PubMed] [Google Scholar]
- 47.Ringe B, Braun F, Moritz M, Zeldin G, Soriano H, Meyers W. Safety and efficacy of living donor liver preservation with HTK solution. Transplant Proc. 2005;37:316–319. doi: 10.1016/j.transproceed.2004.12.009. [DOI] [PubMed] [Google Scholar]


