ABSTRACT
Mycobacterium abscessus accounts for a large proportion of lung disease cases caused by rapidly growing mycobacteria. The association between clarithromycin sensitivity and treatment outcome is clear. However, M. abscessus culture and antibiotic susceptibility testing are time-consuming. Clarithromycin susceptibility genotyping offers an alternate, rapid approach to predicting the efficacy of clarithromycin-based antibiotic therapy. M. abscessus lung disease patients were divided into two groups based upon the clarithromycin susceptibility genotype of the organism isolated. A retrospective analysis was conducted to compare the clinical features, microbiological characteristics, and treatment outcomes of the two groups. Several other potential predictors of the response to treatment were also assessed. Sixty-nine patients were enrolled in the clarithromycin-resistant genotype group, which included 5 infected with rrl 2058-2059 mutants and 64 infected with erm(41)T28-type M. abscessus; 31 were in the clarithromycin-sensitive group, i.e., 6 and 25 patients infected with genotypes erm(41)C28 and erm(41) M type, respectively. The results showed that lung disease patients infected with clarithromycin-sensitive and -resistant M. abscessus genotypes differed significantly in clarithromycin-based combination treatment outcomes. Patients infected with the clarithromycin-sensitive genotype exhibited higher initial and final sputum-negative conversion and radiological improvement rates and better therapeutic outcomes. Multivariate analysis demonstrated that genotyping was a reliable and, more importantly, rapid means of predicting the efficacy of clarithromycin-based antibiotic treatment for M. abscessus lung disease.
KEYWORDS: Mycobacterium abscessus, clarithromycin, genotype, lung disease, treatment outcome
INTRODUCTION
The incidence of infections by nontuberculosis mycobacteria (NTM) has increased significantly in recent years (1–3). Of all NTM infections, treatment of Mycobacterium abscessus infections is the most challenging (4, 5). M. abscessus accounts for 65 to 80% of the cases of lung disease caused by rapidly growing mycobacteria and has emerged as an important pathogen for patients with bronchiectasis, chronic obstructive pulmonary disease, and cystic fibrosis (6–11).
M. abscessus is among the most antibiotic-resistant pathogens known (12). Although some antibiotics, such as amikacin, cefoxitin, and imipenem, are effective, only clarithromycin (CLA) exhibits convincing evidence of clinical efficacy for treatment of M. abscessus lung disease (8). Currently, CLA is the only effective antibiotic administered orally and, therefore, recommended as the core agent for treatment of M. abscessus infections (8).
Genotypic variations influence the sensitivity of M. abscessus to CLA. Two genotypes confer CLA resistance: a point mutation (A to C or A to G) in the 2058-2059 locus of the 23S rRNA (rrl) gene confers acquired resistance (13). An intact erm(41) gene, which exhibits a T/C polymorphism at the 28th nucleotide, confers inducible resistance when the 28th nucleotide is thymidine [erm(41)T28] (14, 15). Alternatively, CLA sensitivity is conferred when cytidine is the 28th nucleotide in intact erm(41), i.e., genotype erm(41)C28 (15). Deletion of erm(41) nucleotides 64 and 65, or deletion of nucleotides 159 to 432, also results in the loss of erm(41) gene function (M type) and a gain in CLA sensitivity (14, 16).
M. abscessus can be divided into M. abscessus subsp. abscessus and M. abscessus subsp. massiliense based upon the integrity or absence of the erm(41) gene. Korean and Japanese researchers first reported that M. abscessus subsp. abscessus and M. abscessus subsp. massiliense exhibited disparate clinical and microbiological characteristics (17, 18). Retrospective analysis and a prospective study conducted in 2017 confirmed these results and suggested that patients infected with a CLA-sensitive [erm(41)C28] genotype had a prognostic advantage (19, 20). Therefore, differences in the A and M subtypes may be due largely to genotypic differences that affect CLA sensitivity (21–23). Here, we report the results of a retrospective analysis undertaken to determine the relationship between genotype, CLA sensitivity, and the outcome of CLA-based treatment of M. abscessus lung disease.
RESULTS
Patient characteristics.
One hundred M. abscessus lung disease patients who conformed to our recruitment criteria were enrolled and divided into CLA-resistant and -sensitive genotype groups according to the rrl and erm(41) sequevar. Sixty-nine (69%) patients were enrolled in the CLA-resistant genotype group, which included 5 (7.2%) rrl 2058-2059 mutant- and 64 (92.8%) erm(41)T28-type-infected patients; 31 (31%) belonged to the CLA-sensitive genotype group, which included 6 (19.4%) erm(41)C28- and 25 (80.6%) erm(41) M-type-infected patients. No significant differences were found in the ages and genders of the two groups (Table 1). The proportion of patients with hemoptysis was higher in the CLA-resistant genotype group than the CLA-sensitive genotype group (22/69 versus 4/31; P = 0.045). Furthermore, a significantly greater incidence of cavity-like manifestations occurred in computed tomography (CT) scans of patients infected with isolates with the CLA-resistant genotype than in patients infected with isolates with the CLA-sensitive genotype (50/69 versus 8/31; P < 0.001). CT scans of the CLA-sensitive-group patients, on the other hand, displayed a higher incidence of a tree-in-bud pattern (14/31 versus 16/69; P = 0.027).
TABLE 1.
Baseline characteristics of patients infected with M. abscessus belonging to CLA-resistant and -sensitive genotypes
| Characteristica | Valueb |
P value | |
|---|---|---|---|
| CLA-resistant group (n = 69) | CLA-sensitive group (n = 31) | ||
| Median age (IQR) (yr) | 58 (44–66) | 56 (32–64) | 0.562 |
| Males | 26 (37.7) | 17 (54.8) | 0.107 |
| BMI (mean ± SD) (kg/m2) | 19.93 ± 0.37 | 19.69 ± 0.57 | 0.729 |
| Underlying disease | |||
| Prior tuberculosisc | 29 (42.0) | 16 (51.6) | 0.373 |
| COPD | 1 (1.4) | 2 (6.5) | 0.226 |
| Hypertension | 11 (15.9) | 3 (9.7) | 0.601 |
| Diabetes | 2 (2.9) | 4 (12.9) | 0.135 |
| CHD | 4 (5.8) | 0 (0) | 0.414 |
| Malignancy | 3 (4.3) | 0 (0) | 0.550 |
| History of surgery | 3 (4.3) | 1 (3.2) | 1 |
| Symptoms | |||
| Cough | 55 (79.7) | 25 (80.6) | 0.914 |
| Sputum | 69 (100.0) | 31 (100.0) | 1 |
| Fever | 15 (21.7) | 4 (12.9) | 0.298 |
| Hemoptysis | 22 (31.9) | 4 (12.9) | 0.045 |
| Radiographic features | |||
| Extent | 0.404 | ||
| Bilateral involvement | 58 (84.0) | 28 (90.3) | |
| Unilateral involvement | 11 (15.9) | 3 (9.7) | |
| Median no. of lobes (IQR) | 4 (3–6) | 4 (2–6) | 0.419 |
| Disease pattern | |||
| Bronchiectasis | 66 (95.7) | 29 (93.4) | 0.655 |
| Cavity | 50 (72.5) | 8 (25.8) | <0.001 |
| Nodules (diam < 1 cm) | 38 (55.0) | 19 (61.3) | 0.561 |
| Nodules (diam > 1 cm) | 39 (56.5) | 16 (51.6) | 0.648 |
| Tree-in-bud pattern | 16 (23.2) | 14 (45.2) | 0.027 |
| Initial AFB smear positivity | 28 (40.6) | 10 (32.3) | 0.428 |
| Initial morphotype | 0.770 | ||
| Rough | 40 (58.0) | 17 (54.8) | |
| Smooth | 29 (42.0) | 14 (45.2) | |
COPD, chronic obstructive pulmonary disease; CHD, coronary heart disease; AFB, acid-fast bacilli; IQR, interquartile range.
Data are the numbers (%) of patients found in the CLA-resistant and -sensitive genotype groups unless otherwise indicated.
Patients treated for tuberculosis prior to the diagnosis of M. abscessus lung disease.
Colony morphology.
M. abscessus isolates manifest two distinct colony morphotypes: smooth and rough. The colony morphology of the isolates associated with both CLA susceptibility groups did not differ significantly (Table 1). Patients infected with M. abscessus characterized by a rough-type colony exhibited a higher incidence of cavities in CT images (P = 0.043) (Table 2). The colony morphotype did not exert a significant effect on any of the other parameters assessed.
TABLE 2.
Relationship between morphotype, results of initial CT scan, and treatment outcome
| Parameter | No. (%) of patientsa |
P value | |
|---|---|---|---|
| Rough n = 57 | Smooth n = 43 | ||
| Radiographic features | |||
| Bronchiectasis | 56 (98.2) | 39 (90.7) | 0.211 |
| Tree-in-bud pattern | 18 (31.6) | 12 (27.9) | 0.692 |
| Cavity | 38 (66.7) | 20 (46.5) | 0.043 |
| Radiological improvement | 26 (45.6) | 21 (48.8) | 0.749 |
| Sputum conversion to negativity | 21 (36.8) | 19 (44.2) | 0.458 |
| Treatment effectiveness | 31 (54.4) | 25 (58.1) | 0.708 |
Number (percentage) of patients infected with isolates that give rise to rough and smooth colony types versus the disease parameter listed.
Comparison of antibiotic sensitivity.
The sensitivity of all the M. abscessus isolates to 10 antibiotics tested is shown in Table 3 and Table S1 in the supplemental material. The five rrl 2058-2059 mutant isolates exhibited acquired resistance to CLA, i.e., they were resistant on day 3 of exposure and prior to induction. Twenty-seven of the 64 erm(41)T28 isolates also exhibited acquired resistance; 36 isolates were induced by 14 days exposure to CLA; and one isolate showed abnormal CLA sensitivity despite expressing an erm(41)T28 gene, albeit with a wild-type rrl gene. In sharp contrast, no CLA resistance was observed within the CLA-sensitive genotype group. Notably, although most isolates in the CLA-resistant genotype group were insensitive to CLA, only one isolate was insensitive to amikacin treatment. A considerable number of isolates in both the CLA-sensitive and CLA-resistant genotype groups were sensitive to linezolid. A large number of isolates in both groups were resistant to moxifloxacin, doxycycline, imipenem, and tobramycin; no significant difference in resistance to these antibiotics was found between groups.
TABLE 3.
Antibiotic resistance of all M. abscessus isolatesa
| Isolate group (n) | Antibiotic | No. of isolates/MIC (mg/ml) of: |
No. (%) resistant isolatesb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | |||
| Resistant (69) | Clarithromycin before induction | 7 | 11 | 13 | 10 | 9 | 19 | 28 (40.6) | ||||||
| Clarithromycin after induction | 1 | 68 | 68 (98.6) | |||||||||||
| Amikacin | 2 | 13 | 35 | 10 | 8 | 1 | 1 (1.4) | |||||||
| Linezolid | 1 | 3 | 2 | 10 | 20 | 33 | 33 (47.8) | |||||||
| Moxifloxacin | 1 | 3 | 65 | 68 (98.6) | ||||||||||
| Doxycycline | 1 | 68 | 68 (98.6) | |||||||||||
| Imipenem | 3 | 14 | 52 | 66 (95.7) | ||||||||||
| Tobramycin | 9 | 22 | 38 | 60 (87.0) | ||||||||||
| Cefoxitin | 3 | 23 | 43 | 43 (62.3) | ||||||||||
| Sulfonamides | 2 | 7 | 22 | 22 | 16 | 38 (55.1) | ||||||||
| Tigecycline | 4 | 10 | 30 | 16 | 9 | ND | ||||||||
| Sensitive (31) | Clarithromycin before induction | 2 | 9 | 4 | 8 | 3 | 3 | 3 | 6 (19.4) | |||||
| Clarithromycin after induction | 7 | 4 | 7 | 4 | 1 | 4 | 4 | 8 (25.8) | ||||||
| Amikacin | 2 | 3 | 15 | 9 | 1 | 1 | 1 (3.2) | |||||||
| Linezolid | 1 | 7 | 10 | 13 | 13 (42.0) | |||||||||
| Moxifloxacin | 1 | 2 | 28 | 30 (96.8) | ||||||||||
| Doxycycline | 1 | 30 | 31 (100) | |||||||||||
| Imipenem | 1 | 4 | 26 | 30 (96.8) | ||||||||||
| Tobramycin | 1 | 9 | 21 | 30 (96.8) | ||||||||||
| Cefoxitin | 2 | 7 | 22 | 22 (71.0) | ||||||||||
| Sulfonamides | 1 | 5 | 7 | 10 | 8 | 18 (58.1) | ||||||||
| Tigecycline | 2 | 6 | 10 | 7 | 6 | ND | ||||||||
The erm(41) sequevar-dependent resistance of 100 M. abscessus isolates to the antibiotics indicated was determined by the microdilution method. The incubation time was 3 days (before) and 14 days (after) induction for CLA and 3 days for the other antibiotics listed.
Resistant isolates were distinguished according to the breakpoint provided by NCCLS document M24-A2. ND, no data. Tigecycline has no recommended breakpoint.
Combination antibiotic treatment and treatment response.
All patients enrolled in the study were treated with a standard combination of antibiotics based upon CLA. Patients infected with the CLA-sensitive genotype group isolates were significantly more likely to demonstrate initial sputum conversion (Fig. 1 and Table 4) (P = 0.011). Times to initial sputum conversion also differed significantly between the CLA-sensitive and CLA-resistant genotype groups (P = 004). Sputum relapse after initial conversion to negative occurred in both groups and did not differ significantly. The proportion of patients whose sputa converted and remained culture-negative during the follow-up period was significantly greater in the CLA-sensitive than in the CLA-resistant genotype group (61.3% versus 30.4%, respectively; P = 0.013). Radiographic improvement rates were significantly higher in patients infected with the CLA-sensitive genotype group isolates than in patients infected with the CLA-resistant genotype group (P = 0.006). The effective treatment response evaluated by radiology and microbiology was also significantly greater for the CLA-sensitive genotype group than for the CLA-resistant genotype group (P < 0.001).
FIG 1.
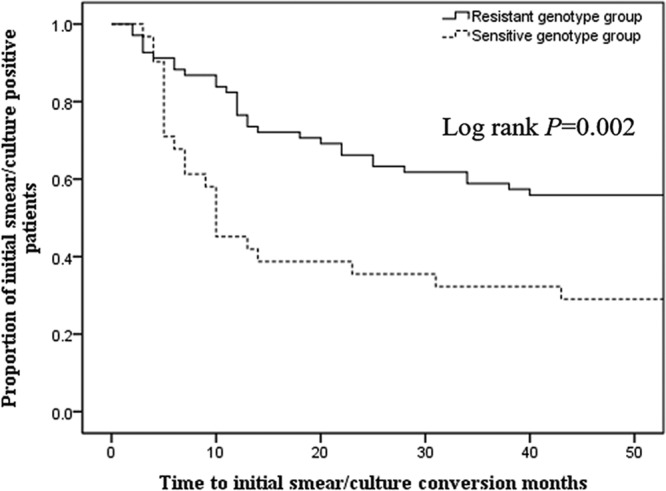
Comparison of initial sputum smear/culture conversion between patients infected with the CLA-resistant [2058-2059 rrl mutant or rrl wild type/erm(41)T28] and -sensitive [rrl wild type/erm(41)C28 or rrl wild type/erm(41) M type] genotype groups. Patients infected with the resistant group isolates showed a significantly longer initial sputum medium conversion time: 12 months versus 7 months for the sensitive group (P = 0.004).
TABLE 4.
Treatment outcomes for CLA-resistant and -sensitive genotype groups
| Parameter | Valuea |
P value | |
|---|---|---|---|
| Resistant group (n = 69) | Sensitive group (n = 31) | ||
| Median duration of treatment [mo (IQR)] | 18 (9–30) | 15 (9–22) | 0.260 |
| Sputum result | 0.013 | ||
| Conversion to stable negative | 21 (30.4) | 19 (61.3) | |
| Failure to convert | 39 (56.5) | 9 (29.0) | |
| Relapse after conversion to negative | 9 (13.0) | 3 (9.7) | |
| Initial smear/culture conversion | |||
| No. of patients who initially converted | 30 (43.5) | 22 (71.0) | 0.011 |
| Median time to initial conversion [mo (IQR)] | 12 (6–23) | 7 (5–11) | 0.004 |
| Radiological result | |||
| Improved | 25 (36.2) | 22 (71.0) | 0.006 |
| No change | 24 (34.8) | 5 (16.1) | |
| Progressed | 20 (30.0) | 4 (12.9) | |
| Final treatment response | |||
| Effective | 30 (43.5) | 26 (83.9) | <0.001 |
| Failure | 39 (56.5) | 5 (16.1) | |
The data are the number and (percentage) of patients in each group unless otherwise indicated.
In multivariate analysis, the genotype was a reliable predictor of the response of M. abscessus lung disease to treatment (odds ratio [OR] = 0.185; 95% confidence interval [CI], 0.059 to 0.579; P = 0.004) (Table 5). All other characteristics, i.e., age, sex, body mass index (BMI), colony morphology, and CT imaging, were nonpredictors.
TABLE 5.
Univariate and multivariate analyses of factors affecting combination antibiotic treatment
| Variable | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|
| Age (yr) | ||||
| >58 | 0.72 (0.33–1.60) | 0.421 | ||
| <58 | 1 | |||
| Sex | ||||
| Male | 1.93 (0.86–4.36) | 0.113 | ||
| Female | 1 | |||
| BMI | ||||
| >20.0 | 1.10 (0.48–2.35) | 0.884 | ||
| <20.0 | 1 | |||
| Resistance of isolates | ||||
| Resistant | 0.15 (0.051–0.431) | <0.001 | 0.185 (0.059–0.579) | 0.004 |
| Sensitive | 1 | 1 | ||
| Initial morphotype | ||||
| Rough | 0.86 (0.39–1.91) | 0.708 | ||
| Smooth | 1 | |||
| Positive AFB smear | ||||
| Yes | 1.13 (0.50–2.56) | 0.765 | ||
| No | 1 | |||
| Bronchiectasis | ||||
| Yes | 1.98 (0.32–12.37) | 0.467 | ||
| No | 1 | |||
| Tree-in-bud pattern | ||||
| Yes | 2.91 (1.14–7.42) | 0.025 | 2.217 (0.810–6.068) | 0.121 |
| No | 1 | 1 | ||
| Cavity | ||||
| Yes | 0.39 (0.17–0.90) | 0.027 | 0.776 (0.298–2.022) | 0.603 |
| No | 1 | 1 | ||
| Completed the initial 4 wk of treatment | ||||
| Yes | 1.38 (0.55–3.45) | 0.498 | ||
| No | 1 |
DISCUSSION
The study reported here was the first undertaken to explore and correlate the differences in treatment outcomes of M. abscessus lung disease patients with the CLA susceptibility genotype of clinical isolates. We found that the treatment results for patients infected with isolates with the CLA-sensitive M. abscessus genotype were far superior to the results for patients infected with isolates with the CLA-resistant genotype evaluated in terms of sputum conversion rate, duration of initial sputum conversion, radiological improvement, and efficacy. Treatment outcome, however, was independent of all other factors examined, which included BMI, colony morphology, and radiological images.
In 2006, the M. abscessus complex was first divided into M. abscessus subsp. abscessus and M. abscessus subsp. massiliense based upon differences in the rpoB gene (24). In 2011, Bastian and coworkers reported that variations in the erm(41) genotype influenced the sensitivity of these subtypes to CLA in vitro (15). The clinical characteristics and treatment outcomes of patients infected with M. abscessus subsp. abscessus and M. abscessus subsp. massiliense differed in subsequent studies (17–20). Patients infected with M. abscessus subsp. massiliense usually responded better to treatment due, in part, to the CLA sensitivity of the organism. Several genotypes are associated with CLA sensitivity and -resistance: rrl mutant/wild type, erm(41)T28, erm(41)C28, and erm(41) M type. In the study described here, clinical isolates were grouped into these genotypes rather than M. abscessus subsp. abscessus and M. abscessus subsp. massiliense, and the responses of patients to standard, CLA-based treatment were assessed and compared. The response of the CLA-sensitive genotype group was significantly superior to that of the CLA-resistant genotype group judged in terms of the sputum conversion rate, radiological improvement, duration of initial sputum conversion results, and treatment efficacy. While lung disease patients infected with M. abscessus subsp. abscessus [erm(41)C28 genotype] isolates may exhibit a better response to combination CLA treatment, the response of patients infected with M. abscessus subsp. massiliense isolates expressing the 2058-2059 rrl mutation was often much worse. As such, CLA susceptibility genotyping is more accurate than subtyping as an approach to predicting the treatment outcomes of patients with M. abscessus lung disease (46.4% versus 42.9% true-positive rates, respectively).
The effect of BMI on the treatment outcomes of patients with NTM lung disease was demonstrated in several studies (25, 26). A recent retrospective study suggested that, in addition to CLA sensitivity, BMI was a factor that affected the success of M. abscessus lung disease treatment (20). This suggestion, however, was not confirmed by the present study. The overall BMIs of M. abscessus lung disease patients enrolled in our study were low; moreover, multivariate analysis failed to support its value in predicting an effective treatment outcome. This finding is consistent with results reported by other investigators (19). Similarly, predictions concerning the outcome of antibiotic therapy based upon symptoms or CT imaging are unrealistic. While hemoptysis and cavity-like manifestations were more common among the patients infected with CLA-resistant genotype M. abscessus, these factors failed to predict the prognosis upon multivariate analysis.
Patients infected with M. abscessus characterized by a rough-type colony exhibited a high incidence of cavities in CT images. This finding is consistent with the conclusion that rough-type strains usually exhibit higher virulence and pathogenicity. Unlike previous studies (19), however, we found that the initial colony morphology failed to correlate with the final radiologic improvement rate or treatment efficacy (Table 2). Jonsson and coworkers reported a significant increase in the number of rough colonies during the course of infection and the occurrence of smooth-to-rough colony conversion (27). Thus, we speculate that colony morphology is associated only with pathogenicity and the pathogenesis of infection and is not a reliable predictor of treatment efficacy.
The study described here has several limitations. First, only a relative small number of isolates exhibited the rrl mutation and erm(41)C28 genotypes; consequently, their characteristics may not be representative. Solidifying their characteristics will require the enrollment of more patients infected with isolates exhibiting the rrl mutant and erm(41)C28 genotypes in future studies. Second, a minority of patients relapsed following initially successful treatment (see Table S2 in the supplemental material). Conceivably, these relapses were due to subsequent infection by a different M. abscessus strain or genotype. In the absence of dynamic follow-up, our study failed to determine whether recurrence occurred due to reinfection by a different M. abscessus strain.
In conclusion, there was a significant difference in treatment outcomes for patients infected with CLA-resistant and -sensitive M. abscessus genotype isolates. The CLA-sensitive genotype group was significantly superior in sputum conversion rate, initial sputum conversion time, radiological improvement, and treatment efficacy. Accurate genotyping is an important factor in predicting the efficacy of combination therapy with CLA-based antibiotics. Rapid genotyping should help clinicians optimize therapeutic strategies, especially in cases of critically ill patients who cannot wait weeks for culture and susceptibility testing. Genotyping would also be effective as a diagnostic approach in areas where facilities for mycobacterial culture and susceptibility testing are unavailable.
MATERIALS AND METHODS
Study population.
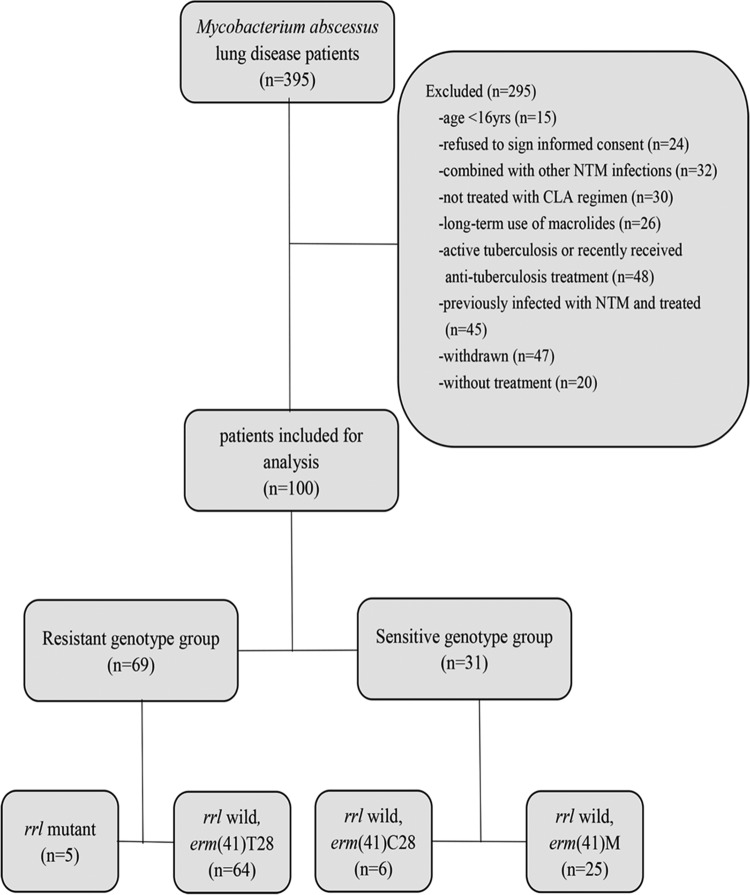
A retrospective review of the medical records of all patients with M. abscessus lung disease was conducted between January 2012 and December 2015 at the Shanghai Pulmonary Hospital. Patient inclusion criteria were as follows: (i) age, >16 years; (ii) underwent initial diagnosis and treatment at the Shanghai Pulmonary Hospital in accordance with the 2007 American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) guidelines; (iii) received oral CLA-based combination treatment; (iv) follow-up period lasted more than 6 months. The exclusion criteria were as follows: (i) age, <16 years; (ii) history of NTM lung disease; (iii) lack of critical visit data (e.g., regular sputum culture or CT examination), failure to follow up, or death from non-M. abscessus lung disease-related causes; (iv) treatment did not include oral CLA; (v) history of long-term macrolide drug treatment; (vi) diagnosed with active tuberculosis or received antituberculosis treatment within 3 months prior to study enrollment; (vii) coinfected with another nontuberculosis mycobacterium; (viii) refused to sign informed consent form; (ix) AIDS. In addition, patients with cystic fibrosis were not included in the study; notably, cystic fibrosis is extremely rare among Asian patients. A detailed, patient enrollment flow chart is shown in Fig. 2. This study was approved by the Ethics Committees of Shanghai Pulmonary Hospital and Tongji University School of Medicine, ethics number K17-150. All participants signed informed consent forms before enrollment.
FIG 2.
Flow diagram of the study. One hundred M. abscessus lung disease patients who conformed to the inclusion criteria were enrolled. Sixty-nine patients were in the CLA-resistant genotype group, including 5 patients infected with rrl 2058-2059 mutants and 64 erm(41)T28-type-infected patients; 31 belonged to the CLA-sensitive group, including 6 erm(41)C28- and 25 erm(41) M-type-infected patients.
Collection, identification, and preservation of bacteria.
All the clinical M. abscessus isolates used in this study were preserved in the Clinical Microbiology Laboratory of Shanghai Pulmonary Hospital. Shanghai Pulmonary Hospital is one of the designated treatment centers for tuberculosis and NTM disease in China, attracting NTM disease cases nationwide. M. abscessus isolates were obtained from sputum and bronchoalveolar lavage fluid. Samples were transferred to Lowenstein-Jensen (L-J) agar plates after treatment with 4% NaOH. Smears prepared from the bacterial colonies that grew were stained and examined microscopically to identify the acid-fast organisms. To select further NTM, positive colonies were inoculated and cultured in L-J medium containing 0.5 mg/ml P-nitrobenzoic acid and 5 mg/ml 2-thiophenecarboxylic acid hydrazide for 1 to 2 weeks at 37°C. Bacterial isolates that grew rapidly were selected for molecular typing by PCR. The bacteria were digested with 1 mg/ml lysozyme and 1 mg/ml proteinase K, and the DNA was extracted with phenol-chloroform. First, the rpoB gene was amplified by PCR, and the DNA sequences were determined. To confirm the M. abscessus complexes, 754 bp of the DNA segment was subjected to BLAST analysis. Second, the erm(41) gene was amplified, and the DNA sequence was analyzed to identify and differentiate M. abscessus subsp. massiliense, M. abscessus subsp. abscessus, and M. abscessus subsp. bolletii. Finally, the PRA-hsp65 gene was compared to an online reference (http://app.chuv.ch/prasite/index.html) to confirm the M. abscessus subsp. abscessus and M. abscessus subsp. bolletii identifications. M. abscessus subsp. bolletii was excluded from the study because it is essentially absent in China. Identified M. abscessus subsp. abscessus and M. abscessus subsp. massiliense isolates, stored at −80°C, were subsequently recovered for microbiology and molecular biology studies.
Identification of colony morphology.
Single colonies were obtained from frozen M. abscessus isolates by growth on Middlebrook 7H10 agar plates supplemented with 10% oleic acid-albumin-dextrose-catalase. The colonies were classified macroscopically as smooth or rough. If isolates gave rise to colonies of both morphotypes, a colony of each type was analyzed separately, and the identity was established by whole-genome sequencing.
Genotype analysis.
Genomic information for all isolates was obtained by whole-genome sequencing. Single nucleotide polymorphism (SNP) analysis was performed using the NCBI GenBank database and BLAST algorithm. The following genotypes were of specific interest: erm(41) [including erm(41)C28, erm(41)T28, and erm(41) M type], rll wild type, and rrl 2058-2059 mutant.
(i) Whole-genome sequencing.
Detailed methods were published previously by us (28). DNA was extracted according to the method of Somerville and coworkers (29), and paired-end libraries with insert sizes of ∼400 bp were prepared following Illumina's standard genomic DNA library preparation protocol (Illumina, San Diego, CA, USA). After shearing, ligating, and PCR, the qualified Illumina paired-end library was used for Illumina HiSeq sequencing (paired-end 150 bp × 2). The default parameters of the SPAdes software (version v.3.6.0) (http://bioinf.spbau.ru/en/spades) were used to assemble the genome draft (30). The assembled product was evaluated using QUAST (version v.2.3) (31; http://quast.bioinf.spbau.ru/).
(ii) SNP analysis.
The NCBI Nucleotide BLAST program was used for SNP analysis. The standard ATCC 19977 (NC_010397.1) M. abscessus strain served as the reference for rrl and erm(41)T28, CR5701 (HQ127366.1) was used as the reference strain for erm(41)C28, and CCUG48898 (AP014547.1) was the reference for M type.
Drug sensitivity assay.
Antibiotic sensitivity was determined by the microdilution method. Sulfonamides, moxifloxacin, cefoxitin, amikacin, doxycycline, tigecycline, CLA, linezolid, imipenem, and tobramycin are among the most common antibiotics used to treat M. abscessus infections; each was tested (TREK Diagnostic Systems, Brooklyn Heights, OH, USA). CLA resistance was assessed at 3 days and 14 days after M. abscessus exposure. Antibiotics' susceptible and resistant breakpoints were interpreted according to Clinical and Laboratory Standards Institute (CLSI) document M24-A2. Staphylococcus aureus (ATCC 29213; American Type Culture Collection, Manassas, VA, USA) served as the control reference strain.
Treatment regimen and efficacy evaluation.
All patients were treated with antibiotics as follows: an initial 4-week course of amikacin (15 mg/kg of body weight/day in two equal doses) combined with cefoxitin (200 mg/kg/day with a maximum of 12 g/day in three equal doses) by intravenous administration. CLA was also administered orally from the beginning of therapy. After 4 weeks, an oral regimen of CLA combined with levofloxacin or moxifloxacin was given. If an adverse reaction to either amikacin or cefoxitin occurred, the regimen was replaced with imipenem (500 mg three times a day), linezolid (600 mg once every 12 h), or tigecycline (100 mg initially, followed by 50 mg every 12 h). CLA was administered continually throughout the course of treatment as recommended in the guidelines.
All the patients underwent chest CT examination, as well as sputum smears and culture, regularly. Therapeutic efficacy was determined according to the results of microbiological examination and radiological changes. The clinical characteristics, sputum culture conversion rate and time, radiological improvement rate, and microbiological characteristics of each genotype group were compared. Culture conversion was defined as three consecutive negative cultures from sputum specimens. Effective treatment was defined as sputum culture negative or significant pulmonary lesion resolution without recurrence during the observation period. Ineffective treatment included failure to achieve culture and smear conversion, recurrence after initial culture conversion, and appearance of increased or stable lesions in CT scans.
Statistical analysis.
All statistical analyses were conducted using SPSS20.0 (IBM, Armonk, NY, USA). The data were compared using Student's t test or the Mann-Whitney U test for continuous variables and the Pearson χ2 test or Fisher exact test for categorical variables. P values of <0.05 were considered statistically significant in a 2-tailed analysis. Times to initial culture conversion were compared using the Kaplan-Meier method. Potential predictors of the treatment response were assessed by multivariable logistic regression. In the logistic regression models, variables with P values of <0.1 in the univariable analysis were included in the multivariable analysis.
Accession number(s).
The accession numbers for all the M. abscessus isolates sequenced in this study are available at DDBJ/ENA/GenBank under BioProject PRJNA398137.
Supplementary Material
ACKNOWLEDGMENTS
We declare no conflict of interest.
This project was supported by grants obtained from the National Natural Science Foundation of China (no. 81672063); the Medical Guide Program of Shanghai Science and Technology Committee (no. 14411962900); the Key Project of Shanghai Municipal Health and Family Planning Commission (no. 201540367); the Youth Project of Shanghai Municipal Health and Family Planning Commission (no. 20164Y0230); and the New Frontier Technology Joint Project of Municipal Hospital, Shanghai Shenkang Hospital Development Center (no. SHDC12017113).
We sincerely thank Stephen H. Gregory (Providence, Rhode Island, USA), who helped write and edit the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02360-17.
REFERENCES
- 1.Prevots DR, Marras TK. 2015. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, Beylis N, Boeree MJ, Cacho J, Chihota V, Chimara E, Churchyard G, Cias R, Daza R, Daley CL, Dekhuijzen PN, Domingo D, Drobniewski F, Esteban J, Fauville-Dufaux M, Folkvardsen DB, Gibbons N, Gomez-Mampaso E, Gonzalez R, Hoffmann H, Hsueh PR, Indra A, Jagielski T, Jamieson F, Jankovic M, Jong E, Keane J, Koh WJ, Lange B, Leao S, Macedo R, Mannsaker T, Marras TK, Maugein J, Milburn HJ, Mlinko T, Morcillo N, Morimoto K, Papaventsis D, Palenque E, Paez-Pena M, Piersimoni C, Polanova M, Rastogi N, Richter E, Ruiz-Serrano MJ, Silva A, da Silva MP, Simsek H, van Soolingen D, Szabó N, Thomson R, Tórtola Fernandez T, Tortoli E, Totten SE, Tyrrell G, Vasankari T, Villar M, Walkiewicz R, Winthrop KL, Wagner D, Nontuberculous Mycobacteria Network European Trials Group. 2013. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 3.Brode SK, Daley CL, Marras TK. 2014. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuberc Lung Dis 18:1370–1377. doi: 10.5588/ijtld.14.0120. [DOI] [PubMed] [Google Scholar]
- 4.Guimaraes T, Chimara E, do Prado GV, Ferrazoli L, Carvalho NG, Simeao FC, de Souza AR, Costa CA, Viana Niero C, Brianesi UA, di Gioia TR, Gomes LM, Spadao FS, Silva MD, de Moura EG, Levin AS. 2016. Pseudooutbreak of rapidly growing mycobacteria due to Mycobacterium abscessus subsp. bolletii in a digestive and respiratory endoscopy unit caused by the same clone as that of a countrywide outbreak. Am J Infect Control 44:e221–e226. doi: 10.1016/j.ajic.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, Verma D, Hill E, Drijkoningen J, Gilligan P, Esther CR, Noone PG, Giddings O, Bell SC, Thomson R, Wainwright CE, Coulter C, Pandey S, Wood ME, Stockwell RE, Ramsay KA, Sherrard LJ, Kidd TJ, Jabbour N, Johnson GR, Knibbs LD, Morawska L, Sly PD, Jones A, Bilton D, Laurenson I, Ruddy M, Bourke S, Bowler IC, Chapman SJ, Clayton A, Cullen M, Dempsey O, Denton M, Desai M, Drew RJ, Edenborough F, Evans J, Folb J, Daniels T, Humphrey H, Isalska B, Jensen-Fangel S, Jonsson B, Jones AM, Katzenstein TL, Lillebaek T, MacGregor G, Mayell S, Millar M, Modha D, Nash EF, O'Brien C, O'Brien D, Ohri C, Pao CS, Peckham D, Perrin F, Perry A, Pressler T, Prtak L, Qvist T, Robb A, Rodgers H, Schaffer K, Shafi N, van Ingen J, Walshaw M, Watson D, West N, Whitehouse J, Haworth CS, Harris SR, Ordway D, Parkhill J, Floto RA. 2016. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354:751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax 71:88–90. doi: 10.1136/thoraxjnl-2015-207983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS, US Cystic Fibrosis Foundation, European Cystic Fibrosis Society. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 71(Suppl 1):i1–22. doi: 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 9.Griffith DE, Girard WM, Wallace RJ Jr. 1993. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis 147:1271–1278. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 10.Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH, Huang YT, Yang PC, Luh KT, Hsueh PR. 2010. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000-2008. Emerg Infect Dis 16:294–296. doi: 10.3201/eid1602.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. 2013. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998-2010. Emerg Infect Dis 19:1889–1891. doi: 10.3201/eid1911.130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 13.Wallace RJ Jr, Meier A, Brown BA, Zhang Y, Sander P, Onyi GO, Bottger EC. 1996. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob Agents Chemother 40:1676–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash KA, Brown-Elliott BA, Wallace RJ Jr. 2009. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastian S, Veziris N, Roux AL, Brossier F, Gaillard JL, Jarlier V, Cambau E. 2011. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother 55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubio M, March F, Garrigo M, Moreno C, Espanol M, Coll P. 2015. Inducible and acquired clarithromycin resistance in the Mycobacterium abscessus complex. PLoS One 10:e0140166. doi: 10.1371/journal.pone.0140166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada T, Akiyama Y, Kurashima A, Nagai H, Tsuyuguchi K, Fujii T, Yano S, Shigeto E, Kuraoka T, Kajiki A, Kobashi Y, Kokubu F, Sato A, Yoshida S, Iwamoto T, Saito H. 2012. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung diseases. J Clin Microbiol 50:3556–3561. doi: 10.1128/JCM.01175-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 19.Koh WJ, Jeong BH, Kim SY, Jeon K, Park KU, Jhun BW, Lee H, Park HY, Kim DH, Huh HJ, Ki CS, Lee NY, Kim HK, Choi YS, Kim J, Lee SH, Kim CK, Shin SJ, Daley CL, Kim H, Kwon OJ. 2017. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis 64:309–316. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Cho J, Lee CH, Han SK, Yim JJ. 2017. Progression and treatment outcomes of lung disease caused by Mycobacterium abscessus and Mycobacterium massiliense. Clin Infect Dis 64:301–308. doi: 10.1093/cid/ciw723. [DOI] [PubMed] [Google Scholar]
- 21.Kim HY, Kim BJ, Kook Y, Yun YJ, Shin JH, Kim BJ, Kook YH. 2010. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol Immunol 54:347–353. doi: 10.1111/j.1348-0421.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 22.Mougari F, Amarsy R, Veziris N, Bastian S, Brossier F, Bercot B, Raskine L, Cambau E. 2016. Standardized interpretation of antibiotic susceptibility testing and resistance genotyping for Mycobacterium abscessus with regard to subspecies and erm41 sequevar. J Antimicrob Chemother 71:2208–2212. doi: 10.1093/jac/dkw130. [DOI] [PubMed] [Google Scholar]
- 23.Mougari F, Bouziane F, Crockett F, Nessar R, Chau F, Veziris N, Sapriel G, Raskine L, Cambau E. 2017. Selection of resistance to clarithromycin in Mycobacterium abscessus subspecies. Antimicrob Agents Chemother 61:e00943-16. doi: 10.1128/AAC.00943-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adekambi T, Berger P, Raoult D, Drancourt M. 2006. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int J Syst Evol Microbiol 56:133–143. doi: 10.1099/ijs.0.63969-0. [DOI] [PubMed] [Google Scholar]
- 25.Bonaiti G, Pesci A, Marruchella A, Lapadula G, Gori A, Aliberti S. 2015. Nontuberculous mycobacteria in noncystic fibrosis bronchiectasis. Biomed Res Int 2015:197950. doi: 10.1155/2015/197950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MR, Yang CY, Chang KP, Keng LT, Yen DH, Wang JY, Wu HD, Lee LN, Yu CJ. 2013. Factors associated with lung function decline in patients with non-tuberculous mycobacterial pulmonary disease. PLoS One 8:e58214. doi: 10.1371/journal.pone.0058214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson BE, Gilljam M, Lindblad A, Ridell M, Wold AE, Welinder-Olsson C. 2007. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J Clin Microbiol 45:1497–1504. doi: 10.1128/JCM.02592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo L, Liu W, Li B, Li M, Huang D, Jing L, Chen H, Yang J, Yue J, Wang F, Chu H, Zhang Z. 2016. Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of Mycobacterium abscessus subspecies according to whole-genome sequencing. J Clin Microbiol 54:2982–2989. doi: 10.1128/JCM.01151-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somerville W, Thibert L, Schwartzman K, Behr MA. 2005. Extraction of Mycobacterium tuberculosis DNA: a question of containment. J Clin Microbiol 43:2996–2997. doi: 10.1128/JCM.43.6.2996-2997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurevich A, Saveliev V, Vyahhi N, Tesler G. 2013. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.