ABSTRACT
When overproduced, the multidrug efflux system MexEF-OprN increases the resistance of Pseudomonas aeruginosa to fluoroquinolones, chloramphenicol, and trimethoprim. In this work, we demonstrate that gain-of-function mutations in the regulatory gene mexT result in oligomerization of the LysR regulator MexT, constitutive upregulation of the efflux pump, and increased resistance in clinical isolates.
KEYWORDS: Pseudomonas aeruginosa, antibiotic resistance, MexEF-OprN, MexT
TEXT
Pseudomonas aeruginosa, an opportunistic pathogen of major clinical importance, is responsible for acute and chronic infections in vulnerable patients. Its intrinsic and/or acquired resistance to a wide range of antibiotics in part relies on constitutive or inducible production of several efflux systems belonging to the resistance-nodulation-cell division (RND) family of drug transporters (1). Among these systems, MexEF-OprN is able to export a rather short list of antimicrobials, including ciprofloxacin (CIP), chloramphenicol (CHL), and trimethoprim (TMP). This efflux pump, which is quiescent in wild-type strains, is overproduced at high levels in nfxC mutants, making them more resistant (from 2- to 32-fold) to the pump substrates (2). The nfxC mutants also exhibit some additional phenotypic traits, such as a decreased susceptibility to carbapenems and a hypersusceptibility to some other β-lactams, which are not related to MexEF-OprN activity but are concomitant to the downregulation of the gene oprD (3) and operon mexAB-oprM, respectively (4). MexEF-OprN production is regulated by MexT, a LysR-type transcriptional regulator (LTTR), whose gene (mexT) is located upstream of operon mexEF-oprN (3). All the mutations identified so far in clinical MexEF-OprN-overproducing strains affect a gene, mexS, which encodes a presumed quinone oxidoreductase, MexS (5–7). The present study reports on the characterization of five nonclonal clinical mutants harboring wild-type mexS genes (6–8). DNA sequencing experiments revealed that these strains contained missense mutations in mexT. Since the impact of these mutations on protein function was unknown, we sought to determine whether amino acid substitutions in the regulator MexT can account for the upregulation of the mexEF-oprN operon and drug resistance.
The relative expression of the gene mexE, as determined by reverse transcriptase quantitative PCR (RT-qPCR) (7), was found to be higher (from 20- to 112-fold) in these bacteria than in the wild-type reference strain PA14 (Table 1). In addition, MIC experiments (9) confirmed that all of the isolates were more resistant to CIP (from 0.5 to 8 μg · ml−1), CHL (from 128 to 2,048 μg · ml−1), and TMP (from 512 to >2,048 μg · ml−1) than PA14 (0.125, 64, and 64 μg · ml−1, respectively) (Table 1).
TABLE 1.
Effects of amino acid substitutions in regulator MexT
| Strain | MexT substitution (304 aaa) | Transcript levelb |
MIC (μg · ml−1)c |
Reference or source | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mexEd | oprD | mexB | CIP | CHL | TMP | IMP | MEM | ATM | |||
| Clinical strains | |||||||||||
| 4177 | R166H | 20 | NDe | 0.6 | 0.5 | 1,024 | 1,024 | 16 | 2 | 32 | 6 |
| 4088 | G257S | 112 | ND | 0.4 | 1 | 2,048 | >2,048 | 8 | 0,25 | 1 | 6 |
| 10-12 | G257A | 26 | NRf | 9.2 | 2 | 128 | >2,048 | 16 | ND | 32 | 8 |
| 0810 | G258D | 32 | ND | ND | 8 | 1,024 | 1,024 | 4 | 1 | ND | 7 |
| 1510 | Y138D plus G258D | 21 | ND | ND | 2 | 256 | 512 | 16 | 8 | ND | 7 |
| Complemented PA14 derivatives | |||||||||||
| PA14 | WTg | 1 | 1 | 1 | 0.125 | 64 | 64 | 1 | 0.5 | 4 | F. Ausubel |
| PA14ΔmexS | WT | 192 ± 9.2 | 0.3 ± 0.1 | 0.4 ± 0.2 | 2 | 2,048 | >2,048 | 4 | 2 | 2 | 7 |
| PA14ΔmexT | −h | 0.4 ± 0.1 | 1.7 ± 0.2 | 2.2 ± 0.2 | 0.125 | 32 | 32 | 1 | 0.5 | 4 | This study |
| PA14ΔmexTPA14 | WT | 2.2 ± 0.7 | 1.1 ± 0.1 | 1.4 ± 0.2 | 0.125 | 64 | 64 | 1 | 0.5 | 4 | This study |
| PA14ΔmexT4177 | R166H | 3.2 ± 0.3 | 2.0 ± 0.2 | 2.6 ± 0.1 | 0.125 | 64 | 64 | 1 | 0.5 | 4 | This study |
| PA14ΔmexT4088 | G257S | 189 ± 5.9 | 0.4 ± 0.1 | 0.9 ± 0.1 | 2 | 2,048 | >2,048 | 2 | 1 | 2 | This study |
| PA14ΔmexT10-12 | G257A | 110 ± 3.6 | 0.4 ± 0.1 | 0.9 ± 0.1 | 1 | 1,024 | 1,024 | 2 | 1 | 2 | This study |
| PA14ΔmexT0810 | G258D | 1.9 ± 0.4 | 0.9 ± 0.1 | 1.4 ± 0.1 | 0.125 | 64 | 64 | 1 | 0.5 | 4 | This study |
| PA14ΔmexT1510 | Y138D plus G258D | 6.3 ± 0.2 | 0.9 ± 0.1 | 1.4 ± 0.1 | 0.25 | 64 | 64 | 1 | 0.5 | 4 | This study |
aa, amino acids.
Mean gene expression values were calculated from two independent bacterial cultures, each of which was assayed in duplicates. They are expressed as a ratio to the gene transcription level in wild-type reference strain PA14.
CIP (ciprofloxacin), CHL (chloramphenicol), and TMP (trimethoprim) are substrates of MexEF-OprN. IMP (imipenem) and MEM (meropenem) are substrates of porin OprD (corepressed with mexEF-oprN overexpression). ATM (aztreonam) is a substrate of MexAB-OprM.
Significant overexpression of mexE (threshold fixed at 20-fold) and increase in resistance to MexEF-OprN substrates is indicated in boldface type.
ND, not determined.
NR, not relevant because of a codon stop in the oprD gene.
WT, wild type.
−, deleted gene.
To investigate the relevance of the observed amino acid changes in MexT, we first deleted gene mexT from PA14 as described previously (7). The mutated alleles from clinical strains were then transferred by conjugation using MiniCTX1-derived recombinant plasmids (10) and were inserted into the chromosome of the mutant strain PA14ΔmexT. Complementation of this mutant with alleles from strains 4177, 0810, and 1510 had no impact on mexE transcription and MIC values (Table 1). In contrast, MexT variants from strains 4088 and 10-12 triggered mexE expression that was 189- and 110-fold, respectively, above the baseline level. As expected, this was associated with an increased resistance of strains PA14ΔmexT4088 and PA14ΔmexT10-12 to CIP (16× and 8×, respectively), CHL (32× and 16×, respectively), and TMP (≥32× and 16×, respectively) compared to that of PA14ΔmexTPA14 (Table 1). These results suggested that these two latter MexT variants are under a constitutively active conformation, able to upregulate the MexEF-OprN pump. They also pointed to the importance of residue G257 in MexT activation, as both variants harbor single-amino acid substitutions at this position (G257S and G257A). Moreover, confirming that when activated, MexT is able to downregulate oprD (3) and mexAB-oprM (4), the mRNA levels of these two loci turned out to be 2.7-fold (oprD) and 1.6-fold (mexB) lower in PA14ΔmexT4088 and PA14ΔmexT10-12, respectively, than in PA14ΔmexTPA14. These results were consistent with a 4-fold-increased resistance to imipenem and meropenem, two antibiotics that selectively diffuse through porin OprD, and a 2-fold higher susceptibility to the MexAB-OprM pump substrate aztreonam (Table 1).
It is known that under oxidative conditions, MexT forms an active oligomer, while reducing conditions result in inactive monomers (11). This is in accordance with the usual mode of action of LTTRs, whereby an active tetramer is formed once a cognate coinducer has bound the inactive monomers (12). To determine whether MexT variants from strains 4088 and 10-12 spontaneously form oligomers (i.e., in the absence of ligand), a bacterial two-hybrid (BACTH) assay (13) was performed in strain DHM1 (cya mutant) of Escherichia coli, with plasmids pUT18 and pKNT25 that code for T18 and T25 subunits, respectively, of CyaA adenylate cyclase. This assay, which has been set up to study protein-protein interactions (13), is based on the reconstitution of adenylate cyclase activity and cAMP synthesis in E. coli. BACTH experiments confirmed that in the absence of a cognate ligand, MexTPA14 occurs as a monomer, as no signal of oligomerization was observed either by using reporter plates or by measuring the β-galactosidase (β-Gal) activity (17 ± 1.66 Miller units) (Table 2). As expected, MexT4088 and MexT10-12 yielded positive results (Table 2), supporting the notion that they can spontaneously form oligomers and activate the expression of mexEF-oprN. To check if other MexT variants are also able to self-oligomerize, three of them (MexT0810, MexT1510, and MexT4177) were subjected to the BACTH assay. The results obtained with MexT0810 and MexT1510 were very similar to that of the wild-type control MexTPA14 (24 ± 9.04 and 32 ± 18.6 Miller units versus 19 ± 1.66, respectively). However, for unclear reasons, a β-Gal activity of 110 ± 20.8 Miller units was recorded with MexT4177, indicating that this variant would also form oligomers, though without impacting mexEF-oprN expression. Whether MexT4177 is able to regulate other target genes of the MexT regulon remains to be investigated (14).
TABLE 2.
MexT oligomerization assayed by bacterial two-hybrid experiments
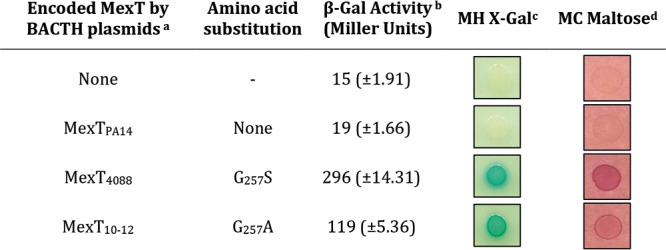
Plasmids pUT18 (ampicillinr) and pKNT25 (kanamycinr), for which the tags are at the C termini of the recombinant proteins, were used in this experiment. Full-length alleles of mexT (915 bp) were cloned using primers TH-MexT Fw (CCATGAACCGAAACGACCTGCG) and TH-MexT Rv (AGAGACTGTCCGGATCGCCGA).
Average values were calculated from five independent bacterial cultures, each assayed in triplicates.
MH, Mueller-Hinton plates containing 40 μg · ml−1 X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; revealing cAMP production in blue), 50 μg · ml−1 kanamycin, and 100 μg · ml−1 ampicillin.
MC, MacConkey plates containing 1% maltose (revealing cAMP production in red), 50 μg · ml−1 kanamycin, and 100 μg · ml−1 ampicillin.
To get insight into what effects the substitutions G257S and G257A may have on MexT oligomerization, we mapped these mutations on a three-dimensional dimeric LTTR model. As the crystal structure of MexT has not been determined yet, we used the dimeric structure of DntR from Burkholderia spp., another LTTR that shares 66% sequence similarity with MexT, according to Clustal Ω results (15). In DntR, position 257 is occupied by a phenylalanine residue within the coinducer binding domain. Interestingly, Phe-257 residues of DntR monomers face each other at the interphase of the dimer (Fig. 1), suggesting that they could play a role in dimer stabilization. Nevertheless, the structural changes caused by amino acid substitutions at position 257 on MexT oligomerization will have to be confirmed once the crystal structure of this regulator is available.
FIG 1.
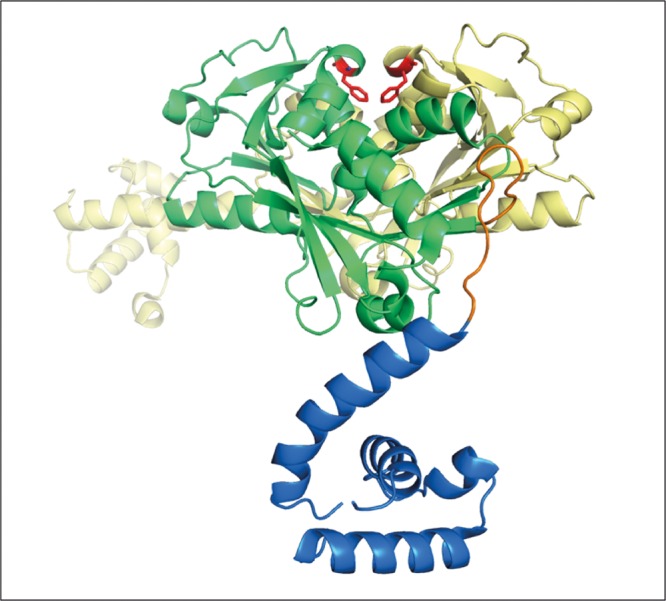
Crystal structure of DntR dimer from Burkholderia spp. (PDB 5AE5) (first reported in reference 15). One monomer is colored to indicate the functions of its constituting domains (green for the coinducer binding domain, blue for the DNA binding domain, and orange for the loop linking the two domains). The second monomer is in yellow. Phenylalanine-257 residues of the two monomers are highlighted in red.
Gain-of-function mutations in LTTRs had already been reported for Salmonella enterica serovar Typhimurium (16, 17) and Acinetobacter baylyi (18). In S. enterica, gene cysB encodes an LTTR controlling the expression of the cysteine regulon. It was found that spontaneous mutants harboring substitutions T149M and T149P in CysB overexpressed genes cysK and cysP and operon cysJIH in the absence of coinducer N-acetyl-l-serine (16, 17). In A. baylyi, the LTTRs CatM and BenM regulate aromatic compound degradation. The ability of these regulators to become constitutively active was studied by site-directed mutagenesis. As a result, substitutions R156H in CatM and R156H plus T157S in BenM yielded mutants that did not require inducers such as benzoate and cis,cis-muconate to activate the catabolic pathway (18). The present study is the first to report on MexT-dependent mutational activation of efflux pump MexEF-OprN in antibiotic-resistant clinical isolates of P. aeruginosa. This observation comes in complement with another study showing that some multidrug-resistant strains of P. aeruginosa upregulate the intrinsic β-lactamase AmpC through a gain-of-function mutation (G154R) in the related LTTR AmpR (19). Altogether, these data highlight the role that LTTRs may play in the emergence of multidrug resistance in this highly adaptive pathogen.
ACKNOWLEDGMENTS
We thank Angélique Joriot for her technical assistance in DNA sequencing.
This work was supported with grants from the French “Ministère de l'Enseignement Supérieur et de la Recherche” and from the cystic fibrosis foundations “Vaincre la Mucoviscidose” and “Grégory Lemarchal.”
We declare no conflict of interest.
Ethical approval was not required for this study.
REFERENCES
- 1.Li XZ, Plesiat P, Nikaido H. 2015. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Köhler T, Michéa-Hamzehpour M, Henze U, Gotoh N, Curty LK, Pechère JC. 1997. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol 23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 3.Köhler T, Epp SF, Curty LK, Pechère JC. 1999. Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J Bacteriol 181:6300–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maseda H, Sawada I, Saito K, Uchiyama H, Nakae T, Nomura N. 2004. Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob Agents Chemother 48:1320–1328. doi: 10.1128/AAC.48.4.1320-1328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobel ML, Neshat S, Poole K. 2005. Mutations in PA2491 (mexS) promote MexT-dependent mexEF-oprN expression and multidrug resistance in a clinical strain of Pseudomonas aeruginosa. J Bacteriol 187:1246–1253. doi: 10.1128/JB.187.4.1246-1253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llanes C, Köhler T, Patry I, Dehecq B, van Delden C, Plésiat P. 2011. Role of the MexEF-OprN efflux system in low-level resistance of Pseudomonas aeruginosa to ciprofloxacin. Antimicrob Agents Chemother 55:5676–5684. doi: 10.1128/AAC.00101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardot C, Juarez P, Jeannot K, Patry I, Plesiat P, Llanes C. 2016. Amino acid substitutions account for most MexS alterations in clinical nfxC mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:2302–2310. doi: 10.1128/AAC.02622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llanes C, Pourcel C, Richardot C, Plesiat P, Fichant G, Cavallo JD, Merens A, GERPA Study Group. 2013. Diversity of beta-lactam resistance mechanisms in cystic fibrosis isolates of Pseudomonas aeruginosa: a French multicentre study. J Antimicrob Chemother 68:1763–1771. doi: 10.1093/jac/dkt115. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Hoang TT, Kutchma AJ, Becher A, Schweizer HP. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59–72. doi: 10.1006/plas.1999.1441. [DOI] [PubMed] [Google Scholar]
- 11.Fargier E, Mac Aogain M, Mooij MJ, Woods DF, Morrissey JP, Dobson AD, Adams C, O'Gara F. 2012. MexT functions as a redox-responsive regulator modulating disulfide stress resistance in Pseudomonas aeruginosa. J Bacteriol 194:3502–3511. doi: 10.1128/JB.06632-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maddocks SE, Oyston PC. 2008. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 154:3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- 13.Battesti A, Bouveret E. 2012. The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli. Methods 58:325–334. doi: 10.1016/j.ymeth.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Tian ZX, Fargier E, Mac Aogain M, Adams C, Wang YP, O'Gara F. 2009. Transcriptome profiling defines a novel regulon modulated by the LysR-type transcriptional regulator MexT in Pseudomonas aeruginosa. Nucleic Acids Res 37:7546–7559. doi: 10.1093/nar/gkp828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devesse L, Smirnova I, Lonneborg R, Kapp U, Brzezinski P, Leonard GA, Dian C. 2011. Crystal structures of DntR inducer binding domains in complex with salicylate offer insights into the activation of LysR-type transcriptional regulators. Mol Microbiol 81:354–367. doi: 10.1111/j.1365-2958.2011.07673.x. [DOI] [PubMed] [Google Scholar]
- 16.Colyer TE, Kredich NM. 1994. Residue threonine-149 of the Salmonella typhimurium CysB transcription activator: mutations causing constitutive expression of positively regulated genes of the cysteine regulon. Mol Microbiol 13:797–805. doi: 10.1111/j.1365-2958.1994.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 17.Colyer TE, Kredich NM. 1996. In vitro characterization of constitutive CysB proteins from Salmonella typhimurium. Mol Microbiol 21:247–256. doi: 10.1046/j.1365-2958.1996.6301347.x. [DOI] [PubMed] [Google Scholar]
- 18.Craven SH, Ezezika OC, Haddad S, Hall RA, Momany C, Neidle EL. 2009. Inducer responses of BenM, a LysR-type transcriptional regulator from Acinetobacter baylyi ADP1. Mol Microbiol 72:881–894. doi: 10.1111/j.1365-2958.2009.06686.x. [DOI] [PubMed] [Google Scholar]
- 19.Cabot G, Ocampo-Sosa AA, Dominguez MA, Gago JF, Juan C, Tubau F, Rodriguez C, Moya B, Pena C, Martinez-Martinez L, Oliver A, Spanish Network for Research in Infectious Diseases (REIPI). 2012. Genetic markers of widespread extensively drug-resistant Pseudomonas aeruginosa high-risk clones. Antimicrob Agents Chemother 56:6349–6357. doi: 10.1128/AAC.01388-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


