Abstract
Arsenate resistance is exhibited by the ericoid mycorrhizal fungus Hymenoscyphus ericae collected from As-contaminated mine soils. To investigate the mechanism of arsenate resistance, uptake kinetics for arsenate (H2AsO4−), arsenite (H3AsO3), and phosphate (H2PO4−) were determined in both arsenate-resistant and -non-resistant H. ericae. The uptake kinetics of H2AsO4−, H3AsO3, and H2PO4− in both resistant and non-resistant isolates were similar. The presence of 5.0 μm H2PO4− repressed uptake of H2AsO4− and exposure to 0.75 mm H2AsO4− repressed H2PO4− uptake in both H. ericae. Mine site H. ericae demonstrated an enhanced As efflux mechanism in comparison with non-resistant H. ericae and lost approximately 90% of preloaded cellular As (1-h uptake of 0.22 μmol g−1 dry weight h−1 H2AsO4−) over a 5-h period in comparison with non-resistant H. ericae, which lost 40% of their total absorbed H2AsO4−. As lost from the fungal tissue was in the form of H3AsO3. The results of the present study demonstrate an enhanced H3AsO3 efflux system operating in mine site H. ericae as a mechanism for H2AsO4− resistance. The ecological significance of this mechanism of arsenate resistance is discussed.
As is ubiquitous in nature with As levels being elevated by mining, industrial, and agricultural activities (Meharg et al., 1994). In the southwest of England, the mining and processing of As ore has led to highly contaminated mine spoil soils. As may be present in these spoil soils at 35 mmol kg−1 with arsenate being the dominant form of available soil As (Colbourn et al., 1975). Within the pH range 2.0 to 6.5 arsenate exists predominantly as H2AsO4− (Fergusson, 1990). The dominant vegetative cover on these mines is Calluna vulgaris, which is present in ericoid mycorrhizal association with the ascomycete fungus Hymenoscyphus ericae (Sharples et al., 2000). Ericoid mycorrhizal symbiosis is considered to be critical to the survival of plants in the order Ericales on natural heathland sites and sites contaminated with toxic metals. The principle benefit conferred upon plants by ericoid mycorrhizal association is fungus-mediated access to otherwise unavailable sources of organic nitrogen and phosphorus, whereas the fungus may also alleviate toxic metal stress under some circumstances (Smith and Read, 1997).
H2AsO4− resistance is exhibited by the ericoid mycorrhizal fungus, H. ericae (Sharples et al., 1999, 2000) and selected angiosperms, including Holcus lanatus (Meharg and Macnair, 1990), Agrostis capillaris, and Deschampsia cespitosa (Meharg and Macnair, 1991, 1992), collected from As-contaminated mine soils. H2AsO4− is a H2PO4− analog and competes with H2PO4− as a substrate for the H2PO4− uptake system in angiosperms (Asher and Reay, 1979; Ullrich-Eberius et al., 1989), fungi (Rothstein and Donovan, 1963; Jung and Rothstein, 1965; Beever and Burns, 1980), mosses (Wells and Richardson, 1985), lichens (Nieboer et al., 1984), and bacteria (Silver and Misra, 1988). Resistance mechanisms to H2AsO4− in the bacteria, Staphylococcus aureous and Eschericha coli, involve reducing cellular concentrations of As and rapidly effluxing them via a plasmid-encoded arsenical pump (Rosen, 1986; Silver and Misra, 1988). Meharg and Macnair (1990) demonstrated that H2AsO4− resistance in the grass H. lanatus was due to suppression of the high affinity H2PO4−uptake system in H2AsO4− tolerant plants, which led to reduced uptake of both H2PO4− and H2AsO4−.
Sharples et al. (2000) recently isolated populations of arsenate-resistant H. ericae from roots of C. vulgaris on As-contaminated mine spoil soils in southwestern England. Populations of the mycorrhizal fungus have evolved resistance to arsenate contamination in parallel with the host plant, and it seems likely that the presence of the mycorrhizal fungus in roots of C. vulgaris is essential its establishment and persistence on As-contaminated sites (Sharples et al., 2000). The present study investigated the mechanism of H2AsO4− resistance in an isolate of H. ericae from an As-contaminated mine site.
RESULTS
Effect of H2AsO4− and H3AsO3 on Biomass Production
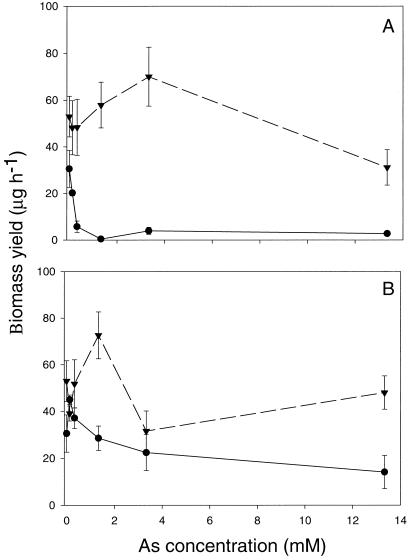
The heathland H. ericae isolate demonstrated significantly greater sensitivity to H2AsO4− and H3AsO3 than the mine site isolate (Fig. 1). Growth of the heathland isolate was almost completely inhibited at 1.33 mm H2AsO4− and above, whereas growth of the mine site isolate was inhibited by only 40% at the highest concentration tested (13.3 mm) (Fig. 1A). Biomass yield of the heathland isolate was 15 μg h−1 in the presence of 13.3 mm H3AsO3, whereas the mine isolate produced a mean biomass yield of approximately 45 μg h−1 at the same H3AsO3 concentration (Fig. 1B). Growth of both isolates was more severely affected by the presence of H2AsO4− than H3AsO3.
Figure 1.
A, Growth of mine site (▾) and heathland (●) H. ericae over a range of H2AsO4− concentrations. Bars = mean ± se (n = 3). B, Growth of mine site (▾) and heathland (●) H. ericae over a range of H3AsO3 concentrations. Bars = mean ± se (n = 3).
Kinetics of High Affinity H2AsO4− and H2PO4− Uptake
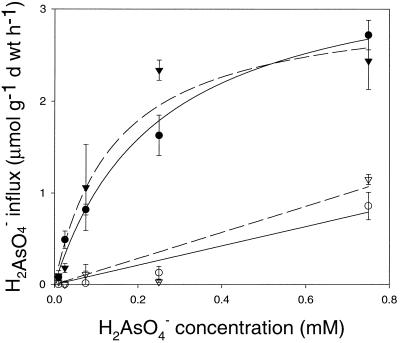
In H2PO4−-deficient tissue the rate of H2AsO4− and H2PO4− uptake was dependent on concentration, and the uptake of both ions displayed saturation kinetics (Figs. 2 and 3). Single Michaelis-Menten functions were fitted to the data, representing the high affinity uptake carrier, which would predominate at low-substrate concentrations used here (Meharg and Macnair, 1990). Kinetics of both H2AsO4− and H2PO4− uptake were similar for the two H. ericae isolates (Table I). There was no significant difference in the uptake of H2AsO4− between the heathland and mine site H. ericae isolates, as determined by ANOVA (data not shown). Growth in the presence of 5 μm H2PO4− prior to uptake suppressed H2AsO4− uptake in both the mine site and heathland isolates (Fig. 2). At 0.75 mm H2AsO4− in inorganic phosphate-sufficient tissue, the rate of H2AsO4− uptake was approximately 2 to 3 times lower than the rate of uptake in the absence of H2PO4− (Fig. 2). Michaelis-Menten kinetic parameters could not be determined for isolates precultured on high concentration H2PO4− media, and a linear regression was fitted to the data (Fig. 2).
Figure 2.
H2AsO4− influx in the absence of H2PO4− for mine site (▾, dashed line) and heathland (●, solid line) H. ericae. H2AsO4− influx after growth in the presence of 5 mmol−3 H2PO4− for mine site H. ericae (▿, dashed line) and heathland H. ericae (○, solid line). Bars = mean ± se (n = 3).
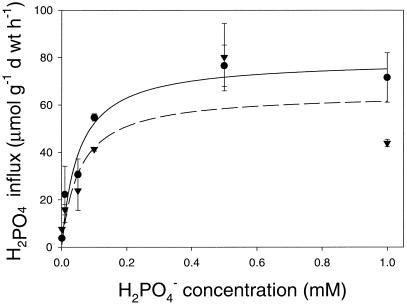
Figure 3.
H2PO4− influx for mine site (▾, dashed line) and heathland (●, solid line) H. ericae. Bars = mean ± se (n = 3).
Table I.
Kinetic parameters for H2AsO4−, H3AsO3−, and H2PO4− influx in mine site and heathland H. ericae
| H. ericae Isolate | Species | Regression | Y0 | A | Km | Vmax | r2 | P |
|---|---|---|---|---|---|---|---|---|
| mol m−3 | μmol g−1 dry wt h−1 | |||||||
| Mine | H3AsO3 | Hyperbola | 3.5 ± 4.6 | 0.86 ± 0.91 | 0.9736 | <0.0001 | ||
| Mine | H3AsO3 | Linear | 0.0068 ± 0.0068 | 0.19 ± 0.014 | 0.9729 | <0.0001 | ||
| Mine | H2AsO4 | Hyperbola | 0.14 ± 0.06 | 3.1 ± 0.5 | 0.9432 | 0.0058 | ||
| Mine | H2PO4− | Hyperbola | 0.055 ± 0.048 | 64.9 ± 13.9 | 0.7734 | 0.0209 | ||
| Heathland | H3AsO3 | Hyperbola | 0.72 ± 0.60 | 0.40 ± 0.17 | 0.9297 | 0.0005 | ||
| Heathland | H3AsO3 | Linear | 0.024 ± 0.016 | 0.22 ± 0.033 | 0.9014 | 0.0011 | ||
| Heathland | H2AsO4 | Hyperbola | 0.28 ± 0.07 | 3.67 ± 0.37 | 0.9887 | 0.0005 | ||
| Heathland | H2PO4− | Hyperbola | 0.051 ± 0.017 | 79.12 ± 6.51 | 0.9571 | 0.0007 |
Figures represent kinetic parameters ± se (n = 3). P is the probability of the source term not being significant.
Increasing H2PO4− concentrations up to 0.1 mm resulted in an increase in the rate of H2PO4− uptake in both isolates (Fig. 3). At concentrations above 0.1 mm, H2PO4− uptake was not further enhanced. Apparent kinetic parameters for both isolates were similar (Table I). There was no significant difference between the rates of H2PO4− uptake in the heathland or mine site isolate as determined by ANOVA (Minitab, SPSS, Chicago).
In comparison with the rate of H2AsO4− uptake, H2PO4− uptake was much greater in both H. ericae isolates at all concentrations. Both isolates demonstrated a much higher Vmax and a lower Km value for H2PO4− in comparison with H2AsO4− (Table I), indicating a much higher affinity for H2PO4− uptake.
Repression of H2AsO4− and H2PO4− Uptake
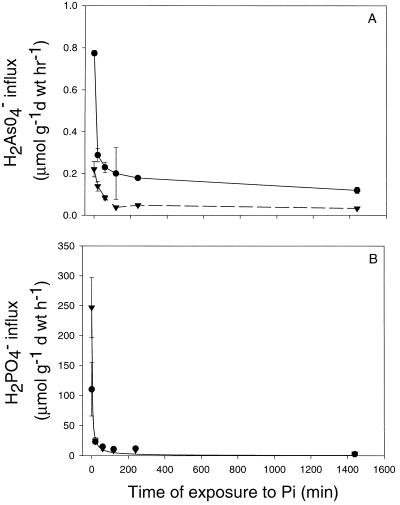
Repression of H2AsO4− uptake by H2PO4− and H2PO4− uptake by H2AsO4− was investigated at fixed concentrations. Uptake of 0.75 mm H2AsO4− from solution in heathland and mine site H. ericae isolates was reduced by pre-exposure of fungal mycelium to H2PO4− (5.0 μm H2PO4−). The initial rate of H2AsO4− uptake in the absence of H2PO4− in mine site H. ericae was 0.22 μmol g−1 dry weight h−1, which was lower than the initial rate of uptake in the heathland isolate (Fig. 4A). After 20 min of exposure to H2PO4−, a rapid decrease in H2AsO4− uptake was observed for both isolates with mine site and heathland isolates demonstrating uptake rates of 0.14 and 0.29 μmol g−1 dry weight h−1, respectively. After 2 h of H2PO4− uptake, H2AsO4− uptake by both isolates was almost completely suppressed (Fig. 4A).
Figure 4.
A, Uptake of 0.75 mm H2AsO4− in mine site (▾) and heathland (●) H. ericae after periods of exposure to 5.0 μm H2PO4−. Bars = mean ± se (n = 3). Second order decay curves were fitted to the data (Sigma Plot, Jandel Scientific). B, Uptake of 0.1 mm H2PO4− in mine site (▾) and heathland (●) H. ericae after periods of exposure to 0.75 mm H2AsO4−. Bars = mean ± se (n = 3). Second order decay curves were fitted to the data (Sigma Plot, Jandel Scientific).
Uptake of 0.1 mm H2PO4− was repressed by pre-exposure to 0.75 mm H2AsO4− (Fig. 4B). Initially, the rate of H2PO4− exposure in the heathland isolate was greater than the mine site isolate, however on exposure to H2AsO4−, rates of H2PO4− uptake decreased in both isolates (Fig. 4B). After 20 min of exposure to H2AsO4−, the rate of H2PO4− uptake was suppressed considerably, however, after 24 h of exposure to H2AsO4−, H2PO4− uptake in both isolates was completely inhibited (Fig. 4B). There was no difference between the effects of H2AsO4− on H2PO4− uptake and H2PO4− on H2AsO4− uptake between the heathland and mine site isolate.
Efflux of As from Fungal Cells
In the methylation experiment, As was not present in the HgCl2 traps for either mine site or heathland isolates. Because HgCl2 complexes all volatile methylated arsines as well as AsH3, this indicates that neither isolate methylated H2AsO4− (data not shown).
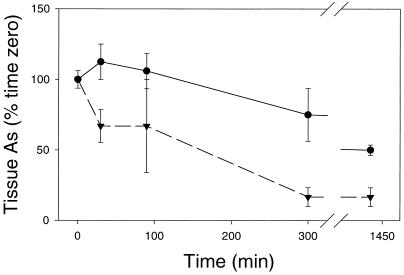
Efflux of As from mine site H. ericae mycelia was more rapid than for the heathland isolate (Fig. 5). After 1 h of incubation in 0.75 mm H2AsO4− (to load cells with As) and transfer to H2AsO4−-free media for time periods of up to 24 h, As concentration in the mine site isolate tissue decreased significantly (P < 0.001) (Fig. 5). After 5 h in H2AsO4−-free media, the mine site isolate lost 83% of its initial As concentration in comparison with the heathland isolate, which lost 13%, showing enhanced As cell efflux in the mine site H. ericae isolate. Similar trends were found after loading for 10 min, 20 min, and 4 h exposure to 0.75 mm H2AsO4− (data not shown). Of the total As effluxed from cells, 71.6% was H3AsO3 with the remainder of As being lost as H2AsO4−. The majority of As lost from the heathland isolate similarly was in the form of H3AsO3 (71.3%). These results indicate an enhanced H3AsO3 efflux mechanism in the mine site H. ericae isolate.
Figure 5.
As content of mine site (▾) and heathland (●) H. ericae tissue over time as a percentage of total As content after incubation in 0.75 mm H2AsO4−. Bars = mean ± se (n = 3).
Uptake of H2AsO4− over Time
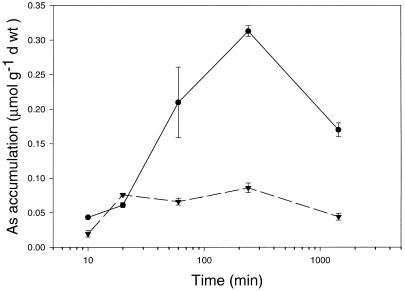
The long-term uptake of H2AsO4− was much greater in the heathland H. ericae isolate than the mine site isolate (Fig. 6). Accumulation of H2AsO4− by the heathland isolate did not differ significantly from linearity with respect to time (r2 = 0.973) over 2 h, however, after 2 h of exposure, decreased accumulation was observed (Fig. 6). This decrease in As accumulation after 2 h seems likely to reflect a response to H2AsO4− toxicity. In contrast, while the mine site isolate demonstrated increased accumulation of As over 20 min there was no increase in accumulation after 2 h and this was sustained for up to 24 h.
Figure 6.
Long-term accumulation of 0.75 mm H2AsO4− in mine site (▾) and heathland (●) H. ericae. Bars = mean ± se (n = 3).
Uptake of H3AsO3
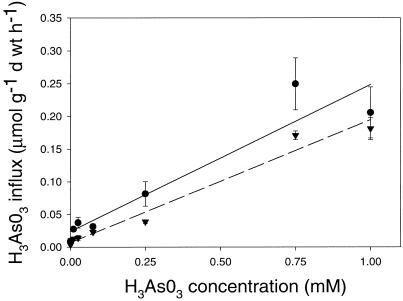
The rates of H3AsO3 uptake by heathland and mine site H. ericae isolates were similar with uptake in both isolates increasing linearly in response to increasing H3AsO3 concentrations (Fig. 7). Although Michaelis-Menten functions could be fitted to the data, linear models demonstrated the best fits (Table I). Uptake of H3AsO3 in mine site and heathland H. ericae isolates at low H3AsO3 concentrations (0.01 mm H3AsO3) was 3- to 4-fold less than the rate of H2AsO4− uptake at the same concentration (Figs. 2 and 6). At 0.75 mm H3AsO3, the rate of H3AsO3 uptake was 15 times less than the rate of H2AsO4− uptake with both isolates have a lower affinity for H3AsO3 than H2AsO4−.
Figure 7.
H3AsO3 influx for mine site (▾) and heathland (●) H. ericae. Bars = mean ± se (n = 3). Linear regressions were fitted to the data (Sigma Plot, Jandel Scientific).
DISCUSSION
Populations of H2AsO4−-resistant H. ericae have been isolated from As/Cu mine soils (Sharples et al., 2000), where soil solution H2AsO4− content is 20 to 75 times higher than natural heathland soils that have not been contaminated by mining and other industrial processes. The present study demonstrates increased H2AsO4− and H3AsO3 resistance in an isolate of H. ericae obtained from an As/Cu mine site in comparison with an isolate from an uncontaminated heathland site. Growth of the heathland isolate was completely inhibited at 1.33 mm H2AsO4−, whereas the mine site H. ericae isolate continued growth at the highest concentration tested (13.3 mm) (Fig. 1A). H3AsO3 is usually considered to be the most toxic form of As available to plants and fungi, however both isolates of H. ericae demonstrated greater sensitivity to H2AsO4− (Fig. 1), although rates of growth of both isolates were similar in the absence of As.
H2AsO4− behaves as a H2PO4− analog (Meharg and Macnair, 1990) and is accumulated by the H2PO4− transport system in a wide range of organisms (Meharg and Macnair, 1992). Inorganic phosphate transport across membranes is carrier-mediated and described by Michaelis-Menten kinetics (Beever and Burns, 1980). Adaptation of the H2PO4− uptake system is a mechanism of H2AsO4− resistance in the grasses H. lanatus, D. cespitosa, and A. capillaris (Meharg and Macnair, 1990, 1991, 1992, 1994). In H. lanatus, H2AsO4− resistance is achieved by constitutive suppression of the high-affinity uptake system by carrier synthesis inhibition and is independent of plant phosphorus status (Meharg and Macnair, 1992). The present study demonstrates similar apparent Km and Vmax parameters for H2AsO4− transport and H2PO4− transport in H2AsO4−-resistant and -non-resistant H. ericae (Table I). Mine site and heathland H. ericae apparent Km values (0.14 ± 0.06 mm and 0.28 ± 0.07 mm, respectively) were higher than values previously reported for H2AsO4−-resistant H. lanatus (0.074 mm) (Meharg and Macnair, 1992) and other fungi (Sharples et al., 1999). For example, Saccharomycetes cerevisiae has a Km for H2AsO4− of 0.004 mm (Jung and Rothstein, 1965), and Candida tropicalis has a Km value of 0.005 mm (Beever and Burns, 1980). The apparent Vmax value for H2AsO4− uptake obtained for H. ericae (Table I) was similar to those reported for S. cerevisiae (10.2 μmol g−1 dry weight h−1), lower than the ectomycorrhizal fungus Hebeloma crustuliniforme (Sharples et al., 1999), yet higher than those found in H2AsO4−-resistant H. lanatus (Meharg and Macnair, 1990).
Both isolates demonstrated similar H3AsO3 transport kinetics, however influx was markedly lower than when compared with the rate of H2AsO4− uptake (Figs. 2 and 7). This decrease was expected because H3AsO3 is not a H2PO4− analog and is therefore not transported by H2PO4− carriers. It is not known how H3AsO3 enters cells, however the present study seems to indicate passive diffusion (Fig. 7).
Pregrowth of H. ericae in the presence of 5 mm H2PO4− significantly suppressed the uptake of H2AsO4− for both mine site and heathland isolates (Fig. 2). Suppression of H2AsO4− uptake by long-term exposure to H2PO4− has also been demonstrated in the plant Hordum vulgare and Silene vulgaris (Lee, 1982; Paliouris and Hutchinson, 1991). In the case of H. vulgare, plants grown in the presence and absence of 0.5 mm H2PO4−, demonstrated H2AsO4− uptake rates of 27.7 and 81.6 nmol g−1 fresh weight h−1, respectively (Lee, 1982). Meharg and Macnair (1991) suggest that H2AsO4− resistance in H. lanatus is due to a decrease in the concentration of protein carriers in the plasma membrane rather than a change in the carrying capacity of the protein.
H2AsO4− uptake was rapidly repressed on exposure to H2PO4− in both mine site and heathland H. ericae (Fig. 4). Similarly, the presence of H2AsO4− rapidly repressed H2PO4− uptake (Fig. 4). Rapid repression of the high affinity H2PO4− uptake system in plants under high plant H2PO4− status has long been reported (Meharg and Macnair, 1992). The nature of this repression differs for different species. Repression may occur by a decrease in Vmax with little or no change in the Km, which occurs in algae (McPharlin and Bieleski, 1987), bacteria (Beever and Burns, 1980), and selected plants (Anghinoni and Barber, 1980; Lee, 1982; Cogliatti and Santa Maria, 1990; Jungk et al., 1990). Repression of the high affinity uptake system in the vesicular arbuscular mycorrhizal fungus Gigaspora margarita is achieved by increase in the apparent Km with little change in the apparent Vmax (Thompson et al., 1990), whereas repression in the plant, Solanum tuberosm, is achieved by both an increase in apparent Km and a decrease in apparent Vmax (Cogliatti and Clarkson, 1983). Changes in H2PO4− uptake with changing H2PO4− status may be under allosteric control (Levebvre and Glass, 1982; Schorring and Jensen, 1984) and by the synthesis and breakdown of transport sites (Jeanjean, 1973; Drew et al., 1984). Because the speed of repression is too rapid to be explained by protein turnover, under short exposure times it is likely that for H. ericae both H2PO4− and H2AsO4− act allosterically.
Short-term uptake of H2AsO4− was similar between isolates, however in the longer term, accumulation of As by the heathland isolate decreased significantly in 24 h (Fig. 6). Such a decrease in the rate of As accumulation in the heathland isolate may reflect cell death in response to H2AsO4− toxicity. Arsenate causes toxicity in fungi and plants by interfering with aerobic phosphorylation, following intracellular reduction of H2AsO4− to H3AsO3, which then breaks down protein sulfydryl groups (Ullrich-Eberius et al., 1989).
The present study demonstrates the ability of an isolate of H. ericae from a mine site to efflux H3AsO3 from its hyphae (Fig. 5), which may provide H2AsO4− resistance to this isolate. H3AsO3 efflux has been reported as a mechanism of H2AsO4− resistance in the bacterium S. aureous (Broer et al., 1993) and the yeast S. cerevisiae (Wysocki et al., 1997) and contrasts to the mechanism of resistance reported in higher plants (Meharg and Macnair, 1990). Arsenate resistance in S. cerevisiae is mediated by an H3AsO3 transporter (Wysocki et al., 1997), and in the case of S. aureous, intracellular H2AsO4− is reduced to H3AsO3 before being actively exported from the cells (Broer et al., 1993). The present study suggests a similar mechanism of As resistance in H. ericae at As-contaminated mine sites. The steady state of As accumulation observed in the mine site isolate after 20 min is probably not due to a suppression of the high affinity uptake system but rapid internal reduction of H2AsO4− to H3AsO3, which then initiates efflux of H3AsO3 from the hyphae. The ability of the mine site H. ericae isolate to efflux H3AsO3from cells into the surrounding soil indicates a need for enhanced resistance to H3AsO3. Arsenate was much more toxic to H. ericae than H3AsO3 (Fig. 1), which supports efflux of H3AsO3 as the mechanism of H2AsO4− resistance in H. ericae.
The mechanism of H2AsO4− resistance we have described in H. ericae is likely to be of ecological importance for its host plant (C. vulgaris) on contaminated mine sites. Arsenite efflux enables the fungus to retain its ability to transport inorganic phosphate from the soil (much of which will, in turn, is transferred to the host plant), whereas effluxing absorbed H2AsO4−. The fungus may thus act as a filter to maintain low plant As levels, while maintaining an adequate supply of phosphorus to the host (Sharples et al., 2000). Efflux of H3AsO3 from the fungal cells into the soil also ensures that re-absorption of As from the soil is limited.
MATERIALS AND METHODS
Fungal Material
The arsenate-resistant Hymenoscyphus ericae genotype was isolated from roots of Calluna vulgaris obtained from the Gawton United mine (Devon, S.W. UK) whereas the non-resistant genotype was obtained from C. vulgaris roots from an uncontaminated natural heathland site at Aylesbeare Common (Devon, S.W. UK). These fungal isolates were randomly selected from approximately 25 isolates from each site and were previously identified by PCR-RFLP analysis (Sharples et al., 2000). Although the characteristics of arsenate absorption and arsenite efflux have only been studied in detail for single isolates from each population, preliminary experiments for absorption and efflux by further isolates indicate consistent patterns for absorption/efflux within the mine site or heathland populations (data not shown). The fungi were maintained on modified Melin Norkrans agar medium (MMN) containing: 3.79 mm (NH4)2HPO4; 2.21 mm KH2PO4; 0.57 mm MgSO4·7H2O; 0.23 mm CaCl2·6H2O; 0.43 mm NaCl; 0.034 mm FeEDTA; 55.5 mm d-Glc; and 0.3 μm thiamine, adjusted to pH 5.5, and incubated at 25°C. In H2AsO4− and H3AsO3 uptake experiments, mycelia were grown in liquid MMN for 17 d and transferred to H2PO4−-free MMN for 48 h prior to uptake analysis. To determine the effects of H2PO4− on H2AsO4− uptake, mycelia were grown in liquid MMN containing 5 μm H2PO4− for 17 d before H2AsO4− uptake. Mycelia for H2PO4− uptake studies were grown on liquid MMN containing 0.01 mm H2PO4− for 17 d at 25°C.
Effect of H2AsO4− and H3AsO3 on Biomass Production
Two plugs (6-mm diameter) of each H. ericae isolate were cut from the edge of actively growing mycelia on MMN and inoculated into 9-cm-diameter Petri dishes containing 25 mL of liquid MMN. After 11 d of incubation at 25°C, fungal plugs were transferred to 25 mL of liquid MMN supplemented with either H2AsO4− or H3AsO3, supplied as Na2HAsO4 and NaAsO2, respectively, at concentrations of 0, 0.67, 1.33, and 4.67 mm. For all treatments, H2PO4− concentration in the medium was adjusted to 0.01 mm. After 7 d of incubation, mycelial mats were removed from basal medium, dried overnight (80°C), and the biomass increase determined gravimetrically. All treatments were replicated three times.
Kinetics of H2AsO4− and H2PO4− Uptake
To determine H2AsO4−, H3AsO3, and H2PO4− uptake, three replicate mycelial mats of each isolate were incubated in 25 mL of aerated test solution for 20 min (except when uptake was determined with respect to time). Test solutions contained 10 mm 2-(N-morpholino) ethanesulfonic acid (MES), 0.5 mm Ca(NO3)2, and different concentrations of either H2AsO4−, H3AsO3, or H2PO4− in the form of Na2HAsO4·7H20, NaAsO2, and Na2HPO4, respectively. In the experiments to determine the rate of H2PO4− uptake, [32P] (as NaH2PO4, supplied by Amersham) was added to give an activity of 37 kBq mL−1. Using the methodology of Meharg and Macnair (1990), uptake was terminated by rinsing mycelia in 25 mL of an ice-cold solution containing 1 mm Na2HPO4, 10 mm MES, and 0.5 mm Ca(NO3)2. Mycelia were transferred to 25 mL of an aerated ice-cold solution of the same composition for 10 min to ensure desorption of H2AsO4−, H3AsO3, or [32P] from the cell-free space. Mycelia were dried (80°C, 24 h) and biomass determined gravimetrically before analysis.
Repression of H2PO4− and H2AsO4− Uptake
To investigate the effects of H2PO4− on H2AsO4− uptake, three replicate mycelial mats of each isolate were pre-incubated in liquid MMN containing 5 μm H2PO4− for 0, 20, 60, 120, 240, or 1,440 min prior to H2AsO4− uptake (as described above). The effects of H2AsO4− on H2PO4− uptake were determined by pre-incubating three replicate mycelia in 0.75 μm H2AsO4− for 0, 20, 60, 120, 240, or 1,440 min before exposure to [32P]-uptake solution.
Methylation and Efflux of As by Fungi
Methylation of H2AsO4− was investigated using a modified method of Gates et al. (1997), involving the chemofocussing of volatile As species on mercuric chloride. Conical flasks were inoculated with 20 mL of a solution containing 10 mm MES, 0.5 mm Ca(NO3)2, and 0.67 mm H2AsO4− for mine site H. ericae mycelia and 0.27 mm H2AsO4− for heathland H. ericae. These H2AsO4− concentrations were the approximate H2AsO4− EC50 values of the mine and heathland population (preliminary data by Sharples et al., 2000) (data not shown). Polyurethane plugs were soaked in 0.1 m HgCl2 and oven dried at 50°C for 12 h before being placed inside a glass condenser fitted to the conical flasks. Three replicate flasks were set up for each isolate. The presence of As was indicated by brown discoloration of the HgCl2 plugs and after 24 h of incubation, HgCl2 plugs were removed and analyzed for As by atomic absorption spectrometry.
To investigate the mechanism of H2AsO4− resistance, mycelia of each isolate (n = 3) were exposed to H2AsO4− uptake solution for 10 min, 20 min, 1 h, 4 h, or 24 h. After termination of uptake, mycelia were transferred to 25 mL of liquid MMN containing no H2PO4− for 0, 30 min, 90 min, 5 h, or 24 h. Mycelia were then dried (80°C, 24 h) and biomass determined gravimetrically before As analysis.
Speciation of As
Three replicate mycelia of each isolate were incubated in 0.75 mm H2AsO4− uptake solution for 1 h. Uptake was terminated and mycelia transferred to a 2-mL test tube containing 1 mL of H2PO4−-free liquid MMN. Fresh liquid MMN was continually pumped into and out of the test tube at a flow rate of 0.7 mL min−1 and removed from the tube at the same rate for 5 h. After 5 h, fungal material was dried, digested, and analyzed for As. Two milliliters of MMN pumped from the test tube was also analyzed for H2AsO4− and H3AsO3 using atomic absorption spectrometry (Glaubig and Goldberg, 1988).
Analysis
To determine [32P]H2PO4− uptake, dried fungal mycelia were placed in 20-mL glass scintillation vials, to which 10 mL of deionized water was added. [32P]-activity was determined by Cherenkov counting using a Tri Carb 2100TR liquid scintillation counter (Packard Bell, Sacramento, CA) with data corrected for quenching.
As was determined by digesting mycelia in 2 mL of concentrated nitric acid (Aristar grade) using a block digester, for 1 h at 120°C followed by 1 h at 180°C, to evaporate the samples to dryness. The As residue was redissolved in 20 mL of a solution containing 5% (v/v) HCl (Analar grade) containing 20 mm potassium iodide. The amount of As present in the digests was determined using hydride generation interfaced with an atomic absorption spectrometer (ThermoUnicam Solaar 929, Cambridge, UK). As species were determined using pH selectivity, H2AsO43− reduced to arsine (AsH3) by NaBH4 at pH < 6, whereas H3AsO3 reduced to AsH3 at pH 7.
Statistical Analysis
Data were analyzed by ANOVA using the computer package Minitab v. 11 (Minitab, State College, PA). Curve fitting was carried out using the fitting regimes within the computer package Sigma Plot (Jandel Scientific, Erkrath, Germany), which uses the Marquardt non-linear curve fitting algorithm (Marquardt, 1963).
LITERATURE CITED
- Anghinoni I, Barber SA. Phosphorus influx and growth characteristics of corn roots as influenced by P supply. Agron J. 1980;72:685–688. [Google Scholar]
- Asher CJ, Reay PF. Arsenic uptake by barley seedlings. Aust J Plant Physiol. 1979;6:459–466. [Google Scholar]
- Beever RE, Burns DWJ. Phosphorus uptake, storage and utilization by fungi. Adv Bot Res. 1980;8:127–219. [Google Scholar]
- Broer S, Ji G, Broer A, Silver S. Arsenic efflux governed by the arsenic resistance determinant of Staphylococcus aureus lasmid pI258. J Bacteriol. 1993;175:3480–3485. doi: 10.1128/jb.175.11.3480-3485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliatti DH, Clarkson DT. Physiological changes in, and phosphate uptake by potato plants during development of, and recovery from phosphate deficiency. Physiol Plant. 1983;58:287–294. [Google Scholar]
- Cogliatti DH, Santa Maria GE. Influx and efflux of phosphate in roots of wheat plants in non-growth limiting conditions. J Exp Bot. 1990;41:601–607. [Google Scholar]
- Colbourn P, Alloway BJ, Thornton I. Arsenic and heavy metals in soils associated with regional geochemical anomalis in South-West England. Sci Total Environ. 1975;4:359–363. [Google Scholar]
- Drew MC, Saker LR, Barber SA, Jenkins WC. Changes in the kinetics of phosphate and potassium absorption in nutrient-deficient barley roots measured by a solution-depletion technique. Planta. 1984;160:490–499. doi: 10.1007/BF00411136. [DOI] [PubMed] [Google Scholar]
- Fergusson JE. The Heavy Elements: Chemistry, Environmental Impact and Health Effects. Oxford: Pergamon Press; 1990. [Google Scholar]
- Gates PN, Harrop HA, Pridham JB, Smethurst B. Can microorganisms convert antimony trioxide or potassium antimonyl tartrate to methylated stibines? Environ Sci Technol. 1997;205:215–221. doi: 10.1016/s0048-9697(97)00203-9. [DOI] [PubMed] [Google Scholar]
- Glaubig RA, Goldberg S. Determination of inorganic arsenic (III) and arsenic (III plus V) using automated hydride-generation atomic-absorption spectrometry. Soil Sci Soc Am J. 1988;52:536–537. [Google Scholar]
- Jeanjean R. The relationship between the rate of phosphate absorption and protein synthesis during phoshate starvation in Chlorella pyrenoidosa. FEBS Lett. 1973;32:149–151. doi: 10.1016/0014-5793(73)80759-8. [DOI] [PubMed] [Google Scholar]
- Jung C, Rothstein A. Arsenate uptake and release in relation to the inhibition of transport and glycolysis in yeast. Biochem Pharmacol. 1965;14:1093–1112. doi: 10.1016/0006-2952(65)90039-0. [DOI] [PubMed] [Google Scholar]
- Jungk A, Asher CJ, Edwards DG, Meyer D. Influence of phosphate status on phosphate uptake kinetics of maize (Zea mays) and soybean (Glycine max) Plant Soil. 1990;124:175–182. [Google Scholar]
- Lee RB. Selectivity of kinetics of ion uptake by barley plants following nutrient deficiency. Ann Bot. 1982;50:429–449. [Google Scholar]
- Levebvre D, Glass DM. Regulation of phosphate influx in barley roots: effects of phosphate deprivation and reduction of influx with provision of orthophosphate. Physiol Plant. 1982;54:199–206. [Google Scholar]
- Marquardt IR. An algorithm for least-squares estimation of non-linear parameters. J Soc Ind Appl Math. 1963;11:431–441. [Google Scholar]
- McPharlin IR, Bieleski RL. Phosphate uptake by Spirodela and Lemna during early stages of phosphate deficiency. Aust J Plant Physiol. 1987;14:561–572. [Google Scholar]
- Meharg AA, Macnair MR. An altered phosphate uptake system in arsenate tolerant Holcus lanatus. New Phytol. 1990;116:29–35. [Google Scholar]
- Meharg AA, Macnair MR. The mechanisms of arsenate tolerance in Deschampsia cespitosa (L.) Beauv. and Agrostis capillaris L. New Phytol. 1991;119:291–297. doi: 10.1111/j.1469-8137.1991.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Meharg AA, Macnair MR. Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot. 1992;43:519–524. [Google Scholar]
- Meharg AA, Naylor J, Macnair MR. Phosphorus nutrition of arsenate-tolerant and nontolerant phenotypes of velvetgrass. J Environ Qual. 1994;23:234–238. [Google Scholar]
- Nieboer E, Padovan D, Lavoie P. Anion accumulation by lichens: II. Competition and toxicity studies involving arsenate, phosphate, sulphate and sulphite. New Phytol. 1984;96:83–94. [Google Scholar]
- Paliouris G, Hutchinson TC. Arsenic, cobalt and nickel tolerances in two populations of Silene vulgaris (Moench.) Garcke from Ontario, Canada. New Phytol. 1991;117:449–459. doi: 10.1111/j.1469-8137.1991.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Rosen BP. Recent advances in bacterial ion transport. Annu Rev Microbiol. 1986;40:263–286. doi: 10.1146/annurev.mi.40.100186.001403. [DOI] [PubMed] [Google Scholar]
- Rothstein A, Donovan K. Interaction of arsenate with the phosphate-transporting system of yeast. J Gen Physiol. 1963;46:1075–1085. doi: 10.1085/jgp.46.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorring JK, Jensen P. Phosphorus nutrition of barley, buckwheat and rape seedlings: II. Influx and efflux of hosphorus by intact roots of different status. Physiol Plant. 1984;61:584–590. [Google Scholar]
- Sharples JM, Meharg AA, Chambers SM, Cairney JWG. Arsenate sensitivity in ericoid and ectomycorrhizal fungi. Environ Toxicol Chem. 1999;18:1848–1855. [Google Scholar]
- Sharples JM, Meharg AA, Chambers SM, Cairney JWG. The symbiotic solution to arsenic contamination. Nature. 2000;404:951–952. doi: 10.1038/35010193. [DOI] [PubMed] [Google Scholar]
- Silver S, Misra TK. Plasmid-mediated heavy metal resistances. Annu Rev Microbiol. 1988;42:717–743. doi: 10.1146/annurev.mi.42.100188.003441. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal Symbiosis. London: Academic Press; 1997. [Google Scholar]
- Thompson BD, Clarkson DT, Brain P. Kinetics of phosphate uptake by germ tubes of the viscular-arbuscular mycorrhizal fungus Gigaspora margarita. New Phytol. 1990;116:647–653. [Google Scholar]
- Ullrich-Eberius CI, Sanz A, Novacky AJ. Evaluation of arsenate- and vandate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba GI. J Exp Bot. 1989;40:119–128. [Google Scholar]
- Wells JM, Richardson DHS. Anion accumulation by the moss Hylocomium splendens: uptake and competition studies involving arsenate, selenate, selenite, phosphate, sulphate, and sulphite. New Phytol. 1985;101:571–583. [Google Scholar]
- Wysocki R, Bobrowicz P, Ulaszewski S. The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J Biol Chem. 1997;272:30061–30066. doi: 10.1074/jbc.272.48.30061. [DOI] [PubMed] [Google Scholar]