ABSTRACT
Newborns with congenital cytomegalovirus (CMV) infection are at high risk for developing permanent sequelae. Intravenous ganciclovir therapy is frequently used for the treatment of congenital CMV infection. A target area under the concentration-time curve from 0 to 24 h (AUC0–24) of 40 to 50 μg · h/ml is recommended. The standard dose has resulted in a large variability in ganciclovir exposure in newborns, indicating the unmet need of dosage individualization for this vulnerable population, but the implementation of this strategy remains challenging in clinical practice. We aim to evaluate the clinical utility of model-based dosage individualization of ganciclovir in newborns using an opportunistic sampling approach. The predictive performance of a published ganciclovir population pharmacokinetic model was evaluated using an independent patient cohort. The individual dose was adjusted based on the target AUC0–24 to ensure its efficacy. A total of 26 newborns with congenital CMV infection were included in the present study. Only 11 (42.3%) patients achieved the target AUC0–24 after being given the standard dose. For all the subtherapeutic patients (achieving <80% of the target AUC) (n = 5), a model-based dosage adjustment was performed using the Bayesian estimation method combined with the opportunistic sampling strategy. The adjusted doses were increased by 28.6% to 60.0% in these five patients, and all adapted AUC0–24 values achieved the target (range, 48.6 to 66.1 μg · h/ml). The clinical utility of model-based dosing individualization of ganciclovir was demonstrated in newborns with congenital CMV infection. The population pharmacokinetic model combined with the opportunistic sampling strategy provides a clinically feasible method to adapt the ganciclovir dose in neonatal clinical practice. (This study has been registered at ClinicalTrials.gov under registration no. NCT03113344.)
KEYWORDS: ganciclovir, neonates, infants, population pharmacokinetics, opportunistic sampling strategy, congenital cytomegalovirus infection, individualized therapy
INTRODUCTION
Cytomegalovirus (CMV) is the most common source of congenital viral infection and is underrecognized as a significant cause of serious morbidity in newborns. The overall incidence of congenital CMV is about 0.7%, and the rates are even higher (>1%) in developing countries (1, 2). Approximately 10% of infants with congenital CMV are symptomatic, more than half of them develop permanent disabilities, and 5% die from consequences of the infection. Even among the asymptomatic infants, 10% to 15% will still develop permanent sequelae (i.e., sensorineural hearing loss, mental retardation) (1, 3–5). According to the previous studies, approximately 30% of cases of deafness in childhood were caused by congenital CMV infection (6–8).
Ganciclovir is recommended for the treatment of congenital CMV infection, although its use is off-label. Ganciclovir is a synthetic guanine derivative and is the first antiviral agent against CMV (8, 9). It has a low protein binding rate (1% to 2%) and is excreted mainly via the kidney through glomerular filtration and active tubular secretion (9, 10). The pharmacokinetic-pharmacodynamic relationship of ganciclovir has been demonstrated in both solid organ transplant adults and symptomatic congenital-CMV newborns (11–13). A target area under the concentration-time curve from 0 to 24 h (AUC0–24) of 40 to 50 μg · h/ml was recommended to enhance the likelihood of efficacy of the antiviral therapy (14–16).
Although an optimal AUC target for rational ganciclovir therapy was determined, few pediatric patients were able to achieve this target drug exposure using the standard dosing regimen. It has been reported that after receiving the standard dosing regimen of 5 mg/kg of body weight twice daily (BID), pediatric transplant patients had a high variable AUC0–24, which ranged from 11.8 to 65.2 μg · h/ml (17). It is expected that newborns would have higher interindividual variability than children. Hence, the standard treatment might bring high risks of therapeutic failure, antiviral resistance, or adverse events to this vulnerable population (18). Therefore, a one-size-fits-all approach is inadequate for neonatal dosing regimens and dosage individualization is strongly recommended to ensure therapeutic target achievement for each individual.
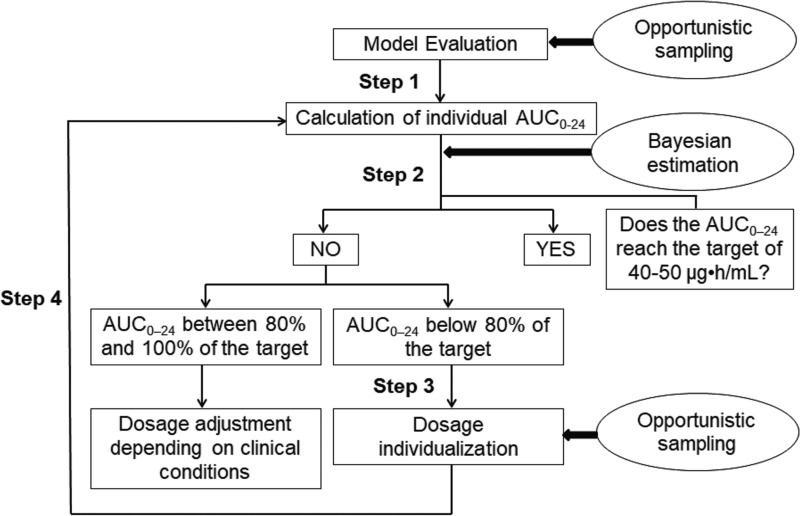
Despite the unmet need of dose individualization of ganciclovir in newborns, there is still a big barrier in application for clinical practice due to the challenges of the small blood volume and the scarcity of sampling allowed in this special population. In an effort to integrate the personalized dose of ganciclovir in clinical practice, we aimed to evaluate the clinical utility of model-based dosage individualization of ganciclovir in neonates and young infants, using an opportunistic sampling approach (Fig. 1).
FIG 1.
Model-based dosage individualization in neonates and young infants.
RESULTS
Twenty-six Chinese newborns with congenital CMV infection were included in the study. Informed-consent forms, signed by the patients' parents or guardians, were obtained from all patients. The patient characteristics are presented in Table 1. A total of 51 ganciclovir concentrations ranging from 0.3 to 5.7 μg/ml were available for analysis (1 patient provided 3 samples, 23 patients provided 2 samples, and 2 patients provided 1 sample). The time between the last dose given and the sample collection ranged from 0.2 to 13.6 h. No ganciclovir-related hematotoxicity and nephrotoxicity were observed during the ganciclovir therapy.
TABLE 1.
Baseline characteristics in 26 Chinese newborns
| Characteristicsa | Mean (SD) | Median (range) |
|---|---|---|
| GA (wk) | 33.0 (4.2) | 32.6 (26.0–39.3) |
| PNA (days) | 28.7 (22.3) | 20.0 (3.0–70.0) |
| PMA (wk) | 37.1 (5.7) | 38.5 (27.1–46.0) |
| Birth wt (g) | 2,161.2 (660.8) | 2,160.0 (1,260.0–4,200.0) |
| Current wt (g) | 2,074.1 (896.1) | 2,350.0 (690.0–3,800.0) |
| Creatinine concn (μmol/liter) in serum | 46.0 (18.5) | 39.0 (26.0–85.0) |
| Ganciclovir concn (μg/ml) in plasma | 1.9 (1.5) | 1.4 (0.3–5.7) |
GA, gestational age at birth; PMA, postmenstrual age; PNA, postnatal age.
Step 1. Model evaluation.
The predictive performance of the model was evaluated using the present data set. The mean prediction error was 7.64% ± 47.80%, and the mean absolute prediction error was 31.30% ± 36.67%. The prediction error was within ±20% and ±30% for 51.0% and 70.6% of measurements, respectively.
Step 2. Calculation of individual AUC0–24 after the standard dosing regimen.
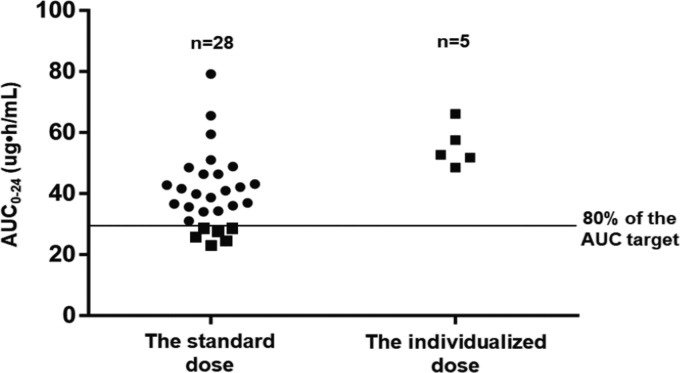
After giving the standard ganciclovir dosing of 5 mg/kg every 12 h, the mean AUC0–24 was 40.6 (22.9 to 79.2) μg · h/ml, and the target AUC0–24 of ≥40 mg · h/liter was achieved in 42.3% (n = 11) of patients.
Step 3. Model-based dosage individualization.
A total of 23.1% (n = 6) of patients with AUC0–24 below 80% of the target were chosen to optimize the ganciclovir dose; one patient who stopped taking ganciclovir after the first dose was excluded. To achieve an AUC0–24 of 40 μg · h/ml, the adjusted doses were increased by 28.6% to 60.0% in these patients.
Step 4. Evaluation of individualized doses.
After administration of the adjusted dose, the AUC0–24 values were calculated from the model and ranged from 48.6 to 66.1 μg · h/ml. As a result, all five patients undergoing model-based dose individualization achieved the therapeutic exposure target. The AUC0–24 values before and after the dose individualization are shown in Fig. 2.
FIG 2.
AUC0–24 before and after model-based dosage individualization.
DISCUSSION
In the current study, the feasibility of dosage individualization of ganciclovir by a model-based approach coupled with an opportunistic sampling strategy in neonates and young infants with congenital CMV infection is presented for the first time.
A target AUC0–24 of 40 to 50 μg · h/ml is recommended in both adult and pediatric patients undergoing CMV prophylaxis and treatment. Ganciclovir pharmacokinetics are highly variable among children (19–22) due to their developmental pharmacology and clinical conditions, which were proved to have profound impacts on the disposition of drugs. Intravenous ganciclovir is regarded as the first-line agent for the treatment of symptomatic congenital CMV infection and is typically initiated with a standard dosing regimen of 5 mg/kg BID. However, the standard dose was established based on limited pediatric pharmacokinetic data and the assumption of an “average child” (9, 21, 23). Consequently, the standard dose might induce side effects or treatment failure or even contribute to CMV resistance. Therefore, dose optimization/individualization is indispensable for pediatric patients to ensure that each individual patient reaches the therapeutic target of ganciclovir exposure.
In the present study, the clinical utility of the developed model has been evaluated, using data from an external validation group. Using only the clinical characteristics of a patient and ganciclovir concentrations obtained with an opportunistic sampling strategy, the model was able to calculate the AUC0–24 for each individual patient. This approach improved the therapeutic target achievement for the cohort of neonates and young infants examined in the current study. Although there were 3 patients with AUC0–24 values ranging from 51.06 to 65.54 μg · h/ml and 4 patients with AUC0–24 values ranging from 51.78 to 66.10 μg · h/ml before and after the dose individualization, respectively, no obvious adverse effects have been observed during the treatment process (9). Thereby, no dosing adjustment was performed for these patients with AUC0–24 above the target. The dosing adjustment should be weighted upon the target attainment rate against drug-related toxicity.
The use of a population pharmacokinetic model combined with the opportunistic sampling strategy in the present study is a reliable alternative strategy to the conventional method for ganciclovir dosage individualization in newborns. The ganciclovir dosing individualization is essential for improving clinical outcome; however, the major barriers to the application of therapeutic drug monitoring (TDM) in newborns include mainly the restriction in the number (and volume) of blood samples that can be obtained and the difficulty in determination of the concentration of drug in plasma at specific times. The proposed approach is an optimal option (more adapted to neonatal clinical practice) for optimizing ganciclovir individual treatment. This approach might provide reasonably precise predictive performance and reliable AUC estimation, reduce both the number (and volume) of blood samples and the risks of multiple blood sampling by exploiting the full potential of residual blood samples, and mitigate parental concern associated with repeated blood sampling. Incorporation of such an approach into a robust clinical dosing support tool can help guide clinicians with dose individualization.
Our study had some limitations. The small number of samples within the dosing interval might introduce bias in predicting the individual AUC of a special patient, requiring repeated dosing adjustment. The methodological aspect of designing an “optimal” opportunistic sampling approach still needs to be evaluated. In addition, the clinical benefits of personalized therapy in treatment efficacy and safety still need to be confirmed in further randomized trials.
Conclusion.
In conclusion, our model-based dosing approach combined with the opportunistic sampling strategy provides a clinically feasible method for ganciclovir dosage individualization in newborns. A close collaboration between neonatologists and pediatric pharmacists/pharmacologists is mandatory to incorporate the ideal model-based dosing individualization approach into clinical practice.
MATERIALS AND METHODS
This open-label pharmacokinetic study was conducted at Shandong Provincial Qianfoshan Hospital and Children's Hospital of Hebei Province between 2016 and 2017 (ClinicalTrials registration no. NCT03113344). Neonates and young infants receiving intravenous ganciclovir therapy (5 mg/kg, BID) for congenital CMV infections were enrolled in this noninterventional study, which was designed in accordance with the legal requirements and the Declaration of Helsinki and was approved by the institute ethics committee. A consent process includes 2 approaches: information given on a flyer/leaflet and a formal informed-consent process. The whole study process included four steps (Fig. 1).
First step: model evaluation.
A previously published ganciclovir population pharmacokinetic model in newborns was used (11). The following modeling information was extracted from the article: a one-compartment model with first-order elimination, interindividual variability for clearance (CL) (% coefficient of variation [%CV], 26.9%) by an exponential error model, residual variability using an exponential model (%CV, 38.2%) and a significant influence of current weight (CW) on CL and volume of distribution (V) with the following regression equations (equations 1 and 2):
| (1) |
| (2) |
The bias and precision of the model were assessed by calculating the median prediction error (MPE) and median absolute prediction error (MAE) (24) (equations 3 and 4). In addition, the numbers of patients with MPE beyond ±20% and ±30% were calculated.
| (3) |
| (4) |
Second step: calculation of individual AUC0–24.
Individual AUC0–24 values were estimated for each patient after administration of the standard dosing regimen of 5 mg/kg BID using a Bayesian estimation. The AUC0–24 was defined as dose/CL, and the individual CL was calculated by a Bayesian estimation (“MAXEVAL” = 0′ and ‘Post hoc’ in the $ESTIMATION step of NONMEM software, first-order conditional estimation interaction option) using population pharmacokinetics parameters obtained from a previously developed model (11).
Third step: model-based dosage individualization.
If the AUC results were substantially lower than the target value of 40 to 50 μg · h/ml (below 80% of the target), an increased dose was calculated based on the target AUC and subsequently given to the patient. For other sub- or overtherapeutic patients, dose adjustment depended on clinical conditions.
Last step: evaluation of individualized dose.
In order to ensure the efficacy of the drug, the ganciclovir concentrations in plasma were measured again after the dose adjustment, and AUC0–24 was calculated using a Bayesian estimation.
Safety evaluation.
The safety evaluation was focused on ganciclovir-related hematotoxicity and nephrotoxicity (8), which was evaluated based on changes in absolute neutrophil count, platelet count, and serum creatinine concentrations from baseline. Hematotoxicity was defined as a 50% reduction in either absolute neutrophil or platelet count (25). Nephrotoxicity was defined as either a 2-fold increase or an increase by at least 0.6 mg/dl of the serum creatinine concentrations (26). Safety evaluation was performed from the start and at various times until the end of ganciclovir therapy.
Opportunistic sampling.
Blood samples during the whole study period were collected using an opportunistic (also called scavenged) sampling strategy (27). During the ganciclovir treatment period, opportunistic samples were collected from blood remaining after routine biochemical tests that were ordered by physicians. No additional blood volume was taken for these samples. Tubes with specific research labels were used, allowing the laboratory staff to identify the opportunistic samples and store them at −80°C after routine testing for a maximum of 48 h before analysis.
Analytical method of ganciclovir.
The analytical method of ganciclovir was adapted from Zhang et al. (28). Concentrations in plasma were determined using high-performance liquid chromatography (HPLC) with UV detection at 254 nm. The calibration graph for ganciclovir ranged from 0.25 to 25 μg/ml (0.25, 0.5, 1, 5, 10, and 25 μg/ml). The interday and intraday coefficients of variation (CVs) were 5.6% and 2.1%, respectively, for controls (2 and 15 μg/ml). The lower limit of quantification (LOQ) was 0.25 μg/ml, with interday and intraday CVs of 5.8% and 1.9%, respectively. The short-term, long-term, and freeze-thaw stabilities of ganciclovir in plasma have been validated in a previous study (29).
ACKNOWLEDGMENTS
This work is supported by the National Science and Technology Major Projects for Major New Drugs Innovation and Development (2017ZX09304029-002), Young Taishan Scholars Program and Young Scholars Program of Shandong University (2015WLJH49).
We have no conflicts of interest to declare.
REFERENCES
- 1.Dollard SC, Grosse SD, Ross DS. 2007. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 17:355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 2.Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 3.Stowell JD, Forlin-Passoni D, Cannon MJ. 2010. Congenital cytomegalovirus: an update. Contemp Pediatr 27:38–51. [Google Scholar]
- 4.Michaels MG. 2007. Treatment of congenital cytomegalovirus: where are we now? Expert Rev Anti Infect Ther 5:441–448. doi: 10.1586/14787210.5.3.441. [DOI] [PubMed] [Google Scholar]
- 5.Schleiss MR. 2008. Congenital cytomegalovirus infection: update on management strategies. Curr Treat Options Neurol 10:186–192. doi: 10.1007/s11940-008-0020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malm G, Engman ML. 2007. Congenital cytomegalovirus infections. Semin Fetal Neonatal Med 12:154–159. doi: 10.1016/j.siny.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Boppana SB, Fowler KB, Pass RF, Rivera LB, Bradford RD, Lakeman FD, Britt WJ. 2005. Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. J Pediatr 146:817. doi: 10.1016/j.jpeds.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 8.Stockmann C, Roberts JK, Knackstedt ED, Spigarelli MG, Sherwin CM. 2015. Clinical pharmacokinetics and pharmacodynamics of ganciclovir and valganciclovir in children with cytomegalovirus infection. Expert Opin Drug Metab Toxicol 11:205–219. doi: 10.1517/17425255.2015.988139. [DOI] [PubMed] [Google Scholar]
- 9.Genentech USA, Inc. 2010. Cytovene-IV package insert. Genentech USA, Inc, San Francisco, CA. [Google Scholar]
- 10.Faulds D, Heel RC. 1990. Ganciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in cytomegalovirus infections. Drugs 39:597–638. doi: 10.2165/00003495-199039040-00008. [DOI] [PubMed] [Google Scholar]
- 11.Acosta EP, Brundage RC, King JR, Sánchez PJ, Sood S, Agrawal V, Homans J, Jacobs RF, Lang D, Romero JR, Griffin J, Cloud G, Whitley R, Kimberlin DW, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. 2007. Ganciclovir population pharmacokinetics in neonates following intravenous administration of ganciclovir and oral administration of a liquid valganciclovir formulation. Clin Pharmacol Ther 81:867. doi: 10.1038/sj.clpt.6100150. [DOI] [PubMed] [Google Scholar]
- 12.Kimberlin DW, Acosta EP, Sánchez PJ, Sood S, Agrawal V, Homans J, Jacobs RF, Lang D, Romero JR, Griffin J, Cloud GA, Lakeman FD, Whitley RJ, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. 2008. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J Infect Dis 197:836. doi: 10.1086/528376. [DOI] [PubMed] [Google Scholar]
- 13.Wiltshire H, Paya CV, Pescovitz MD, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Zuideveld KP, Valganciclovir Solid Organ Transplant Study Group. 2005. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation 79:1477. doi: 10.1097/01.TP.0000164512.99703.AD. [DOI] [PubMed] [Google Scholar]
- 14.Åsberg A, Bjerre A, Neely M. 2014. New algorithm for valganciclovir dosing in paediatric solid organ transplant recipients. Pediatr Transplant 18:103–111. doi: 10.1111/petr.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pescovitz MD, Ettenger RB, Strife CF, Sherbotie JR, Thomas SE, McDiarmid S, Bartosh S, Ives J, Bouw MR, Bucuvalas J. 2010. Pharmacokinetics of oral valganciclovir solution and intravenous ganciclovir in paediatric renal and liver transplant recipients. Transpl Infect Dis 12:195. doi: 10.1111/j.1399-3062.2009.00478.x. [DOI] [PubMed] [Google Scholar]
- 16.Villeneuve D, Brothers A, Harvey E, Kemna M, Law Y, Nemeth T, Gantt S. 2013. Valganciclovir dosing using area under the curve calculations in paediatric solid organ transplant recipients. Pediatr Transplant 17:80–85. doi: 10.1111/petr.12030. [DOI] [PubMed] [Google Scholar]
- 17.Launay E, Théôret Y, Litalien C, Duval M, Alvarez F, Lapeyraque AL, Phan V, Larocque D, Poirier N, Lamarre V, Ovetchkine P. 2012. Pharmacokinetic profile of valganciclovir in paediatric transplant recipients. Pediatr Infect Dis J 31:405. doi: 10.1097/INF.0b013e3182463a19. [DOI] [PubMed] [Google Scholar]
- 18.Torre-Cisneros J, Aguado JM, Caston JJ, Almenar L, Alonso A, Cantisán S, Carratalá J, Cervera C, Cordero E, Fariñas MC, Fernández-Ruiz M, Fortún J, Frauca E, Gavaldá J, Hernández D, Herrero I, Len O, Lopez-Medrano F, Manito N, Marcos MA, Martín-Dávila P, Monforte V, Montejo M, Moreno A, Muñoz P, Navarro D, Pérez-Romero P, Rodriguez-Bernot A, Rumbao J, San Juan R, Vaquero JM, Vidal E, Spanish Society of Transplantation (SET), Group for Study of Infection in Transplantation of the Spanish Society of Infectious Diseases and Clinical Microbiology (GESITRA-SEIMC), Spanish Network for Research in Infectious Diseases (REIPI). 2016. Management of cytomegalovirus infection in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant Rev (Orlando) 30:119–143. doi: 10.1016/j.trre.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Launay E, Théôret Y, Litalien C, Duval M, Alvarez F, Lapeyraque AL, Phan V, Larocque D, Poirier N, Lamarre V, Ovetchkine P. 2012. Pharmacokinetic profile of valganciclovir in paediatric transplant recipients. Pediatr Infect Dis J 31:405–407. doi: 10.1097/INF.0b013e3182463a19. [DOI] [PubMed] [Google Scholar]
- 20.Wei Z, Baudouin V, Zhang D, Deschênes G, Guellec CL, Jacqzaigrain E. 2009. Population pharmacokinetics of ganciclovir following administration of valganciclovir in paediatric renal transplant patients. Clin Pharmacokinet 48:321–328. doi: 10.2165/00003088-200948050-00004. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Fakhoury M, Fila M, Baudouin V, Deschênes G, Jacqz-Aigrain E. 2012. Individualization of valganciclovir prophylaxis for cytomegalovirus infection in paediatric kidney transplant patients. Ther Drug Monit 34:326–330. doi: 10.1097/FTD.0b013e3182509e3a. [DOI] [PubMed] [Google Scholar]
- 22.Vethamuthu J, Feber JA, Lampe D, Filler G. 2007. Unexpectedly high inter- and intrapatient variability of ganciclovir levels in children. Pediatr Transplant 11:301–305. doi: 10.1111/j.1399-3046.2006.00669.x. [DOI] [PubMed] [Google Scholar]
- 23.Neely M, Jelliffe R. 2008. Practical therapeutic drug management in HIV-infected patients: use of population pharmacokinetic models supplemented by individualized Bayesian dose optimization. J Clin Pharmacol 48:1081. doi: 10.1177/0091270008321789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Meer AF, Marcus MA, Touw DJ, Proost JH, Neef C. 2011. Optimal sampling strategy development methodology using maximum a posteriori Bayesian estimation. Ther Drug Monit 33:133–146. doi: 10.1097/FTD.0b013e31820f40f8. [DOI] [PubMed] [Google Scholar]
- 25.Whitley RJ, Cloud G, Gruber W, Storch GA, Demmler GJ, Jacobs RF, Dankner W, Spector SA, Starr S, Pass RF, Stagno S, Britt WJ, Alford C Jr, Soong S, Zhou XJ, Sherrill L, FitzGerald JM, Sommadossi JP. 1997. Ganciclovir treatment of symptomatic congenital cytomegalovirus infection: results of a phase II study. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis 175:1080–1086. [DOI] [PubMed] [Google Scholar]
- 26.Spivey JF, Singleton D, Sweet S, Storch GA, Hayashi RJ, Huddleston CB, Danziger-Isakov LA. 2007. Safety and efficacy of prolonged cytomegalovirus prophylaxis with intravenous ganciclovir in paediatric and young adult lung transplant recipients. Pediatr Transplant 11:312–318. doi: 10.1111/j.1399-3046.2006.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leroux S, Turner MA, Guellec CB, Hill H, van den Anker JN, Kearns GL, Jacqz-Aigrain E, Zhao W, TINN (Treat Infections in NeoNates) and GRiP (Global Research in Paediatrics) Consortiums. 2015. Pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet. 54:1273–1285. doi: 10.1007/s40262-015-0291-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, Lapeyraque AL, Popon M, Loirat C, Jacqzaigrain E. 2003. Pharmacokinetics of ganciclovir in paediatric renal transplant recipients. Pediatr Nephrol 18:943–948. doi: 10.1007/s00467-003-1226-x. [DOI] [PubMed] [Google Scholar]
- 29.Chu F, Kiang CH, Sung ML, Huang B, Reeve RL, Tarnowski T. 1999. A rapid, sensitive HPLC method for the determination of ganciclovir in human plasma and serum. J Pharm Biomed Anal 21:657–667. doi: 10.1016/S0731-7085(99)00161-2. [DOI] [PubMed] [Google Scholar]