ABSTRACT
Combination therapy is an attractive option for the treatment of multidrug-resistant (MDR) Pseudomonas aeruginosa infections; however, limited data are available on combinations with ceftolozane-tazobactam (C-T). The in vitro pharmacodynamic chemostat model was employed to compare human-simulated exposures of C-T at 3 g every 8 h alone or in combination with amikacin at 25 mg/kg of body weight daily or colistin at 360 mg daily against four MDR P. aeruginosa isolates. C-T alone resulted in 24-h changes in the number of CFU of −0.02 ± 0.21, −1.81 ± 0.55, −1.44 ± 0.40, and +0.62 ± 0.05 log10 CFU/ml against isolates with C-T MICs of 4, 4, 8, and 16 μg/ml, respectively. Amikacin and colistin monotherapy displayed various results. The addition of amikacin to C-T resulted in −2.00 ± 0.23 (P < 0.001, additive)-, −1.50 ± 0.83 (P = 0.687, indifferent)-, −2.84 ± 0.08 (P = 0.079, indifferent)-, and −2.67 ± 0.54 (P < 0.001, synergy)-log10 CFU/ml reductions, respectively. The addition of colistin to C-T resulted in −3.02 ± 0.22 (P < 0.001, additive)-, −3.21 ± 0.24 (P > 0.05, indifferent)-, −4.6 ± 0.11 (P = 0.002, synergy)-, and −3.01 ± 0.28 (P < 0.001, synergy)-log10 CFU/ml reductions, respectively, against the MDR P. aeruginosa isolates with these MICs. Greater overall reductions in bacterial burden, including additive or synergistic interactions at 24 h, with C-T plus amikacin or colistin were observed against 3 out of 4 MDR P. aeruginosa strains tested, particularly those strains that were intermediate or resistant to C-T. Further studies assessing combination regimens containing C-T against MDR P. aeruginosa are warranted.
KEYWORDS: cephalosporin, aminoglycoside, polymyxin, synergy
INTRODUCTION
Pseudomonas aeruginosa is a nonfermenting, Gram-negative, rod-shaped bacterium commonly associated with the four most frequent types of hospital-acquired infections, including pneumonia and skin and soft tissue, urinary tract, and bloodstream infections (1, 2). In 2009, the Infectious Diseases Society of America (IDSA) defined the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) group of pathogens, in which P. aeruginosa is represented; these pathogens portray a high level of multidrug resistance (MDR) with few antibiotic options for treatment (3). Antibiotic resistance in P. aeruginosa can be caused by a number of mechanisms (4). For most β-lactam antibiotics, resistance is secondary to stable derepression of AmpC cephalosporinases in combination with active efflux pumps or loss of porins that permit entry through the outer membrane into the periplasmic space. Extended-spectrum β-lactamases (ESBLs) and carbapenemases (e.g., metallo-β-lactamases) can also be present but more rarely cause resistance in P. aeruginosa. Unfortunately, β-lactam resistance is often associated with reduced susceptibility to other antimicrobials as well, including fluoroquinolones and aminoglycosides (4). Recent surveillance in Europe reported that 17.8% (range by country, 0 to 66.3%) of P. aeruginosa isolates are resistant to carbapenems and 12.9% are resistant to at least 3 of the 5 primary antibiotic classes used clinically (5). The Centers for Disease Control and Prevention (CDC) reported similar rates (13%) of MDR P. aeruginosa (6), and the rate of carbapenem resistance is frequently observed to be 20 to 30% among U.S. surveillance studies (7, 8).
As a result of multiple global efforts, the antimicrobial pipeline addressing ESKAPE pathogens has only recently become bountiful (9). One of these new compounds is ceftolozane-tazobactam (C-T; Zerbaxa; Merck & Co, Inc., Kenilworth, NJ), a novel antipseudomonal cephalosporin combined with tazobactam, a well-established β-lactamase inhibitor (10). This combination has demonstrated potent in vitro activity against Gram-negative bacteria, with particular activity against MDR strains of P. aeruginosa and ESBL-producing strains of some Enterobacteriaceae (11, 12). Of interest is C-T's ability to retain activity against most (76.9 to 84.9%) MDR P. aeruginosa strains, including strains nonsusceptible to ceftazidime, cefepime, piperacillin-tazobactam, and meropenem (13). C-T is approved for the treatment of complicated intra-abdominal and urinary tract infections, including acute pyelonephritis, at a dose of 1.5 g every 8 h (q8h); furthermore, clinical trials of C-T for hospital-acquired bacterial pneumonia are under way using a higher dose of 3 g every 8 h (10). As a result of its in vitro potency and to preserve its future effectiveness, C-T is often reserved by hospitals for use against MDR or extensively drug resistant (XDR) P. aeruginosa infections (14–18).
Because C-T is used in the direst clinical situations for resistant P. aeruginosa infections, it is natural to question whether to optimize its utility by adding a second drug with activity. Studies addressing the benefits of combination therapy have rarely found a clinical benefit, as long as at least one antibiotic with activity was prescribed; however, only a few studies with MDR P. aeruginosa have been conducted, and none have included newer, potent antibiotics, such as C-T (19, 20). Nonetheless, many patients described in case series and isolated reports receive combination therapy (14–17), presumably because this reduces the trepidation associated with using a newer agent when few other options remain. Munita and colleagues (16) presented retrospective data on 35 patients treated with C-T for carbapenem-resistant P. aeruginosa infections. Although not statistically significantly different, the rate of clinical success was 70% versus 87% in patients receiving mono- versus combination therapy, respectively. Furthermore, the authors observed clinical failure in all patients harboring a C-T-nonsusceptible isolate (MIC > 4 μg/ml).
Of the available antibiotics with activity against P. aeruginosa, the combination of an antipseudomonal β-lactam with an aminoglycoside or a fluoroquinolone is among the most frequently recommended. Combinations including colistin are also more widely used due to increasing MDR prevalence; furthermore, such combinations are now recommended in the treatment of critically ill patients with ventilator-associated pneumonia in the most recent IDSA guidelines (21). This study aimed to evaluate the antibacterial efficacy of C-T alone and in combination with amikacin or colistin, two antipseudomonal antibiotics often reserved for MDR isolates, in an in vitro pharmacodynamic model simulating the free drug concentration profiles of aggressive dosing regimens for each antibiotic.
RESULTS
P. aeruginosa susceptibility.
The four isolates selected for this experiment were all meropenem, ceftazidime, cefepime, and piperacillin-tazobactam resistant and susceptible to amikacin and colistin. Two of the four isolates were susceptible to C-T, one was intermediate, and one was resistant (Table 1).
TABLE 1.
Antibiotic MICs against the four P. aeruginosa isolates
| Isolate | Modal MIC (μg/ml)a |
||||||
|---|---|---|---|---|---|---|---|
| C-T | AMK | CST | MEM | CAZ | FEP | TZP | |
| PSA C8-21 | 4 | 8 | 1 | 32 | 128 | 64 | 256 |
| PSA C28-5 | 4 | 8 | 1 | 32 | 128 | 64 | 512 |
| PSA C45-10 | 8 | 2 | 1 | 8 | 128 | 64 | 512 |
| PSA C14-22 | 16 | 8 | 2 | 16 | 128 | 32 | 256 |
C-T, ceftolozane-tazobactam; AMK, amikacin; CST, colistin; MEM, meropenem; CAZ, ceftazidime; FEP, cefepime; TZP, piperacillin-tazobactam.
Antibiotic exposures.
The observed concentrations and the calculated pharmacokinetic parameters for all antibiotic regimens over the 24-h experiments are represented in Table 2. Overall, the target concentrations were achieved for all antibiotics.
TABLE 2.
Observed versus target concentration pharmacokinetic parameters achieved in the in vitro modelc
| Antibiotic | Free peak concn (μg/ml) |
Free trough concn (μg/ml) |
Free AUC0–24 (μg · h/ml) |
t1/2 (h) |
||||
|---|---|---|---|---|---|---|---|---|
| Target | Observed | Target | Observed | Target | Observed | Target | Observed | |
| Ceftolozanea | 55.6 | 65.9 ± 5.7 | 11.2 | 12.3 ± 1.9 | 679 | 779 ± 35 | 3.7 | 3.5 ± 0.4 |
| Amikacin | 63.6 | 62.5 ± 4.0 | 0.9 | 0.9 ± 0.3 | 348 | 358 ± 11 | NC | NC |
| Colistinb | 0.73 | 0.72 ± 0.12 | NC | NC | NC | NC | NC | NC |
Concentration and pharmacokinetic data were targeted only for the ceftolozane component of ceftolozane-tazobactam.
Colistin was administered as a continuous infusion during the experiments; therefore, the free peak concentration is the mean concentration over the 24-h experiment.
Data are presented as the mean ± standard deviation. AUC0–24, area under the curve for the 24-h experiment; t1/2, half-life; NC, not calculated.
Antibacterial results.
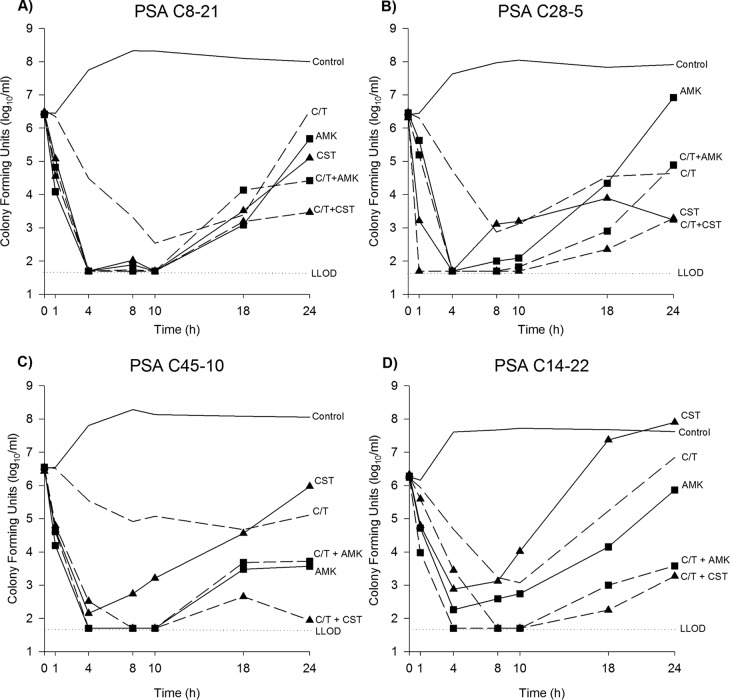
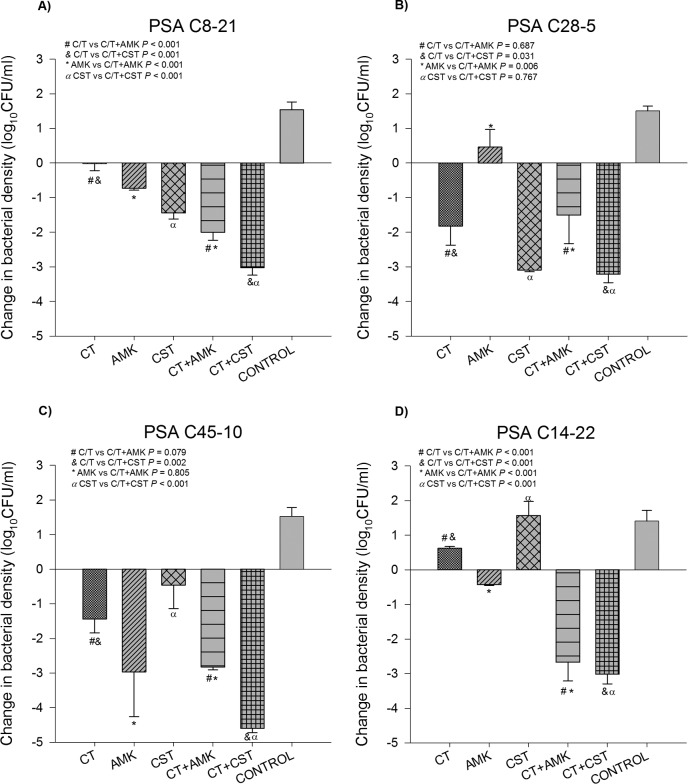
The mean bacterial density of the starting inoculum was 6.42 ± 0.12 log10 CFU/ml for all experiments. For controls, the isolates grew to 7.90 ± 0.27 log10 CFU/ml over 24 h. Time-kill curves are presented in Fig. 1 for each P. aeruginosa isolate and each regimen studied. Additionally, the observed difference in bacterial density at 24 h by isolate is provided in Fig. 2. All treatment regimens reduced the number of CFU at 24 h to a level significantly greater than that for the controls, with the exception of colistin monotherapy against PSA C14-22. Overall, no antagonism was observed with any of the combinations.
FIG 1.
Mean number of CFU over 24 h by P. aeruginosa isolate and for each antibiotic regimen. Data are plotted as the mean number of CFU from two experiments for each treatment regimen and the mean number of CFU for all growth controls for each isolate. (A) PSA C8-21 (MICs, C-T, 4 μg/ml; amikacin [AMK], 8 μg/ml; colistin [CST], 1 μg/ml); (B) PSA C28-5 (MICs, C-T, 4 μg/ml; amikacin, 8 μg/ml; colistin, 1 μg/ml); (C) PSA C45-10 (MICs, C-T, 8 μg/ml; amikacin, 2 μg/ml; colistin, 1 μg/ml); (D) PSA C14-22 (MICs, C-T, 16 μg/ml; amikacin, 8 μg/ml; colistin, 2 μg/ml). Straight line/no symbol, growth control; dashed line/no symbol, C-T monotherapy; straight line/square, amikacin monotherapy; straight line/triangle, colistin monotherapy; dashed line/square, C-T plus amikacin combination; dashed line/triangle, C-T plus colistin combination; dotted line, lowest limit of detection (LLOD).
FIG 2.
Mean change at 24 h in the number of CFU from that at 0 h by P. aeruginosa isolate and for each antibiotic regimen. (A) PSA C8-21 (MICs, C-T, 4 μg/ml; amikacin, 8 μg/ml; colistin, 1 μg/ml); (B) PSA C28-5 (MICs, C-T, 4 μg/ml; amikacin, 8 μg/ml; colistin, 1 μg/ml); (C) PSA C45-10 (MICs, C-T, 8 μg/ml; amikacin, 2 μg/ml; colistin, 1 μg/ml); (D) PSA C14-22 (MICs, C-T, 16 μg/ml; amikacin, 8 μg/ml; colistin, 2 μg/ml). The results for all antibiotic regimens were significantly different from the results for the control (P < 0.05) except CST monotherapy against PSA C14-22. Statistical results between monotherapy and combination therapy regimens are provided in the upper left corner of each panel.
Both PSA C8-21 and PSA C28-5 were C-T susceptible with an MIC of 4 μg/ml. C-T monotherapy resulted in −0.02 ± 0.21- and −1.82 ± 0.55-log10 CFU/ml reductions for the two strains at 24 h, respectively. The addition of amikacin to C-T against PSA C8-21 demonstrated a significant reduction in the number of CFU compared with the reductions achieved with C-T and amikacin alone (P < 0.001 for both); the additional −1.27-log10 CFU/ml difference over the reduction achieved with the more active antibiotic (i.e., amikacin) was sufficient to classify this combination as additive. The addition of colistin to C-T against PSA C8-21 demonstrated a significant reduction in the number of CFU compared with that achieved with C-T and colistin alone (P < 0.001 for both); the additional −1.58-log10 CFU/ml difference over the reduction achieved with colistin alone was additive. The addition of amikacin or colistin to C-T against PSA C28-5 resulted in indifferent interactions for both combinations.
PSA C45-10 was intermediate resistant to C-T with an MIC of 8 μg/ml. Despite this phenotypic classification, C-T monotherapy resulted in a −1.44 ± 0.40-log10 CFU/ml reduction at 24 h. The addition of amikacin to C-T did not demonstrate any significant increase in activity compared with that of C-T or amikacin alone (P = 0.079 and 0.805, respectively), and this combination was indifferent. The addition of colistin to C-T demonstrated a significant reduction in the number of CFU compared with the numbers achieved with both C-T and colistin monotherapy (P = 0.002 and P < 0.001, respectively). The additional −3.17-log10 CFU/ml difference compared with the reduction achieved with C-T alone qualified this combination as synergistic.
PSA C14-22 was C-T resistant with an MIC of 16 μg/ml. C-T monotherapy resulted in regrowth to 0.62 ± 0.05 log10 CFU/ml. Both amikacin and colistin also demonstrated little antibacterial efficacy by 24 h (Fig. 1). The C-T plus amikacin and C-T plus colistin combinations resulted in significant increases in the reduction in the number of CFU compared with the number of CFU achieved with each of the antibiotics alone (P < 0.001 for all comparisons). Notably, both of these combinations were synergistic, with additional reductions of −2.24 and −3.64 log10 CFU/ml, respectively, compared with the reduction achieved with the most active single antibiotic.
AUCFU.
The log ratio (LR) for the area under the curve for CFU (AUCFU) data are presented in Table 3. This method provides the log difference in the total bacterial burden over the duration of the study for the test method (e.g., combination therapy) relative to that for the reference treatment (e.g., control or single antibiotic alone) (22). A 1-log reduction is equivalent to a 10-fold, or 90%, reduction in the bacterial burden over time relative to that achieved with the reference, a 2-log reduction is equivalent to a 100-fold reduction (i.e., 99%), etc. Relative to control isolate growth, all monotherapy antibiotic regimens achieved substantial reductions in the bacterial burden over time (−1.62 to −3.18), with the exception of colistin alone against PSA C14-22 (−0.46, or an ∼3-fold reduction). Combination regimens were compared with each antibiotic alone as a reference. Compared with the reduction achieved with C-T alone, the combination of C-T plus amikacin achieved greater than 1-log reductions against PSA C8-21 and PSA C14-22. Referenced to the reduction achieved with amikacin alone, the reductions were −0.24 and −0.53 against these isolates, respectively. The combination of C-T plus colistin also achieved greater than 1-log reductions against PSA C8-21 and PSA C14-22 compared with those achieved with C-T alone. AUCFU reductions with this combination were <1 log relative to the reduction achieved with colistin alone for all isolates, except PSA C14-22, where the combination resulted in more than a 99% reduction (i.e., 2.36 logs) relative to that achieved with colistin alone.
TABLE 3.
LR of AUCFU for each antibiotic alone (test) compared with the control (reference) and for each antibiotic combination (test) compared with each antibiotic alone (reference)
| Isolate | Log difference in AUCFUa |
||||||
|---|---|---|---|---|---|---|---|
| C-T/control | AMK/control | CST/control | C-T + AMK/C-T alone | C-T + AMK/AMK alone | C-T + CST/C-T alone | C-T + CST/CST alone | |
| PSA C8-21 | −2.25 | −3.13 | −3.12 | −1.02 | −0.24 | −1.01 | −0.11 |
| PSA C28-5 | −2.55 | −1.62 | −3.18 | −0.48 | −1.23 | −0.62 | 0.11 |
| PSA C45-10 | −2.26 | −3.16 | −2.54 | −0.77 | −0.10 | −0.78 | −0.50 |
| PSA C14-22 | −1.58 | −2.44 | −0.46 | −1.42 | −0.53 | −1.16 | −2.36 |
The log difference is presented as the log ratio (LR), which is equal to the number of log10 CFU(test/reference). AUCFU, area under the curve for CFU; C-T, ceftolozane-tazobactam; AMK, amikacin; CST, colistin.
DISCUSSION
C-T is a recent addition to our antibiotic armamentarium targeting MDR Gram-negative bacteria, most notably, P. aeruginosa. The majority of the real-world use of C-T has been for treatment of off-label infections (predominantly pneumonia) caused by P. aeruginosa strains resistant to most currently available β-lactams (14–18). Although reported outcomes have generally been favorable, many patients received combination therapy; furthermore, the MIC for those MDR P. aeruginosa strains tended to be well below the current susceptibility breakpoint of 4 μg/ml. The rate of clinical success was 70% versus 87% in patients receiving mono- versus combination therapy, respectively, in the case series by Munita and colleagues; furthermore, the authors observed clinical failure in all patients harboring a C-T-nonsusceptible isolate (16). Observations such as these beseech the question of whether combination therapy with newer antibiotics, such as C-T, should be advocated. Herein, we used an in vitro pharmacodynamic model to evaluate the antibacterial efficacy of C-T monotherapy compared with that of combination regimens of amikacin or colistin. Although no antagonism was observed with any combinations, varied results were achieved by isolate; synergistic interactions were noted for 2 of the 4 isolates.
The four P. aeruginosa isolates selected for this experiment were resistant to all β-lactams tested, including carbapenems, ceftazidime, cefepime, and piperacillin-tazobactam. Two isolates had C-T MICs at the susceptibility breakpoint, which account for ∼5% of P. aeruginosa isolates in surveillance studies (23). The other two strains selected were nonsusceptible with MICs of 8 and 16 μg/ml, which each accounted for ∼1% of isolates. Thus, although still rare in the Unites States, isolates with this level of resistance are exactly the types to which combination therapy would be targeted.
An additional reason for selection of these four isolates was the availability of time-kill synergy results for comparison (24). Using a ceftolozane concentration of 7.5 μg/ml (roughly a free trough concentration) and reported trough concentrations of other antipseudomonal antibiotics, synergy was observed with colistin against PSA C28-5, PSA C45-10, and PSA C14-22; additivity was observed against PSA C8-21. In contrast, when combined with the trough concentrations of amikacin, indifference was observed for all isolates, except PSA C45-10, against which synergy was demonstrated. Those observations varied significantly from our findings using the in vitro pharmacodynamic model. When using a human-simulated free concentration profile of 3 g q8h of C-T, 25 mg/kg of body weight once daily amikacin, and 360 mg daily of colistin base activity, synergy was observed with the C-T plus colistin regimen only against the two C-T-nonsusceptible isolates, PSA C45-10 and PSA C14-22. The addition of colistin resulted in additivity or indifference against the two susceptible strains. The combination of C-T plus amikacin resulted in synergy only against PSA C14-22, whereas additivity or indifference was observed against all other strains. The most practical reason for these differences lies in the drug concentration profiles used during each experiment. Unless administered as continuous infusions, the concentration profiles simulated in time-kill synergy studies cannot be replicated in vivo. Notably, no antagonism by C-T and amikacin or colistin against any of these P. aeruginosa isolates was observed in either study.
When assessed using the LR difference in AUCFU method (22), similar conclusions could be made for both combinations against most isolates (Table 3). We used C-T as the primary reference for combination regimens since the clinical intent would be to add the other agents to the β-lactam backbone; furthermore, monotherapy with an aminoglycoside or colistin against P. aeruginosa would be strongly discouraged. Compared with the reduction achieved with C-T alone, combination therapy with amikacin and colistin resulted in an additional −1.42- and −1.16-log reduction in the overall bacterial burden against PSA C14-22, respectively. Against PSA C8-21, where the 24-h CFU-per-milliliter reduction endpoint resulted in additivity for both combinations, relative agreement was observed, as the AUCFU method identified additional −1.02 and −1.01 log reductions, respectively, compared with the reduction achieved with C-T alone. Finally, agreement was also observed against PSA C28-5; −0.48- and −0.62-log reductions were achieved with combination therapy, which was concordant with the 24-h CFU-per-milliliter reduction results for indifference. In contrast, a ≥1-log AUCFU reduction was not observed for C-T plus colistin against PSA C45-10, whereas the 24-h data suggested synergy. This was likely due to an observed bump in the number of CFU at 18 h for the C-T plus colistin combination (Fig. 1C), which contributed to the AUCFU of this regimen, followed by a reduction approaching the lower limit of detection at 24 h.
As a β-lactam, ceftolozane's pharmacodynamic profile is similar to that of other cephalosporins and best correlated with the percentage of the dosing interval that the free plasma concentrations exceed the MIC (fTMIC). The fTMIC required for 1-log kill in the classic neutropenic murine thigh infection model was 39% (range, 20 to 47%) against 14 P. aeruginosa strains, although this level of kill was unachievable for 2 of the included strains (25). Bulik and colleagues (26) simulated ceftolozane exposures of 37.5 to 100% fTMIC in an immunocompetent murine thigh infection model against 8 diverse P. aeruginosa strains. At least 1-log10 CFU/ml reductions were observed against all strains at 24 h, including those with ceftolozane MICs of 8 and 16 μg/ml. The mean free drug trough concentration observed in critically ill patients receiving C-T at 3 g q8h and simulated herein was ∼11.2 μg/ml (27), thereby resulting in a 100% fTMIC for the three isolates with MICs of ≤8 μg/ml and an ∼90% fTMIC for the isolate with an MIC of 16 μg/ml. Despite these high exposures in the in vitro pharmacodynamic model, C-T monotherapy resulted in a ≥1-log10 CFU/ml kill against only PSA C28-5 and PSA C45-10. The results of the in vitro and in vivo murine studies should not be directly compared due to a number of differences, including the use of different isolates, the presence of a fully or partially active immune system in animals, as well as the simulation of ideal growth conditions for P. aeruginosa in vitro. Indeed, the in vitro pharmacodynamic chemostat model is perhaps among the most challenging test of antibacterial activity, and therefore, it is promising to observe antibacterial effects at least up to the MIC of 8 μg/ml with the 3-g-q8h dosing regimen.
All four P. aeruginosa strains selected were susceptible to amikacin, an observation consistent with observations from contemporary surveillance studies. With the exception of colistin and now C-T, amikacin often remains the most active antibiotic against P. aeruginosa. Among 236 meropenem-resistant P. aeruginosa strains in the United States, the strains displayed the greatest susceptibility to amikacin at a rate of 91%, whereas the rate of susceptibility to tobramycin was 74% (28). Amikacin was simulated in the model as a high-dose, extended-interval regimen consistent with a 25-mg/kg daily dose (29). When tested alone, amikacin displayed the greatest antibacterial effect against PSA C45-10 (a −3-log10 CFU/ml reduction at 24 h and a −3.16 log reduction in AUCFU relative to the values for the control), the isolate against which it was most active (MIC = 2 μg/ml). When combined with C-T, additivity was observed against one C-T-susceptible isolate, indifference was observed against the remaining susceptible isolate as well as PSA C45-10, and synergy was observed against the resistant C-T isolate (PSA C14-22) (Fig. 2). Although synergy at 24 h was not observed with all isolates, it should be noted that this combination provided rapid killing to the lower limit of detection by 4 to 8 h against all strains (Fig. 1), whereas C-T alone never reached this level of kill over the 24-h experiment. Drusano and colleagues have proposed that achievement of rapid kill (≥2 log10 CFU/ml) early after starting antibiotic therapy permits attainment of the saturable threshold needed for granulocytes to contribute optimally to bacterial clearance (30). As a result, there may be value to combining the two antibiotics clinically even in the absence of synergy. The addition of aminoglycosides to β-lactams has demonstrated additivity, synergy, as well as the suppression of resistance emergence in several studies (31, 32).
Lastly, colistin was selected for this study due to its utility as an agent of last resort against MDR Gram-negative bacteria. All four isolates were susceptible to colistin with MICs of 1 to 2 μg/ml. Consistent with the findings of other in vitro pharmacodynamic studies (33), colistin simulated at an aggressive dose of 360 mg daily attained rapid bactericidal activity against all P. aeruginosa strains, followed by the regrowth of three of the four strains. The combination of C-T plus colistin resulted in synergy at 24 h against two of the four strains (notably, the C-T-nonsusceptible ones) and indifference or additivity against the two C-T-susceptible isolates. This combination also resulted in rapid early killing (Fig. 1) and numerically the largest reductions in the number of CFU against each isolate over 24 h (Fig. 2). These observations are again consistent with those in the in vitro combination therapy literature when colistin is added to other β-lactams against P. aeruginosa (33). No clinical studies have ever identified a benefit to administering colistin in combination with a β-lactam against MDR P. aeruginosa; however, dosing may not have been optimized for the individual agents in these studies (33). Nonetheless, the potential benefit of its use must be weighed against its well-described toxicity.
Some limitations of this study should be noted. These experiments were conducted in vitro, although we would argue that the use of human-simulated concentration profiles gets closer to what might be observed in the clinic than the use of time-kill or checkerboard studies. These studies are conducted in the presence of ideal growth conditions for the organism with no contribution of any immune system. Studies were conducted only over 24 h; longer-duration experiments are needed to assess the emergence of resistance or the ability of combination therapy to suppress this endpoint. Only four P. aeruginosa strains were included, and all of the strains had C-T MICs much higher those for than the majority of isolates seen clinically; therefore, these observations may not apply to isolates with lower MICs. Finally, aggressive dosing regimens for all three antibiotics were simulated. The use of lower doses may result in different conclusions, including additional observations of synergy, because it is easier to meet the >2-log10 CFU/ml threshold if the monotherapy regimens perform poorly. However, for the treatment of MDR P. aeruginosa infections with few other options, we believe that the pharmacokinetics and pharmacodynamics should be optimized for each individual antibiotic administered so as to achieve a maximum contribution.
In summary, we observed greater overall reductions in bacterial burdens, including additivity or synergy at 24 h, with C-T plus amikacin and C-T plus colistin combination regimens against 3 out of 4 MDR P. aeruginosa strains tested, particularly those strains that were intermediate or resistant to C-T, compared to the reductions achieved with monotherapy. These data suggest that combination therapy could prove useful in the treatment of MDR P. aeruginosa infections. Further studies are justified to fully evaluate the clinical benefits of these regimens.
MATERIALS AND METHODS
Bacterial strains.
Four clinical P. aeruginosa isolates with an MDR resistance pattern isolated during a surveillance study in the United States were used in this study (PSA C8-21, PSA C28-5, PSA C45-10, PSA C14-22). The isolates were selected on the basis of meropenem, ceftazidime, cefepime, and piperacillin-tazobactam resistance and the presence of a C-T MIC at or within 2 dilutions above the susceptibility breakpoint of 4 μg/ml. The MICs of C-T, amikacin, colistin, meropenem, ceftazidime, cefepime, and piperacillin-tazobactam were determined by broth microdilution according to Clinical and Laboratory Standards Institute (CLSI) recommendations, as described previously by Sutherland and Nicolau (23).
Antibiotics.
Ceftolozane-tazobactam (Zerbaxa; lot number SP1348; expiration date, December 2017) was obtained from Merck & Co., Inc. (Kenilworth, NJ). Amikacin analytical powder (amikacin sulfate, USP; lot number 115837/B; expiration date, April 2017) was obtained from Medisca (Plattsburgh, NY). Colistin analytical powder (colistin sulfate; lot number SLBN5158V-SLBT0851; expiration date, March 2021) was obtained from Sigma-Aldrich (St. Louis, MO). Before all in vitro pharmacodynamic experiments, antibiotic stock solutions were prepared according to the pharmaceutical instructions and immediately frozen in cryovials. The amount of solution needed for each experiment was defrosted just prior to commencement of daily experiments.
Simulated drug exposures.
C-T was administered to simulate the peak and trough free ceftolozane concentrations achieved in the plasma of critically ill patients with suspected pneumonia receiving the 3-g (2 g ceftolozane, 1 g tazobactam)-every-8-h (q8h) dosage regimen (27). After applying a protein binding of 16%, the target ceftolozane concentration was a free peak concentration of 55.6 μg/ml with a half-life (t1/2) of 3.7 h, thereby resulting in an 8-h trough concentration of 11.2 μg/ml. Although present in the pharmaceutical formulation, we did not humanize or consider tazobactam exposure since it has a limited role in ceftolozane's activity against P. aeruginosa (10). Amikacin was simulated to achieve a free peak concentration and a free drug area under the curve for the 24-h experiment (fAUC0–24) consistent with that achieved with a 25-mg/kg daily dose in a 70-kg critically ill patient with severe sepsis (29). After considering a protein binding of 11%, these values were 63.6 μg/ml and 348.0 μg · h/ml, respectively. The elimination rate was set to be consistent with that of C-T; a supplemental amikacin bolus dose was administered at 8 h to raise the concentration from 12.8 to 22.0 μg/ml in order to achieve the target fAUC0–24. Colistin was simulated as a continuous infusion to achieve the mean steady-state average concentration (Css,avg) for adult critically ill patients receiving the currently recommended European Medicines Agency (EMA) dosing regimen of 360 mg daily of colistin base activity (34). Assuming a protein binding of 50%, the target free Css,avg was 0.73 μg/ml.
In vitro pharmacodynamic model.
A one-compartment in vitro chemostat model was used for all experiments. Briefly, each bug-drug combination experiment consisted of three independent glass reactor models studied simultaneously; two of them contained experimental treatments in duplicate, and one was an antibiotic-free growth control. The reactors were placed in a water bath with a temperature modulator set for 35°C, and magnetic stirrers were placed inside to ensure consistent mixing. All reactors were filled with 300 ml of cation-adjusted Mueller-Hinton broth (CAMHB; Becton Dickinson and Company, Sparks, MD). The reactors were inoculated with 106 log10 CFU/ml of each isolate 30 min prior to the administration of the antibiotic(s) to allow the bacteria to enter the log phase of growth. After the bolus of the antibiotic(s) at 0 h to achieve the target free peak concentration(s) was added, fresh broth was infused into the models via a peristaltic pump (Masterflex model 7519-05 and Masterflex L/S model 7519-15; Cole-Palmer Instrument Company, Vernon Hills, IL), which was set to achieve the human-simulated half-life of C-T. During colistin experiments, the infused broth contained the target free Css,avg of 0.73 μg/ml to maintain this concentration in the reactor while simulating the C-T t1/2. Each experiment was conducted over 24 h.
To assess bacterial density over time, samples were taken from each reactor at a series of time points (0, 1, 4, 8, 10, 18, and 24 h) and serially diluted in normal saline. To perform quantitative culture, the diluted samples were immediately plated into Trypticase soy agar with 5% sheep blood plates (BBL Stacker Plate; 90-mm diameter; Becton Dickinson and Company, Sparks, MD) and incubated at 37°C for 16 to 24 h, after which the colony counts were read. The lower limit of quantification was 1.7 log10 CFU/ml.
Antibiotic concentrations and exposure determination.
The antibiotic concentrations obtained inside the reactors were measured at established time points (0, 2, 8, 10, 16, 18, and 24 h). All samples were immediately stored at −80°C until analysis. The concentration of the ceftolozane component of C-T was assessed by a high-performance liquid chromatography (HPLC) method at the Center for Anti-Infective Research and Development (Hartford, CT, USA) (35). The ceftolozane assay was linear over a concentration of 0.5 to 50 μg/ml. Any concentrations above the upper limit of the assay were diluted accordingly to fit within the standard curve range. The mean intraday coefficients of variation (CVs) for low (1 μg/ml) and high (40 μg/ml) quality control samples were 4.57% and 2.10%, respectively. For the interday quality control samples, the CVs were 3.69% and 4.78%, respectively. Amikacin samples were analyzed by Quest Diagnostics (Chantilly, PA, USA) using an enzyme multiple-immunoassay technique (EMIT; on an AU680 analyzer using the Syva 2000 reagent kit from Beckman Coulter). Colistin samples were analyzed through a validated liquid chromatography-tandem mass spectrometry (LC/MS-MS) assay by Keystone Bioanalytical, Inc. (North Wales, PA, USA). Both colistin A and colistin B concentrations were measured and summed to equate colistin concentrations. Observed concentrations were reported for free peak and trough values for all antibiotics; for colistin this was the Css,avg. The fAUC0–24 was calculated for ceftolozane and amikacin using the log trapezoidal rule. The terminal half-life (t1/2) was calculated for ceftolozane as 0.693/k, where k was the estimated elimination rate constant between the measured concentrations.
Statistical analyses.
To evaluate the antibiotic activity of each regimen, bacterial density was measured by the change in the number of log10 CFU per milliliter between 0 and 24 h for each simulated regimen. Time-kill curves were constructed by plotting the mean bacterial density (number of CFU per milliliter) for each isolate and regimen against time. The observed change in the number of log10 CFU per milliliter over 24 h was compared between antibiotic regimens for each isolate using analysis of variance (ANOVA) with the Holm-Sidak method for multiple comparisons on SigmaPlot (version 13.0) software (Systat Software Inc., San Jose, CA, USA). Statistical significance was defined a priori as a P value of <0.05. Synergy for combination regimens was defined as a difference of >2 log10 CFU/ml at 24 h from the reduction achieved with the most active agent of the combination administered alone; additivity was defined as a 1- to 2-log10 CFU/ml difference at 24 h from the reduction achieved with the most active agent of the combination (36, 37). Indifference was defined by log10 CFU-per-milliliter changes of less than 1. Finally, antagonism was defined as a statistically significant difference resulting in a worsening log10 CFU-per-milliliter value from the value achieved with the least active agent alone.
As an alternative endpoint, the LR difference in AUCFU (22) was calculated for each antibiotic alone referenced to the control isolate and for each antibiotic combination relative to the antibiotic alone as LR = log10(AUCFUtest/AUCFUreference), where AUCFUtest is the AUCFU for the test isolate and AUCFUreference is the AUCFU for the reference isolate, No definitions for synergy, additivity, etc., have been established for the LR of the AUCFU method; however, one may consider the additional difference in the total bacterial burden reduction that is observed. Therefore, a 1-log reduction would be a 10-fold (or 90%) reduction in the overall bacterial burden. For combination therapies, we considered those regimens achieving a ≥1-log difference in AUCFU compared with that achieved with C-T alone to be noteworthy (i.e., the combination results in a 10-fold further reduction in the overall bacterial burden compared with that achieved with C-T alone), since, clinically, amikacin or colistin would be added to the β-lactam backbone and monotherapy with amikacin or colistin against P. aeruginosa is strongly discouraged.
ACKNOWLEDGMENTS
We acknowledge Jennifer Tabor-Rennie, Christina Sutherland, Elizabeth Cyr, Michelle Insignares, Sara Giovagnoli, Kimelyn Greenwood, and Sean Stainton from the Center for Anti-Infective Research and Development, Hartford, CT, for their assistance with the conduct of the study.
This study was funded by an investigator-initiated grant from Merck & Co., Inc., Kenilworth, NJ.
D.P.N. is a consultant and member of the speakers' bureau for Merck & Co., Inc. The other authors have nothing to disclose.
REFERENCES
- 1.Gupta R, Malik A, Rizvi M, Ahmed M, Singh A. 2017. Epidemiology of multidrug-resistant Gram-negative pathogens isolated from ventilator-associated pneumonia in ICU patients. J Glob Antimicrob Resist 9:47–50. doi: 10.1016/j.jgar.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Thabit AK, Crandon JL, Nicolau DP. 2015. Antimicrobial resistance: impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin Pharmacother 16:159–177. doi: 10.1517/14656566.2015.993381. [DOI] [PubMed] [Google Scholar]
- 3.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 4.Bonomo RA, Szabo D. 2006. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis 43(Suppl 2):S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control. 2017. Antimicrobial resistance surveillance in Europe—2016. European Centre for Disease Prevention and Control, Stockholm, Sweden: https://ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2016 Accessed 15 November 2017. [Google Scholar]
- 6.CDC. 2013. Antibiotic resistance threats in the United States, 2013. CDC, Atlanta, GA: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf#page=69 Accessed 11 October 2017. [Google Scholar]
- 7.Sader HS, Casanheira M, Flamm RK. 2017. Antimicrobial activity of ceftazidime-avibactam against Gram-negative bacteria isolated from patients hospitalized with pneumonia in U.S. medical centers, 2011 to 2015. Antimicrob Agents Chemother 61:e02083-16. doi: 10.1128/AAC.02083-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lob SH, Hackel MA, Kazmierczak KM, Young K, Motyl M, Karlowsky JA, Sahm DF. 2017. In vitro activity of imipenem-relebactam against Gram-negative ESKAPE pathogens isolated by clinical laboratories in the United States in 2015 (results from the SMART Global Surveillance Program). Antimicrob Agents Chemother 61:e02209-16. doi: 10.1128/AAC.02209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falagas ME, Mavroudis AD, Vardakas KZ. 2016. The antibiotic pipeline for multi-drug resistant gram negative bacteria: what can we expect? Expert Rev Anti Infect Ther 18:747–763. doi: 10.1080/14787210.2016.1204911. [DOI] [PubMed] [Google Scholar]
- 10.Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, Lagace-Wiens PR, Rubinstein E, Gin AS, Walkty A, Hoban DJ, Lynch JP III, Karlowsky JA. 2014. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 74:31–51. doi: 10.1007/s40265-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 11.Farrell DJ, Sader HS, Flamm RK, Jones RN. 2014. Ceftolozane/tazobactam activity tested against Gram-negative bacterial isolates from hospitalized patients with pneumonia in US and European medical centres (2012). Int J Antimicrob Agents 43:533–539. doi: 10.1016/j.ijantimicag.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Sader HS, Farrell DJ, Castanheira M, Flamm RK, Jones RN. 2014. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011-12). J Antimicrob Chemother 69:2713–2722. doi: 10.1093/jac/dku184. [DOI] [PubMed] [Google Scholar]
- 13.Shortridge D, Castanheira M, Pfaller MA, Flamm RK. 2017. Ceftolozane/tazobactam activity against Pseudomonas aeruginosa clinical isolates from US hospitals: report from the PACTS Antimicrobial Surveillance Program. Antimicrob Agents Chemother 61:e00465-17. doi: 10.1128/AAC.00465-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuti JL, Ghazi IM, Quintiliani R Jr, Shore E, Nicolau DP. 2016. Treatment of multidrug-resistant Pseudomonas aeruginosa with ceftolozane/tazobactam in a critically ill patient receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents 48:342–343. doi: 10.1016/j.ijantimicag.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Dinh A, Wyplosz B, Kerneis S, Lebeaux D, Bouchand F, Duran C, Beraud G, Lazaro P, Davido B, Henard S, Canoui E, Ferry T, Wolff M. 2017. Use of ceftolozane/tazobactam as salvage therapy for infections due to extensively drug-resistant Pseudomonas aeruginosa. Int J Antimicrob Agents 49:782–783. doi: 10.1016/j.ijantimicag.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Munita JM, Aitken SL, Miller WR, Perez F, Rosa R, Shimose LA, Lichtenberger PN, Abbo LM, Jain R, Nigo M, Wanger A, Araos R, Tran TT, Adachi J, Rakita R, Shelburne S, Bonomo RA, Arias CA. 2017. Multicenter evaluation of ceftolozane/tazobactam for serious infections caused by carbapenem-resistant Pseudomonas aeruginosa. Clin Infect Dis 26:158–161. doi: 10.1093/cid/cix014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xipell M, Bodro M, Marco F, Marinez JA, Soriano A. 2017. Successful treatment of three severe MDR or XDR Pseudomonas aeruginosa infections with ceftolozane/tazobactam. Future Microbiol 12:1323–1326. doi: 10.2217/fmb-2017-0018. [DOI] [PubMed] [Google Scholar]
- 18.Wright H, Bonomo RA, Paterson DL. 2017. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect 23:704–712. doi: 10.1016/j.cmi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Paul M, Lador A, Grozinsky-Glasberg S, Leibovi L. 2014. Beta lactam antibiotic monotherapy versus beta lactam aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 1:CD003344. doi: 10.1002/14651858.CD003344.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul M, Carmeli Y, Durante-Mangoni E, Mouton JW, Tacconelli E, Theuretzbacher U, Mussine C, Leibovici L. 2014. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother 69:2305–2309. doi: 10.1093/jac/dku168. [DOI] [PubMed] [Google Scholar]
- 21.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeny DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratala J, El Solh AA, Ewig S, Fey PD, File TM Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the America Thoracic Society. Clin Infect Dis 63:575–582. doi: 10.1093/cid/ciw504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao GG, Li J, Garonzik SM, Nation RL, Forrest A. 18 December 2017. Assessment and modeling of antibacterial combination regimens. Clin Microbiol Infect. doi: 10.1016/j.cmi.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Sutherland CA, Nicolau DP. 2015. Susceptibility profile of ceftolozane/tazobactam and other parenteral antimicrobials against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa from US hospitals. Clin Ther 37:1564–1571. doi: 10.1016/j.clinthera.2015.05.501. [DOI] [PubMed] [Google Scholar]
- 24.Monogue ML, Nicolau DP. 18 December 2017. Antibacterial activity of ceftolozane/tazobactam alone and in combination with other antimicrobial agents against MDR Pseudomonas aeruginosa. J Antimicrob Chemother. doi: 10.1093/jac/dkx483. [DOI] [PubMed] [Google Scholar]
- 25.Lepak AJ, Reda A, Marchillo K, Van Hecker J, Craig WA, Andes D. 2014. Impact of MIC range for Pseudomonas aeruginosa and Streptococcus pneumoniae on the ceftolozane in vivo pharmacokinetic/pharmacodynamic target. Antimicrob Agents Chemother 58:6311–6314. doi: 10.1128/AAC.03572-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulik CC, Tessier PR, Keel RA, Sutherland CA, Nicolau DP. 2012. In vivo comparison of CXA-101 (FR264205) with and without tazobactam versus piperacillin-tazobactam using human simulated exposures against phenotypically diverse Gram-negative organisms. Antimicrob Agents Chemother 56:544–549. doi: 10.1128/AAC.01752-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caro L, Larson KB, Nicolau D, De Waele J, Kuti J, Mosquera B, Gadzicki E, Adedoyin A, Zeng Z, Rhee EG. 2017. Pharmacokinetics/pharmacodynamics and safety of 3 g ceftolozane/tazobactam in critically ill adult patients, abstr 1882 IDWeek Abstr 2017. [Google Scholar]
- 28.Sutherland CA, Verastegui JE, Nicolau DP. 2016. In vitro potency of amikacin and comparators against E. coli, K. pneumoniae, and P. aeruginosa respiratory and blood isolates. Ann Clin Microbiol Antimicrob 15:39. doi: 10.1186/s12941-016-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmoudi L, Mohammadpour AH, Ahmadi A, Niknam R, Mojtahedzadeh M. 2013. Influence of sepsis on higher daily dose of amikacin pharmacokinetics in critically ill patients. Eur Rev Med Pharmacol Sci 17:285–291. [PubMed] [Google Scholar]
- 30.Drusano GL, Liu W, Fikes S, Cirz R, Robbins N, Kurhanewicz S, Rodriquez J, Brown D, Baluya D, Louie A. 2014. Interaction of drug- and granulocyte-mediated killing of Pseudomonas aeruginosa in a murine pneumonia model. J Infect Dis 210:1319–1324. doi: 10.1093/infdis/jiu237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drusano GL, Bonomo RA, Bahniuk N, Bulitta JB, Vanscoy B, Defiglio H, Fikes S, Brown D, Drawz SM, Kulawy R, Louie A. 2012. Resistance emergence mechanism and mechanism of resistance suppression by tobramycin for cefepime for Pseudomonas aeruginosa. Antimicrob Agents Chemother 56:231–242. doi: 10.1128/AAC.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louie A, Liu W, Fides S, Brown D, Drusano GL. 2013. Impact of meropenem in combination with tobramycin in a murine model of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother 57:2788–2792. doi: 10.1128/AAC.02624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergen PJ, Bulman ZP, Landersdorfer CB, Smith N, Lenhard JR, Bulitta JB, Nation RL, Li J, Tsuji BT. 2015. Optimizing polymyxin combinations against resistant gram-negative bacteria. Infect Dis Ther 4:391–415. doi: 10.1007/s40121-015-0093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nation RL, Garonzik SM, Li J, Thamlikitkul V, Giamarellos-Bourboulis EJ, Paterson DL, Turnidge JD, Forrest A, Silveira FP. 2016. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 62:552–558. doi: 10.1093/cid/civ964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutherland CA, Nicolau DP. 2016. Development of an HPLC method for the determination of ceftolozane/tazobactam in biological and aqueous matrixes. J Chromatogr Sci 54:1037–1040. doi: 10.1093/chromsci/bmw047. [DOI] [PubMed] [Google Scholar]
- 36.Hagihara M, Housman T, Nicolau DP, Kuti JL. 2014. In vitro pharmacodynamics of polymyxin B and tigecycline alone and in combination against carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 58:874–879. doi: 10.1128/AAC.01624-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albur MS, Noel A, Bowker K, MacGowan A. 2015. The combination of colistin and fosfomycin is synergistic against NDM-1-producing Enterobacteriaceae in in vitro pharmacokinetic/pharmacodynamic model experiments. Int J Antimicrob Agents 46:560–567. doi: 10.1016/j.ijantimicag.2015.07.019. [DOI] [PubMed] [Google Scholar]