ABSTRACT
A major obstacle to development of an effective AIDS vaccine is that along with the intended beneficial responses, the immunization regimen may activate CD4+ T cells that can facilitate acquisition of human immunodeficiency virus (HIV) by serving as target cells for the virus. Lu et al. (W. Lu et al., Cell Rep 2:1736–1746, 2012, https://doi.org/10.1016/j.celrep.2012.11.016) reported that intragastric administration of chemically inactivated simian immunodeficiency virus SIVmac239 and Lactobacillus plantarum (iSIV-L. plantarum) protected 15/16 Chinese-origin rhesus macaques (RMs) from high-dose intrarectal SIVmac239 challenge at 3 months postimmunization. They attributed the observed protection to induction of immune tolerance, mediated by “MHC-Ib/E-restricted CD8+ regulatory T cells that suppressed SIV-harboring CD4+ T cell activation and ex vivo SIV replication in 15/16 animals without inducing SIV-specific antibodies or cytotoxic T.” J.-M. Andrieu et al. (Front Immunol 5:297, 2014, https://doi.org/10.3389/fimmu.2014.00297) subsequently reported protection from infection in 23/24 RMs immunized intragastrically or intravaginally with iSIV and Mycobacterium bovis BCG, L. plantarum, or Lactobacillus rhamnosus, which they ascribed to the same tolerogenic mechanism. Using vaccine materials obtained from our coauthors, we conducted an immunization and challenge experiment with 54 Indian RMs and included control groups receiving iSIV only or L. plantarum only as well as unvaccinated animals. Intrarectal challenge with SIVmac239 resulted in rapid infection in all groups of vaccinated RMs as well as unvaccinated controls. iSIV-L. plantarum-vaccinated animals that became SIV infected showed viral loads similar to those observed in animals receiving iSIV only or L. plantarum only or in unvaccinated controls. The protection from SIV transmission conferred by intragastric iSIV-L. plantarum administration reported previously for Chinese-origin RMs was not observed when the same experiment was conducted in a larger cohort of Indian-origin animals.
IMPORTANCE Despite an increased understanding of immune responses against HIV, a safe and effective AIDS vaccine is not yet available. One obstacle is that immunization may activate CD4+ T cells that may act as target cells for acquisition of HIV. An alternative strategy may involve induction of a tolerance-inducing response that limits the availability of activated CD4+ T cells, thus limiting the ability of virus to establish infection. In this regard, exciting results were obtained for Chinese-origin rhesus macaques by using a “tolerogenic” vaccine, consisting of intragastric administration of Lactobacillus plantarum and 2,2′-dithiodipyridine-inactivated SIV, which showed highly significant protection from virus transmission. In the present study, we administered iSIV-L. plantarum to Indian-origin rhesus macaques and failed to observe any protective effect on virus acquisition in this experimental setting. This work is important because it contributes to the overall assessment of the clinical potential of a new candidate AIDS vaccine platform based on iSIV-L. plantarum.
KEYWORDS: SIV vaccine, oral vaccines
INTRODUCTION
With an estimated 37 million individuals infected with human immunodeficiency virus (HIV) worldwide and no cure for this infection, the development of a safe and effective vaccine remains a key priority in contemporary research on HIV/AIDS. However, the enormous variability of HIV in the human population, the flexible, mutation-tolerant structure of the HIV envelope (Env) protein, the ability of HIV to rapidly escape cellular immune responses, and the persistence of the virus in an immunologically silent latent form have created unique scientific challenges to the design of a successful HIV vaccine that have not yet been overcome (1, 2). In addition, the design of an effective AIDS vaccine is further complicated by the fact that candidate immunization regimens may result in the activation and expansion of CD4+ cells in mucosal tissues that are the portal of entry for HIV. These vaccine-induced activated CD4+ T cells often express the main HIV coreceptor, CCR5, and therefore have the potential to act as targets for the virus, thereby increasing the risk of its acquisition (3, 4). Altogether, these complex, multifaceted biological challenges have so far precluded the successful clinical development of an AIDS vaccine.
The current paradigm in the field of HIV vaccinology is predicated on the premise that optimal immunogens must elicit high and durable titers of broadly neutralizing HIV-1 Env-specific antibodies in concert with robust antiviral cellular immune responses and in the absence of major CD4+ T cell activation in mucosal tissues to achieve meaningful protection (1, 2). While some protection from virus transmission and/or early replication has been observed with several immunization regimens in preclinical studies of rhesus macaques (RMs) of Indian origin, a model of HIV infection widely used in the United States, none of these approaches has yet proved to be consistently successful in humans. More recently, a very intense research effort has focused on the design of HIV Env immunogens that can elicit the production of broadly neutralizing antibodies (bnAbs). This effort was prompted by the observations that (i) a number of bnAbs targeting different epitopes in the Env protein have been observed in the sera of a subset of HIV-infected individuals and (ii) the passive administration of such bnAbs to RMs has conferred strong protection from intravenous and mucosal simian-human immunodeficiency virus (SHIV) challenges (5–7). However, the generation of durable high titers of bnAbs in healthy humans or RMs after administration of specific HIV Env-based immunogens has so far been elusive, and it appears that a deeper understanding of the molecular mechanisms responsible for the generation of these bnAbs is needed in order to generate such effective immunogens.
Given these premises, it is not surprising that the results of clinical trials aimed at testing the efficacy of candidate HIV/AIDS vaccines in humans have so far been disappointing. Earlier clinical trials, such as the AIDSVAX (based on recombinant gp120-Env), Step and Phambili (both based on human adenovirus 5 [Ad5] vectors expressing the HIV antigens Gag, Pol, and Nef), and HVTN-505 (based on DNA expressing HIV antigens Gag, Pol, Nef, and Env and Ad5 vectors expressing a Gag-Pol fusion protein and Env) trials, showed no protection from HIV acquisition (8, 9). Note that the Step, Phambili, and HVTN-505 trials all showed a trend toward increased risk of HIV infection in vaccinated individuals. While the mechanisms responsible for this effect remain unclear, it has been proposed that the vectors used for these trials increased the level of CD4+ T cell activation in mucosal tissues, as observed in preclinical studies of similar vectors in RMs (3). In contrast, the RV-144/Thai trial, which tested an immunization regimen consisting of a “prime” vaccine called ALVAC-HIV (vCP1521) followed by a boost with the AIDSVAX gp120 envelope protein (subtypes B and E), showed limited (∼31% [P = 0.039] according to a “modified intent-to-treat” statistical analysis, 26.4% [P = 0.08] according to an “intent-to-treat” statistical analysis, and 25% [P = 0.16] according to a “per-protocol” statistical analysis) but significant protection from HIV infection in a population of low-risk individuals (10). While the results of the RV144 trial have widely been seen as encouraging, the relatively low and transient level of protection, which appeared to fade after 4 to 6 months, clearly indicates that more candidate HIV vaccines, both concepts and products, should be developed and tested in preclinical models (11).
In this study, we tested an innovative vaccine concept based on immune modulation that was originally developed by the laboratory of Jean-Marie Andrieu (12). The rationale for this approach is the idea that an infectious inoculum must find permissive target cells in order to establish a systemic and spreading infection in the host. For HIV and simian immunodeficiency virus (SIV), the preferred permissive target cells are activated CD4+ T cells that also express CCR5, and upon initial mucosal infection, inflammatory immune responses recruit additional activated CD4+ T cells in a process that may contribute to the ability of the initial infection to expand, spread, and disseminate throughout the host. A conceptually alternative vaccination paradigm may therefore involve induction of a tolerance-inducing response that limits the availability of susceptible activated CD4+ T cells, thus limiting the ability of the inoculum to establish a spreading infection. In the original study by Lu et al. (12), intragastric vaccination of Chinese-origin RMs was performed with a combination of Lactobacillus plantarum, a commensal bacterium that favors immune tolerance, and 2,2′-dithiodipyridine (Aldrithiol-2)-inactivated SIV (iSIV). The hypothesis was that L. plantarum would promote immune tolerance to SIV, thus preventing the establishment of SIV infection by lowering the number of activated CD4+ target cells. In the study of Lu et al., the vaccine-induced protective effects were attributed to CD8+ regulatory T cells that suppressed CD4+ T cell activation and ex vivo SIV replication in 15 of 16 RMs without inducing SIV-specific antibodies or robust cytotoxic T lymphocyte (CTL) responses. Of 16 Chinese-origin RMs that were challenged intrarectally at a high dose (i.e., 100,000 50% tissue culture infective dose [TCID50]) with the vaccine-homologous virus SIVmac239 or the heterologous strain SIVB670, 15 showed sterile protection. Moreover, for four animals that were rechallenged intravenously, plasma SIV levels peaked slightly and then dropped to undetectable levels.
In addition, the Andrieu-Lu team asked an independent expert (Gianfranco Pancino of the Pasteur Institute) to confirm these results by rechallenging, with a very high intrarectal SIV dose, seven RMs that were vaccinated 3 years earlier and had already resisted SIV infection 2 years before. This experiment showed that the seven Chinese RMs remained fully protected, while four animals that were used as controls became infected (Pancino, unpublished report to the Scientific Council of Paris-Descartes University, March 2013 [available on request]).
In the current study, we performed an immunization and challenge experiment with a cohort of 54 RMs of Indian origin, using immunogens provided by the Andrieu team, and included additional control groups receiving iSIV only or L. plantarum only. Note that vaccination did not trigger any B cell immune response even in the control group that received iSIV only. Intrarectal challenge with SIVmac239 resulted in similarly rapid infection in all groups of vaccinated RMs as well as unvaccinated controls. No apparent protective effect on virus acquisition was conferred by the tested iSIV-L. plantarum-based vaccination in this experimental setting. Furthermore, SIV-infected Indian-origin RMs that were vaccinated with iSIV-L. plantarum showed peak and postpeak viral loads similar to those observed in animals receiving iSIV only or L. plantarum only or in unvaccinated controls. The protection from SIV transmission conferred by intragastric iSIV-L. plantarum immunization reported by Lu et al. (12) for Chinese-origin RMs was not observed when the same vaccine was tested in a larger cohort of Indian-origin animals.
RESULTS
Immunization and challenge study design.
In this study, we evaluated potential protection from rectal SIVmac239 challenge conferred by intragastric administration of 2,2′-dithiodipyridine-inactivated SIV (iSIV) and Lactobacillus plantarum. The study design is shown in Fig. 1, including immunization regimens and the challenge time. Briefly, four groups of Mamu-B*08-negative and Mamu-B*17-negative Indian-origin RMs were immunized as follows: (i) 17 animals received iSIV and L. plantarum, (ii) 10 received iSIV only, (iii) 10 received L. plantarum only, and (iv) 17 received a sham intragastric immunization. Six months following the final immunization, all RMs were challenged intrarectally with a single dose of SIVmac239 (10,000 TCID50). All infected animals were monitored for 6 to 8 weeks after the first detection of SIV viremia (>1,000 copies/ml of plasma) to monitor the early clinical, virological, and immunological course of the infection.
FIG 1.
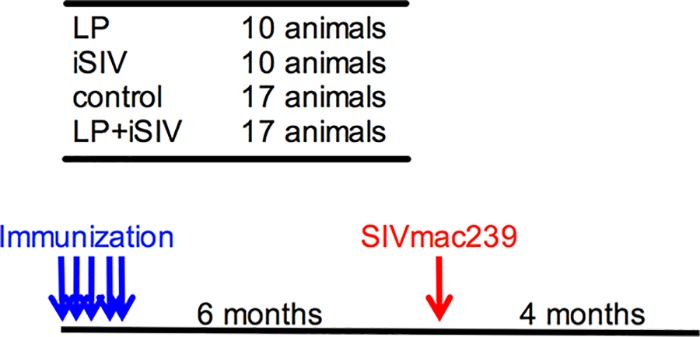
Study design, including oral intragastric immunization regimens and schematic representation of immunization and challenge. Four groups of Mamu-B*08-negative and Mamu-B*17-negative adult Indian RMs were immunized daily for 5 days (blue arrows), as follows: 10 received L. plantarum (LP) only, 10 received iSIV only, 17 received a sham intragastric immunization, and 17 animals received iSIV and L. plantarum. Six months following the final immunization, all RMs were challenged intrarectally with a single dose of SIVmac239 (10,000 TCID50) (red arrow). All infected animals were monitored for up to 4 months after the first detection of SIV viremia of >1,000 copies/ml. Blood was collected at intervals throughout the study.
Anti-SIV Env antibody responses postvaccination.
Anti-SIV gp140 antibodies were measured by enzyme-linked immunosorbent assay (ELISA) prior to challenge for all immunized animals. Anti-gp140 antibodies were found to be below the limit of detection for all 54 animals for plasma collected at the latest time point prior to challenge. These data led us to conclude that no anti-gp140 antibodies were generated after immunization, including in the control group vaccinated with iSIV alone (Fig. 2), thus identifying a clear difference between Indian and Chinese RMs in the ability to mount an immune response to an intragastric SIV vaccine.
FIG 2.
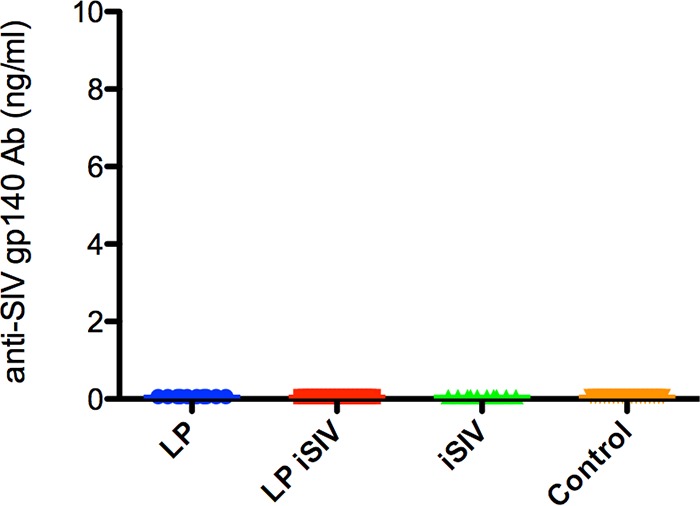
Anti-SIV envelope antibody responses postvaccination. Binding Ab titers were measured in sera collected prior to challenge. The amount of anti-SIV gp140 is expressed in nanograms per milliliter for each immunization group. The color scheme is as follows: blue, L. plantarum only; red, L. plantarum-iSIV; green, iSIV only; and orange, controls.
Protection from virus transmission.
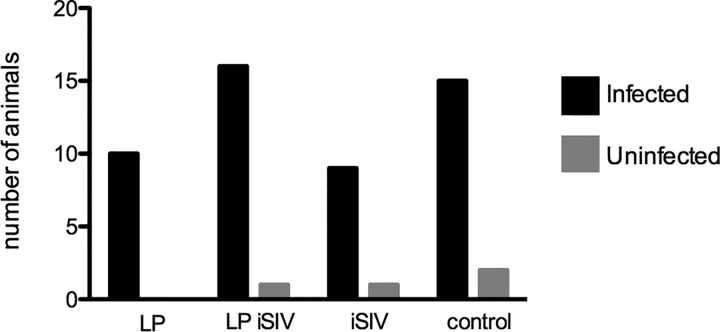
Six months following the last oral immunization, we performed mucosal (i.e., intrarectal) challenge with a single dose of 10,000 TCID50 SIVmac239. This SIV challenge resulted in rapid productive infection in the vast majority of RMs (50 of 54 animals), with all groups of vaccinated animals and controls showing the same rate of infection (Fig. 3). The four RMs that remained ostensibly uninfected (i.e., did not experience two separate time points at which plasma viremia was above 103 copies/ml) belonged to the following groups: (i) the iSIV-L. plantarum group (one animal with a single blip of 1.7 × 103 copies/ml [at week 2 postchallenge] that then remained negative for the rest of the follow-up), (ii) the iSIV group (one animal that always remained negative), and (iii) the control group (one animal that always remained negative and one animal that experienced two blips of <103 copies/ml of plasma [i.e., 1.1 × 102 copies/ml at week 2 postchallenge and 2.1 × 102 copies/ml at week 4 postchallenge] and then remained negative for the rest of the follow-up). Overall, these data indicate that in the current experiment, intragastric immunization with iSIV-L. plantarum (or with each of the individual components alone) did not confer protection from a single intrarectal challenge with 10,000 TCID50 of SIVmac239 in our cohort of Indian-origin RMs.
FIG 3.
SIV transmission and viral acquisition. After SIVmac239 challenge, the numbers of animals that were infected (black bars) and that remained uninfected (gray bars) were plotted. LP, L. plantarum.
Protection from virus replication.
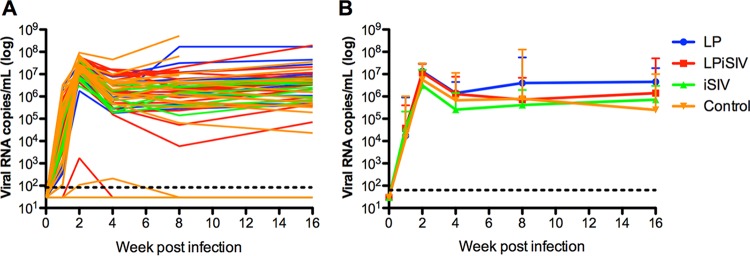
To determine whether the performed immunization with iSIV-L. plantarum (or the individual components) conferred any improved postacquisition control of virus replication, we next measured plasma viremia longitudinally in all SIV-infected RMs included in this study. As shown in Fig. 4A and B, SIV-infected animals that belonged to all four experimental groups showed very similar kinetics of peak viremia, postpeak decline, and set-point viral loads. In particular, the trends of SIV viremia were similar among the three immunization groups (iSIV-L. plantarum, iSIV alone, and L. plantarum alone) compared to each other and to the unvaccinated control group. Overall, these results indicate that the tested immunization regimens did not induce any significant control of postacquisition virus replication in the SIV-infected RMs.
FIG 4.
Viral loads (individual and groups). (A) Individual SIV plasma viral loads (expressed as numbers of copies per milliliter of plasma) were measured by real-time PCR at weeks 1, 2, 4, 8, and 16 after infection. (B) Average viral loads of the immunized groups compared to those of the control animals. Error bars represent SD. The color scheme is as follows: blue, L. plantarum only; red, L. plantarum-iSIV; green, iSIV only; and orange, controls.
Protection from SIV-induced CD4+ T cell decline.
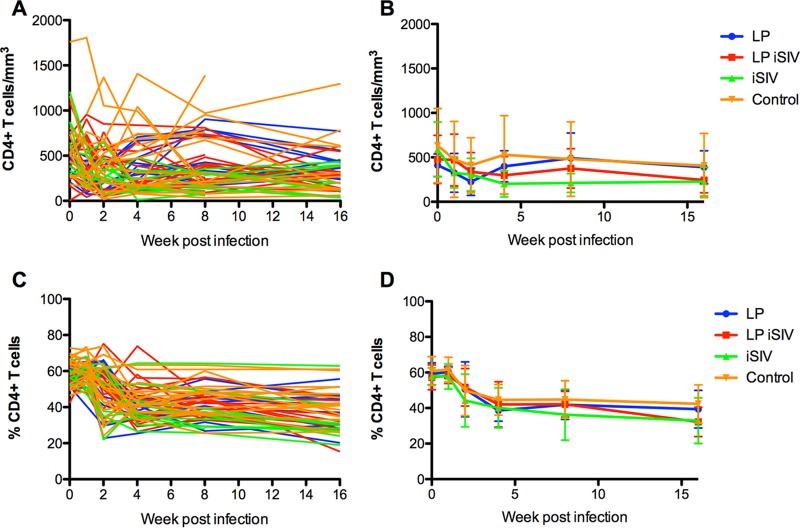
To investigate whether intragastric immunization with iSIV-L. plantarum (or the individual components) had any impact on SIV pathogenesis and associated immune deficiency, we next measured CD4+ T cell counts longitudinally (both as percentages of CD3+ T cells and as absolute numbers of cells per cubic millimeter of blood) after SIV infection in all RMs included in the current study. As shown in Fig. 5A to D, we found that the trends in CD4+ T cell counts, measured as both percentages and absolute counts, were very similar for all four experimental groups (i.e., no statistically significant differences were observed). Based on these data, we concluded that intragastric immunization of Indian RMs with iSIV-L. plantarum did not appear to induce any protection from viral pathogenesis, as assessed by longitudinal CD4+ T cell trends, after intrarectal transmission of SIVmac239.
FIG 5.
CD4 counts (percentage and absolute, individual and groups). A longitudinal assessment of CD4+ T cell levels in peripheral blood was performed. (A) After SIV challenge, individual levels of CD4+ T cells in blood (expressed as numbers of cells per cubic millimeter) were calculated. (B) Average CD4+ T cell counts were calculated for each immunization group. (C and D) Individual and average frequencies of CD4+ T cells were tracked (expressed as percentages of CD3+ T cells). Error bars represent SD. The color scheme is as follows: blue, L. plantarum only; red, L. plantarum-iSIV; green, iSIV only; and orange, controls.
DISCUSSION
As reported by Lu and colleagues, intragastric immunization of Chinese-origin RMs with iSIV-L. plantarum protected 15 of 16 animals from intrarectal challenge with SIVmac239 as well as with the heterologous strain SIVB670 (12). They also reported that in four animals protected from intrarectal challenge that were subsequently rechallenged intravenously, plasma SIV RNA levels showed a minor transient peak and then declined to levels that were below the limit of detection. Moreover, an independent study showed that 7 of 7 vaccinated Chinese RMs that were protected after a first challenge (performed 1 year after vaccination) remained protected after a second challenge performed 2 years later (i.e., 3 years after vaccination). Importantly, in their paper, Lu et al. showed that the group of four Chinese RMs vaccinated with iSIV only had a clear anti-SIV antibody response (IgM and IgG); moreover, Chinese RMs vaccinated with iSIV-L. plantarum had a strong suppressive activity generated by major histocompatibility complex E (MHC-E)-restricted regulatory and/or suppressive CD8+ T cells. These cells were measurable only in fresh cells (12, 28) and were not evaluated in the current study.
These exciting and provocative results, reported for the use of an unconventional AIDS vaccine approach, prompted the Bill & Melinda Gates Foundation to support a larger follow-up study in which Indian-origin RMs were used and two additional groups were included (each immunized with one individual component of the regimen, i.e., iSIV alone or L. plantarum alone). The choice of using Indian RMs (instead of the Chinese RMs used in the previous study) reflects the fact that this subspecies of RMs has been used for the vast majority of preclinical nonhuman primate (NHP) studies of candidate HIV/AIDS vaccines. Note that the immunogens used in the current study were produced by the group of W. Lu and J.-M. Andrieu as described in the original study, and an extensive characterization of these reagents was performed in the laboratories of the AIDS and Cancer Virus Program (Frederick National Laboratory for Cancer Research) and T. R. Klaenhammer for iSIV and L. plantarum, respectively.
The overall results of the current study are quite straightforward in that they indicate that the intragastric immunization of Indian RMs with iSIV-L. plantarum (or with any of the individual components, i.e., iSIV only or L. plantarum only) did not confer any protection from virus transmission after intrarectal challenge with SIVmac239 or result in an attenuated outcome of SIV infection as determined by either levels of virus replication (peak or set point) or CD4+ T cell counts. Taken as a whole, these results are in strong contrast to those observed by the Lu-Andrieu team for Chinese RMs, in which 15/16 animals were protected from a very high intrarectal dose of SIVmac239. This rather striking difference in outcome between the initial study by Lu et al. and the current experiment prompted us to carefully consider any potential differences that could be responsible for these discordant experimental results.
The first study was conducted at the Nonhuman Primate Laboratory of the Gaoyao Experimental Animal Center, while the second was conducted at the Yerkes National Primate Research Center of Emory University. While differences between facilities can have an impact on the results of NHP studies, differences in protocol-specific procedures seem unlikely to account for the observed difference in results. Indeed, prior to the second study, all experimental procedures, including in particular the preparation and intragastric administration of the immunogens and the intrarectal challenge with SIVmac239, were reviewed in detail with Lu and Andrieu.
A second potential difference was the challenge virus used. Although SIVmac239 was used for both studies, different stocks were used in the two studies, i.e., one produced by W. Lu in the first study and the other kindly provided by K. Van Rompay of the California National Primate Research Center of the University of California at Davis for use in the second study. While use in the second study of the identical challenge stock used by Lu et al. in the first study would have been optimal, there was not a sufficient amount of the original SIVmac239 stock available to conduct the second experiment. Any potential differences between the SIVmac239 stocks used in the two experiments are mitigated by the fact that both were prepared from the SIVmac239 molecular clone and therefore are unlikely to differ enough from each other to account for the observed dramatic difference in virological outcomes. Furthermore, the challenge dose of 10,000 TCID50 used in the current experiment was chosen to be 10-fold lower than the dose used in the original study by Lu et al. to minimize the prospect of weak protection not being observed as a result of using a different and possibly more infectious preparation of SIVmac239.
Perhaps the most important difference between the two studies was in the genetic makeup of the RMs used, i.e., Chinese origin in the first study and Indian origin in the second experiment. As mentioned above, this difference reflected an explicit decision to attempt to extend the observations reported from the initial study of Chinese-origin RMs to the Indian-origin RM system that is most commonly used as a preclinical NHP model for HIV/AIDS vaccine development.
In this context, the absence of an antibody response to SIV Env in all 54 Indian RMs included in this study represents an important finding. In particular, the lack of response observed in the 10 RMs that were intragastrically vaccinated with iSIV alone is in strong contrast to the anti-SIV Env antibody response observed in the four Chinese RMs vaccinated with iSIV alone (12). In addition, the absence of viral suppression in the 17 Indian RMs that received iSIV-L. plantarum indicates that the CD8+ T cell-mediated regulatory responses that elicited viral suppression in Chinese RMs were not induced (or were not functional) in vaccinated Indian RMs. It is conceivable that this lack of immune responses in Indian RMs contributed to their lack of protection against SIV challenge.
The observed differences in immune responses between Indian and Chinese RMs after intragastric immunization are most likely of genetic origin. At this time, full-genome sequences of both Indian- and Chinese-origin RMs are available, and comparative analyses of these two subspecies at the genetic level have identified a number of differences and polymorphisms in immune-related genes that may potentially explain the protection observed in Chinese RMs and the absence of protection observed in Indian RMs (13, 14). Consistent with the vaccination success observed in Chinese RMs, several studies published over the last decade have suggested that the immunogenetic background of Chinese RMs is much closer to that of humans than to that of Indian RMs (15–19).
Overall, the results of the current study are very straightforward, as the oral iSIV-L. plantarum vaccine protected none of the Indian RMs tested, while the same vaccine prepared by the same team protected most of the Chinese RMs tested. As mentioned above, it is possible that this discrepancy was caused by the different immunogenetic responses elicited in the two subspecies of RMs by the same vaccine. Note that the strong CD8 regulatory T cell activity that prevents CD4+ T cell activation and thus suppresses SIV replication that was discovered in vaccinated Chinese RMs by Andrieu and Lu has also been found in the so-called “elite controllers,” a rare population (<1/100) of HIV-infected individuals who maintain undetectable viral loads without antiretroviral therapy (20). It is therefore possible that Indian RMs are not the appropriate model for studying the effects of an oral suppressive vaccine (such as iSIV-L. plantarum), as they do not have a genetic background allowing a suppressive/regulatory CD8+ T cell response.
MATERIALS AND METHODS
Animals.
Fifty-four healthy, SIV-uninfected, Mamu-B*08-negative, Mamu-B*17-negative Indian rhesus macaques (RMs) were used in this study. All animals were housed at the Yerkes National Primate Research Center and maintained in accordance with NIH guidelines. These studies were approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
Preparation of iSIV and L. plantarum.
Detailed methods are given in the original paper by Lu et al. (12). SIVmac239 was grown in CEM174 cells, and culture supernatant containing an estimated 1 × 1012 RNA copies was inactivated first with 250 μM 2,2′-dithiodipyridine and then by heating at 56°C for 30 min. The inactivated virus was then used to inoculate CEM174 cells to verify the lack of measurable residual infectivity. L. plantarum (ATCC 8014) was cultured at 37°C in MRS medium with a rotation rate of 200 rpm. To obtain L. plantarum at the logarithmic (mid-log) phase of bacterial culture, bacteria were cultured until they reached an optical density at 600 nm of 1.0, with a final L. plantarum concentration of ∼1010 CFU/ml (∼3.5 h) (21).
Characterization of iSIV.
Characterization of the inactivated virus preparation provided by Andrieu also included SDS-PAGE and immunoblot analysis under reducing and nonreducing conditions, which showed a complex profile of mostly non-SIV proteins but did confirm the presence of SIV gp120(SU), gp41(TM), p28(CA), and p8(NC) in the preparation, with the nonreducing analysis showing apparent cross-linking of p28(CA) and p8(NC) proteins, as expected for 2,2′-dithiodipyridine-treated virions (22–24). p28(CA) content was estimated to be approximately 15 μg/ml based on SDS-PAGE analysis. The SIV gag RNA content was estimated at approximately 3 × 1011 copies/ml, based on the averaged values for triplicate determinations across a 3-log dilution series in a quantitative reverse transcription-PCR (qRT-PCR) assay, as described in detail previously (25).
The residual 2,2′-dithiodipyridine concentration in the preparation provided, determined by high-pressure liquid chromatography (HPLC) analysis, was 4 to 5 ng/ml. The provided reagent was confirmed to be SIVmac251 by sequence analysis (99.1% Env similarity with SIVmac239, provided by N. L. Letvin and D. H. Barouch of Harvard University; 98.5% similarity with virus provided by R. S. Veazey of Tulane University; 98.7% similarity with virus provided by C. J. Miller of UC Davis; 97.9% similarity with virus provided by R. C. Desrosiers of the University of Miami; and 97.9% similarity with virus provided by the German Primate Center). No residual virus infectivity was observed as assessed using the Tzm/bl cell assay.
Independent verification of L. plantarum.
Freeze-dried L. plantarum powder was received and stored at −80°C until processing. For 16S rRNA gene sequencing, powder was streaked onto the following three media for colony isolation, for purity analysis, and to obtain the 16S rRNA gene sequence: (i) plate count agar (PCA) (incubated aerobically at 30°C), (ii) Luria-Bertani (LB) agar (incubated aerobically at 37°C), and (iii) MRS plates (incubated anaerobically at 37°C). L. plantarum grew on all media. A total of 11 different colonies from the 3 plates were picked for 16S rRNA gene colony PCR. BLAST searches showed that all 11 colonies exhibited 100% identity to L. plantarum. For cell counts, 0.5 g of powder was diluted in 49.5 ml of 0.1× MRS medium in triplicate, and serial dilutions were performed in that diluent. The dilutions were spiral plated onto MRS plates and incubated anaerobically at 37°C for 3 days, and the white colonies were counted on a protocol counter. The average ± standard deviation (SD) colony count was 3.03 × 1010 ± 3.92 × 109 CFU/0.5 g of powder. In summary, identification by 16S rRNA gene sequencing showed that the colonies were all L. plantarum, indicating sample purity. The bacterial count of the freeze-dried powder was approximately 6 × 1010 CFU/g of sample.
Immunization regimen.
All immunizations were delivered to anesthetized animals via oral intragastric delivery. Indian-origin RMs were divided into four experimental groups: (i) 17 animals received iSIV and L. plantarum, (ii) 10 received iSIV only, (iii) 10 received L. plantarum only, and (iv) 17 received a sham intragastric immunization. All animals received each dose of iSIV and/or L. plantarum every 30 min for 3 h, for five consecutive days. The amount of L. plantarum administered was 5.4 × 1011 CFU/day, for a total of 2.7 × 1012 CFU over 5 days. The amount of iSIV administered was 7.2 × 109 copies/day, for a total dose of 3.6 × 1010 copies over 5 days. Control animals received a sham immunization via the same method.
Viral challenge.
Six months following the final immunization, all RMs were challenged intrarectally with SIVmac239 (10,000 TCID50), provided by K. Van Rompay of the California National Primate Research Center, Davis, CA. Animals were considered to be infected if they had >1,000 copies/ml sustained for multiple time points.
Plasma viral load determination.
qRT-PCR to determine the SIVmac239 load was performed as previously described (26). The sensitivity of the assay as performed is 60 copies/ml of plasma.
Anti-SIV Env antibodies.
SIV Env-specific binding Abs were measured by use of an enzyme-linked immunosorbent assay (ELISA) with commercially purchased SIVmac239 gp140 antigen (Immune Technology Corp., New York, NY) as described previously (27). Briefly, ELISA plates (Costar; Corning Life Sciences, Lowell, MA) were coated with SIVmac239 gp140 (0.5 μg/ml) overnight at 4°C. Plates were washed and blocked for 1 h (phosphate-buffered saline [PBS]–Tween with 4% whey and 5% dry milk). Test sera was added to duplicate wells in serial 3-fold dilutions and incubated for 2 h. Plates were then washed, and bound Ab was detected using peroxidase-conjugated anti-monkey IgG (Accurate Chemical and Scientific, Westbury, NY) and tetramethylbenzidine substrate (KPL, Gaithersburg, MD). Reactions were stopped by addition of 100 μl of 1 N H3PO4. Each plate included a standard curve generated using goat anti-monkey IgG and rhesus macaque IgG (both from Accurate Chemical and Scientific Corp.). Standard curves were fitted, and sample concentrations were interpolated to nanograms of Ab per milliliter of serum by using SOFTmax 2.3 software (Molecular Devices, Sunnyvale, CA). The concentrations of IgG are relative to our standard curve and are not absolute values.
Power calculation.
Power calculations to determine the appropriate animal number for this experiment were conducted in collaboration with Steve Self of the University of Washington, Seattle, WA. In particular, the number 17 for the iSIV-L. plantarum and control groups was powered at 60% efficacy and with good robustness to deal with the possibility of 1 or 2 noninfections in the control group. For the single-component arms (i.e., iSIV alone and L. plantarum alone), the number 10 was chosen with the awareness that the smaller group size might increase the possibility of type 1 errors of the tests in the comparison with the other groups. This decision was made in order to be able to address whether (i) each single-component arm is “noninferior” to the two-component arm, (ii) each single-component arm has a positive efficacy relative to that of placebo, and (iii) there is a positive interaction between iSIV and L. plantarum that delivers a level of protective efficacy greater than that predicted from the efficacies of the single-component arms. This assessment involved outcomes for all four experimental groups for which a formal test for interaction was made and defined the real scientific issues that motivated inclusion of the two single-component arms in the design.
Statistical analyses.
Measurements among all treatment groups were performed in GraphPad Prism (version 5.0f), using the parametric t test or nonparametric Mann-Whitney test as well as one-way analysis of variance (ANOVA) with Bonferroni multiple-comparison adjustments. Mixed-effects regression analysis of viral loads, CD4 counts, and CD4 percentages was also performed in R (v3.3.3). The slopes for each immunization group were compared to those for the control group to determine the statistical significance of the impact of each immunization on CD4+ T cell counts and viral loads.
ACKNOWLEDGMENTS
We thank Adrien Nivoliez, from Biose Industrie, Aurillac, France, who prepared the lyophilized L. plantarum; Todd Klaenhammer and Rosemary Sanozky-Dawes, from North Carolina State University, for their characterization of the used Lactobacillus preparation; David Roser, Julian W. Bess, Jr., Elena Chertova, Kelli Oswald, Brandon Keele, and Jeffery Lifson, from the AIDS and Cancer Virus Program of the Frederick National Laboratory for Cancer Research, Frederick, MD, for their characterization of the 2,2′-dithiodipyridine-treated SIV prepared by the Andrieu laboratory; Amy Weiner for helpful discussions; B. Cervasi and K. P. Gill for technical support; S. Ehnert for study organization and scheduling; and the Yerkes National Primate Center veterinary and animal care staff for caring for the animals and performing all experimental procedures.
This study was supported by the Bill & Melinda Gates Foundation, under a grant for “Inactivated AT-2 Treated SIVmac239 and Lactobacillus plantarum as Candidate A,” as well as grant P51 OD011132 to the Yerkes National Primate Research Center, the Emory Center for AIDS Research; by NIH grant P30-AI-504; and in part by federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E (Jeffery Lifson).
REFERENCES
- 1.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. 2012. A blueprint for HIV vaccine discovery. Cell Host Microbe 12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes BF, Shaw GM, Korber B, Kelsoe G, Sodroski J, Hahn BH, Borrow P, McMichael AJ. 2016. HIV-host interactions: implications for vaccine design. Cell Host Microbe 19:292–303. doi: 10.1016/j.chom.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnathan DG, Wetzel KS, Yu J, Lee ST, Johnson BA, Paiardini M, Yan J, Morrow MP, Sardesai NY, Weiner DB, Ertl HC, Silvestri G. 2015. Activated CD4+CCR5+ T cells in the rectum predict increased SIV acquisition in SIVGag/Tat-vaccinated rhesus macaques. Proc Natl Acad Sci U S A 112:518–523. doi: 10.1073/pnas.1407466112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haase AT. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol 5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 5.Bonsignori M, Liao HX, Gao F, Williams WB, Alam SM, Montefiori DC, Haynes BF. 2017. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol Rev 275:145–160. doi: 10.1111/imr.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussenzweig MC, Martin MA. 2016. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Ghneim K, Sok D, Bosche WJ, Li Y, Chipriano E, Berkemeier B, Oswald K, Borducchi E, Cabral C, Peter L, Brinkman A, Shetty M, Jimenez J, Mondesir J, Lee B, Giglio P, Chandrashekar A, Abbink P, Colantonio A, Gittens C, Baker C, Wagner W, Lewis MG, Li W, Sekaly RP, Lifson JD, Burton DR, Barouch DH. 2016. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 353:1045–1049. doi: 10.1126/science.aag0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Excler JL, Michael NL. 2016. Lessons from HIV-1 vaccine efficacy trials. Curr Opin HIV AIDS 11:607–613. doi: 10.1097/COH.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 9.Tomaras GD, Plotkin SA. 2017. Complex immune correlates of protection in HIV-1 vaccine efficacy trials. Immunol Rev 275:245–261. doi: 10.1111/imr.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Excler JL, Michael NL. 2015. Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annu Rev Med 66:423–437. doi: 10.1146/annurev-med-052912-123749. [DOI] [PubMed] [Google Scholar]
- 12.Lu W, Chen S, Lai C, Guo W, Fu L, Andrieu JM. 2012. Induction of CD8+ regulatory T cells protects macaques against SIV challenge. Cell Rep 2:1736–1746. doi: 10.1016/j.celrep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O'Brien WE, Prüfer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. 2007. Evolutionary and biomedical insights from the rhesus macaque genome. Science 316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 14.Yan G, Zhang G, Fang X, Zhang Y, Li C, Ling F, Cooper DN, Li Q, Li Y, van Gool AJ, Du H, Chen J, Chen R, Zhang P, Huang Z, Thompson JR, Meng Y, Bai Y, Wang J, Zhuo M, Wang T, Huang Y, Wei L, Li J, Wang Z, Hu H, Yang P, Le L, Stenson PD, Li B, Liu X, Ball EV, An N, Huang Q, Zhang Y, Fan W, Zhang X, Li Y, Wang W, Katze MG, Su B, Nielsen R, Yang H, Wang J, Wang X, Wang J. 2011. Genome sequencing and comparison of two nonhuman primate animal models, the cynomolgus and Chinese rhesus macaques. Nat Biotechnol 29:1019–1023. doi: 10.1038/nbt.1992. [DOI] [PubMed] [Google Scholar]
- 15.Ling B, Veazey RS, Luckay A, Penedo C, Xu K, Lifson JD, Marx PA. 2002. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS 16:1489–1496. doi: 10.1097/00002030-200207260-00005. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Bao R, Haigwood NL, Persidsky Y, Ho WZ. 2013. SIV infection of rhesus macaques of Chinese origin: a suitable model for HIV infection in humans. Retrovirology 10:89. doi: 10.1186/1742-4690-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mothe BR, Southwood S, Sidney J, English AM, Wriston A, Hoof I, Shabanowitz J, Hunt DF, Sette A. 2013. Peptide-binding motifs associated with MHC molecules common in Chinese rhesus macaques are analogous to those of human HLA supertypes and include HLA-B27-like alleles. Immunogenetics 65:371–386. doi: 10.1007/s00251-013-0686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Southwood S, Solomon C, Hoof I, Rudersdorf R, Sidney J, Peters B, Wahl A, Hawkins O, Hildebrand W, Mothe BR, Sette A. 2011. Functional analysis of frequently expressed Chinese rhesus macaque MHC class I molecules Mamu-A1*02601 and Mamu-B*08301 reveals HLA-A2 and HLA-A3 supertypic specificities. Immunogenetics 63:275–290. doi: 10.1007/s00251-010-0502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon C, Southwood S, Hoof I, Rudersdorf R, Peters B, Sidney J, Pinilla C, Marcondes MC, Ling B, Marx P, Sette A, Mothe BR. 2010. The most common Chinese rhesus macaque MHC class I molecule shares peptide binding repertoire with the HLA-B7 supertype. Immunogenetics 62:451–464. doi: 10.1007/s00251-010-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu W, Chen S, Lai C, Lai M, Fang H, Dao H, Kang J, Fan J, Guo W, Fu L, Andrieu JM. 2016. Suppression of HIV replication by CD8(+) regulatory T-cells in elite controllers. Front Immunol 7:134. doi: 10.3389/fimmu.2016.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Baarlen P, Troost FJ, van Hemert S, van der Meer C, de Vos WM, de Groot PJ, Hooiveld GJ, Brummer RJ, Kleerebezem M. 2009. Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci U S A 106:2371–2376. doi: 10.1073/pnas.0809919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arthur LO, Bess JW Jr, Chertova EN, Rossio JL, Esser MT, Benveniste RE, Henderson LE, Lifson JD. 1998. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res Hum Retroviruses 14(Suppl 3):S311–S319. [PubMed] [Google Scholar]
- 23.Chertova E, Crise BJ, Morcock DR, Bess JW Jr, Henderson LE, Lifson JD. 2003. Sites, mechanism of action and lack of reversibility of primate lentivirus inactivation by preferential covalent modification of virion internal proteins. Curr Mol Med 3:265–272. doi: 10.2174/1566524033479889. [DOI] [PubMed] [Google Scholar]
- 24.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW Jr, Vasquez GM, Wiltrout TA, Chertova E, Grimes MK, Sattentau Q, Arthur LO, Henderson LE, Lifson JD. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol 72:7992–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen SG, Piatak M, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, Gilliam AN, Xu G, Whizin N, Burwitz BJ, Planer SL, Turner JM, Legasse AW, Axthelm MK, Nelson JA, Fruh K, Sacha JB, Estes JD, Keele BF, Edlefsen PT, Lifson JD, Picker LJ. 2017. Addendum: immune clearance of highly pathogenic SIV infection. Nature 547:123–124. doi: 10.1038/nature22984. [DOI] [PubMed] [Google Scholar]
- 26.Cartwright EK, Spicer L, Smith SA, Lee D, Fast R, Paganini S, Lawson BO, Nega M, Easley K, Schmitz JE, Bosinger SE, Paiardini M, Chahroudi A, Vanderford TH, Estes JD, Lifson JD, Derdeyn CA, Silvestri G. 2016. CD8(+) lymphocytes are required for maintaining viral suppression in SIV-infected macaques treated with short-term antiretroviral therapy. Immunity 45:656–668. doi: 10.1016/j.immuni.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwa S, Lai L, Gangadhara S, Siddiqui M, Pillai VB, Labranche C, Yu T, Moss B, Montefiori DC, Robinson HL, Kozlowski PA, Amara RR. 2014. CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge. J Virol 88:9579–9589. doi: 10.1128/JVI.00975-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrieu J-M, Chen S, Lai C, Guo W, Lu W. 2014. Mucosal SIV vaccines comprising inactivated virus particles and bacterial adjuvants induce CD8+ T-regulatory cells that suppress SIV-positive CD4+ T-cell activation and prevent SIV infection in the macaque model. Front Immunol 5:297. doi: 10.3389/fimmu.2014.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]