ABSTRACT
Ceftazidime-avibactam was used to treat 77 patients with carbapenem-resistant Enterobacteriaceae (CRE) infections at our center. Thirty- and 90-day survival rates were 81% and 69%, respectively; these rates were higher than those predicted by SAPS II and SOFA scores at the onset of infection. Clinical success was achieved for 55% of patients but differed by the site of infection. Success rates were lowest for pneumonia (36%) and higher for bacteremia (75%) and urinary tract infections (88%). By multivariate analysis, pneumonia (P = 0.045) and receipt of renal replacement therapy (RRT) (P = 0.046) were associated with clinical failure. Microbiologic failures occurred in 32% of patients and occurred more commonly among patients infected with KPC-3-producing CRE than among those infected with KPC-2-producing CRE (P = 0.002). Pneumonia was an independent predictor of microbiologic failure (P = 0.007). Ceftazidime-avibactam resistance emerged in 10% of patients, including 14% of those infected with Klebsiella pneumoniae and 32% of those with microbiologic failure. RRT was an independent predictor of the development of resistance (P = 0.009). Resistance was identified exclusively among K. pneumoniae bacteria harboring variant KPC-3 enzymes. Upon phylogenetic analysis of whole-genome sequences, resistant isolates from 87.5% (7/8) of patients clustered within a previously defined sequence type 258 (ST258) clade II sublineage; resistant isolates from one patient clustered independently from other ST258 clade II isolates. In conclusion, our report offers new insights into the utility and limitations of ceftazidime-avibactam across CRE infection types. Immediate priorities are to identify ceftazidime-avibactam dosing and therapeutic regimens that improve on the poor outcomes among patients with pneumonia and those receiving RRT.
KEYWORDS: CRE, Klebsiella pneumoniae, ceftazidime-avibactam, failure, outcomes, pneumonia, renal replacement therapy
INTRODUCTION
Ceftazidime-avibactam (Cef-Avi) has been commercially available in the United States for more than 2 years following Food and Drug Administration (FDA) approval for treatment of complicated urinary tract and intra-abdominal infections. Avibactam, a β-lactamase inhibitor, is active against class A (e.g., KPC), class C (e.g., AmpC), and certain class D (e.g., OXA-48) carbapenemases but not against class B metallo-β-lactamases (e.g., VIM, IMP, and NDM). Clinical experience with ceftazidime-avibactam against carbapenem-resistant Enterobacteriaceae (CRE) infections is accumulating, but important knowledge gaps remain, and real-world experience reported in the literature is limited. We have published a study of 37 patients treated with ceftazidime-avibactam for a variety of CRE infections (1) and a subsequent study comparing patients treated with ceftazidime-avibactam to those receiving other treatment options for CRE bacteremia (2). In our experience, ceftazidime-avibactam was efficacious and well tolerated, but microbiologic failures occurred in 27% of patients and ceftazidime-avibactam resistance emerged in 8% (1). Ceftazidime-avibactam resistance arose in KPC-3-producing Klebsiella pneumoniae isolates of a novel sequence type (ST), ST258, clade II sublineage, which are phylogenetically distinct from previously characterized ST258 clade II isolates (3).
Important questions remain about the effectiveness of ceftazidime-avibactam across CRE infection types, the clinical and microbiologic features associated with poor treatment outcomes, and the emergence of resistance. The objectives of this study were to report our ongoing experience with ceftazidime-avibactam in the treatment of CRE infections, determine risk factors associated with treatment failure and the emergence of resistance, and describe resistance mechanisms and the genomics of resistant isolates.
RESULTS
Patients, microbiology, and treatment regimens.
Seventy-seven consecutive patients treated with ≥3 days of ceftazidime-avibactam were evaluated. The median age was 62 years (range, 19 to 91); 61% (47/77) were men. The median Charlson Comorbidity Index score was 4 (range, 0 to 10). Twenty-six percent (20/77) of patients were transplant recipients (18 solid-organ and 2 bone marrow transplants). At the onset of infection, median SAPS II and SOFA scores were 41 (range, 8 to 81) and 5 (range, 0 to 20), respectively. Infections included pneumonia (43% [33/77]), primary bacteremia (26% [20/77]), urinary tract infection (UTI) (10% [8/77]), intra-abdominal infection (9% [7/77]), skin/soft tissue infection (8% [6/77]), and mediastinitis, subdural empyema/ventriculitis, and purulent tracheobronchitis (1% [1/77] each). Seventy-nine percent (26/33) and 21% (7/33) of pneumonia cases were ventilator and health care associated, respectively. Pyelonephritis was present in 50% (4/8) of UTIs; 25% (2/8) of UTIs resulted in secondary bacteremia.
K. pneumoniae was the predominant pathogen (78% [60/77]), followed by Escherichia coli (12% [9/77]), Enterobacter cloacae (6% [5/77]), and Enterobacter aerogenes, Serratia marcescens, and Klebsiella oxytoca (1 case each). All baseline CRE isolates were resistant to at least one carbapenem, as determined by the University of Pittsburgh Medical Center (UPMC) clinical microbiology laboratory, and susceptible to ceftazidime-avibactam, as determined using broth microdilution (Table 1) (median MIC, 1 μg/ml; range, 0.12 to 8 μg/ml). By broth microdilution methods, 96% (74/77), 88% (68/77), and 78% (60/77) of isolates were nonsusceptible to ceftazidime, ertapenem, and meropenem, respectively. Seventy-five percent (58/77) of isolates harbored a blaKPC gene, including 93% (56/60) of K. pneumoniae isolates. Fifty-eight percent (35/60) and 35% (21/60) of K. pneumoniae isolates carried blaKPC-2 and blaKPC-3, respectively. No isolates had genes encoding NDM, VIM, IMP, or OXA-48-like carbapenemases.
TABLE 1.
Characteristics of CRE isolates from patients treated with ceftazidime-avibactam
| Pathogen (no. of isolates) | Median (range) MIC (μg/ml) |
No. (%) of isolates with the following β-lactamase: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Ceftazidime-avibactam | Ceftazidime | Ertapenema | Meropenema | CTX-M | OXAb | SHV | TEM | KPCc | |
| K. pneumoniae (60) | 1 (0.12 to 2) | 256 (32 to >512) | 32 (0.06 to >64) | 16 (0.06 to >64) | 7 (12) | 11 (18) | 56 (93) | 53 (88) | 56 (93) |
| E. coli (9) | 1 (0.25 to 2) | 128 (2 to >512) | 2 (0.06 to 64) | 0.12 (0.06 to 4) | 8 (89) | 5 (56) | 0 (0) | 1 (11) | 0 (0) |
| E. cloacae (5) | 0.5 (0.5 to 8) | 32 (0.5 to 128) | 0.5 (0.06 to 8) | 0.12 (0.06 to 0.5) | 3 (60) | 2 (40) | 1 (20) | 3 (60) | 1 (20) |
| E. aerogenes (1) | 0.5 | 128 | 4 | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| K. oxytoca (1) | 0.5 | 128 | 8 | 0.25 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| S. marcescens (1) | 1 | 128 | 1 | 0.25 | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total (77) | 1 (0.12 to 8) | 256 (0.5 to >512) | 32 (0.06 to >64) | 16 (0.06 to >64) | 19 (25) | 18 (23) | 57 (74) | 57 (74) | 58 (75) |
Eighty-five percent (58/68) and 92% (55/60) of isolates that were not susceptible to ertapenem and meropenem, respectively, produced KPC. Non-KPC-producing isolates (n = 19) generally carried extended-spectrum CTX-M (n = 15) or AmpC (n = 1) variant β-lactamases; 3 isolates did not harbor KPC or ESBL.
No isolate harbored an OXA-48 variant.
Of KPC-producing isolates, 62% (36/58) had KPC-3 and 38% (22/58) had KPC-2.
Ceftazidime-avibactam was administered as monotherapy or in combination regimens to 69% (53/77) or 31% (24/77) of patients, respectively. Combinations included intravenous (i.v.) (n = 10), inhaled (n = 7), or both i.v. and inhaled (n = 2) gentamicin, i.v. (n = 1) or intrathecal (n = 1) colistin, i.v. amikacin (n = 1), i.v. ciprofloxacin (n = 1), and i.v. tigecycline (n = 1). The median ceftazidime-avibactam treatment duration was 14 days (range, 4 to 71 days).
Treatment outcomes.
Thirty- and 90-day survival rates were 81% (62/77) and 69% (54/72), respectively. Survival rates were higher than those predicted by SAPS II and SOFA scores, in particular for scores in the ranges of 30 to 64 for SAPS II and 10 to 14 for SOFA (Table 2). Clinical success was achieved for 55% (42/77) of patients and did not differ between those receiving monotherapy (56% [30/53]) and those receiving combination therapy (50% [12/24], P = 0.62). Failures were due to death (n = 15), recurrence (n = 11), or the absence of clinical improvement (n = 9). Success rates were 88% (7/8) for patients with urinary tract infections, 75% (15/20) for primary bacteremia, 67% (4/6) for skin/soft tissue infections, 43% (3/7) for intra-abdominal infections, 36% for pneumonia (12/33), and 33% (1/3) for other infections. Seventeen percent (7/42) of patients with 30-day clinical success had subsequent relapsing CRE infections at the same or contiguous infection sites within 90 days; the median time to relapse was 38 days (range, 34 to 84 days). By multivariate analysis, pneumonia (odds ratio [OR], 3.10; 95% confidence interval [CI], 1.03 to 9.34; P = 0.045) and receipt of renal replacement therapy (RRT) (OR, 4.78; 95% CI, 1.03 to 22.20; P = 0.046) were independent predictors of clinical failure (Table 3). The time to initiation of ceftazidime-avibactam treatment was not associated with clinical failure.
TABLE 2.
Predicted versus actual mortality among patients treated with ceftazidime-avibactam for CRE infection
TABLE 3.
Risk factors for clinical failure of ceftazidime-avibactam therapy among patients with CRE infection
| Risk factora | Valueb for patients with: |
P value | Multivariate P value (OR, 95% CI)c | |
|---|---|---|---|---|
| Success (n = 42) | Failure (n = 35) | |||
| Demographics and underlying conditions | ||||
| Male gender | 29 (69) | 18 (51) | 0.16 | |
| Age (yr) | 64 (19–91) | 59 (26–79) | 0.22 | |
| Solid-organ transplant recipient | 9 (21) | 9 (26) | 0.79 | |
| Charlson Comorbidity Index | 4 (0–10) | 5 (0–10) | 0.86 | |
| Severity of illness | ||||
| ICU at disease onset | 17 (40) | 27 (77) | 0.001 | Excluded |
| Renal replacement therapy | 3 (7) | 13 (37) | 0.002 | 0.046 (4.78, 1.03–22.2) |
| SOFA score | 5 (0–18) | 8 (0–20) | 0.0007 | 0.13 |
| SAPS II score | 37 (8–62) | 43 (17–81) | 0.04 | 0.98 |
| Infection characteristics | ||||
| Infection type | ||||
| Pneumonia | 12 (29) | 21 (60) | 0.01 | 0.045 (3.09, 1.03–9.34) |
| Primary bacteremia | 15 (36) | 5 (14) | 0.04 | |
| Urinary tract infection | 7 (17) | 1 (3) | 0.07 | |
| Intra-abdominal infection | 3 (7) | 4 (11) | 0.70 | |
| Skin/soft tissue infection | 4 (10) | 2 (6) | 0.68 | |
| Other | 1 (2) | 2 (6) | 0.59 | |
| CRE pathogen | ||||
| K. pneumoniae | 34 (81) | 25 (71) | 0.42 | |
| E. coli | 5 (12) | 4 (11) | 1.00 | |
| E. cloacae | 2 (5) | 3 (9) | 0.65 | |
| Other | 2 (5) | 2 (6) | 1.00 | |
| Presence of blaKPC | ||||
| KPC-2 | 15 (36) | 7 (20) | 0.20 | |
| KPC-3 | 19 (45) | 17 (49) | 0.82 | |
| KPC negative | 8 (19) | 11 (31) | 0.29 | |
| Treatment characteristics | ||||
| Time to treatment initiation (h) | 71 (15–162) | 76 (3–168) | 0.87 | |
| Ceftazidime-avibactam monotherapy | 30 (71) | 23 (66) | 0.63 | |
Statistically significant risk factors for clinical failure of ceftazidime-avibactam are shown in boldface. ICU, intensive care unit.
Values for categorical variables are numbers (percentages) of patients with the risk factor. Values for continuous variables are medians (ranges).
OR, odds ratio; CI, confidence interval.
Microbiologic failures occurred in 32% (25/77) of patients, due to recurrent/ongoing infection (n = 14; within 30 days), relapsing infection (n = 9; within 31 to 90 days), or colonization (n = 2). The median time to microbiologic failure was 21 days (range, 7 to 84 days) from the initiation of ceftazidime-avibactam therapy. Failures were caused by K. pneumoniae (n = 22; 8 KPC-2- and 14 KPC-3-producing isolates) and E. coli (n = 3). For K. pneumoniae specifically, microbiologic failures were more common among KPC-3-producing isolates (67% [14/21]) than among KPC-2-producing isolates (23% [8/35]) (P = 0.002). Across all patients, pneumonia was the only independent predictor of microbiologic failure (OR, 2.71; 95% CI, 1.53 to 14.57; P = 0.007).
Ceftazidime-avibactam resistance and toxicity.
Ceftazidime-avibactam resistance developed in 10% (8/77) of all patients and in 14% (8/59) of patients infected with K. pneumoniae. Thirty-two percent (8/25) of isolates associated with microbiologic failure were ceftazidime-avibactam resistant. Resistance emerged in 22% (8/37) and 0% (0/19) of KPC-3- and KPC-2-producing K. pneumoniae infections, respectively (P = 0.04). Resistant isolates carried mutant blaKPC-3 encoding variant KPC-3 enzymes (Table 4; see also Table S1 in the supplemental material). Most mutations were within the KPC-3 Ω-loop; a variant with a tyrosine-for-aspartic acid substitution at Ambler amino acid position 179 (D179Y), alone or in combination with other mutations, was identified in 88% (7/8) of patients. Less commonly, mutations were identified outside the Ω-loop, including a glycine-for-valine substitution at amino acid 240 (V240G), which corresponds to KPC-8. Meropenem MICs against isolates carrying variant KPC-3 enzymes were decreased by ≥4-fold (Table S1). Resistance developed following a median of 15 days (range, 7 to 31 days) of ceftazidime-avibactam therapy. Receipt of RRT was a strong independent predictor of the development of ceftazidime-avibactam resistance among patients with microbiologic failures (Table 5) (OR, 26.67; 95% CI, 2.24 to 317.1; P = 0.009). All cases of resistance were due to KPC-3, so this variable was excluded from multivariate analysis.
TABLE 4.
Clinical and microbiologic characteristics of patients in whom ceftazidime-avibactam resistance developed
| Pta | Age (sex)b | Underlying diseasec | CRE pathogend | Type of initial infection | Baseline creatinine clearance (ml/min)e | Initial treatmentf (days) | Clinical outcome at 30 days | Time to microbiologic failure (days) | Causes of microbiologic failure and outcome at 90 days | C-A MIC (μg/ml) for: |
KPC-3 variant of posttreatment isolate | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment isolate | Posttreatment isolate | |||||||||||
| 1 | 49 (F) | Double lung transplant | ST258, KPC-3-producing CRKP | Pneumonia | 51 | C-A, 2.5 g i.v. q8h (10) | Failure | 14 | Reinfection: pneumonia, treated with C-A for an additional 14 days | 2 | 1) 32 | 1) D179Y T243M |
| 2) 256g | 2) D179Y T243M | |||||||||||
| 28 | Persistent pneumonia, treated with meropenem and gentamicin until death | Not applicable | 256 | D179Y T243M | ||||||||
| 2 | 58 (F) | Morbid obesity s/p gastric sleeve surgery | ST258, KPC-3-producing CRKP | Intra-abdominal infection | CRRT | C-A, 0.94 g i.v. q12h (19) | Failure | 41 | Urine colonization, not treated and survived | 4 | 1) 32 | 1) V240G |
| 2) >256g | 2) D179Y | |||||||||||
| 3 | 73 (M) | Esophageal cancer s/p esophagectomy | ST258, KPC-3-producing CRKP | Pneumonia | 113 | C-A, 2.5 g i.v. q8h (15) | Success | 34 | Respiratory colonization, not treated | 2 | 2 | Wild type |
| 58 | Respiratory colonization, not treated | Not applicable | 1) 64 | 1) D179Y | ||||||||
| 2) 128g | 2) D179Y | |||||||||||
| 76 | Recurrent pneumonia, treated with meropenem and colistin for 14 days and survived | Not applicable | 1) 2 | 1) Wild type | ||||||||
| 2) 64g | 2) D179Y | |||||||||||
| 4 | 67 (M) | Esophageal cancer s/p esophagectomy | ST258, KPC-3-producing CRKP | Pneumonia | CRRT | C-A, 1.25 g i.v. q8h (15); inhaled gentamicin (15) | Failure | 25 | Relapsing intra-abdominal infection, treated with C-A for an additional 15 days | 1 | 256 | A177E D179Y |
| 72 | Relapsing bacteremia, treated with meropenem for 14 days and survived | Not applicable | 128 | A177E D179Y | ||||||||
| 5 | 43 (F) | Double lung transplant | ST258, KPC-3-producing CRKP | Pneumonia | iHD | C-A, 0.94 g i.v. q48h (11) | Failure | 74 | Relapsing pneumonia, treated with C-A for 4 additional days prior to death | 2 | 64 | D179Y |
| 6 | 59 (M) | Coronary artery disease, diabetes mellitus | ST258, KPC-3-producing CRKP | Pneumonia | 47 | C-A, 2.5 g i.v. q8h (11) | Failure | 7 | Persistent pneumonia, treated with doripenem and gentamicin for 14 days | 2 | 64 | D179Y |
| 26 | Respiratory colonization, not treated and survived | Not applicable | 1) 4 | 1) Wild type | ||||||||
| 2) 32g | 2) D179Y | |||||||||||
| 7 | 60 (F) | Double lung transplant | ST258, KPC-3-producing CRKP | Pneumonia | iHD | C-A, 0.94 g i.v. q48h (7) | Failure | 7 | Breakthrough bacteremia, treated with C-A for an additional 18 days | 4 | 1 | Wild type |
| 101 | Respiratory colonization, not treated and survived | Not applicable | 64 | 166–167 EL ins | ||||||||
| 8 | 68 (M) | Double lung transplant | ST258, KPC-3-producing CRKP | Pneumonia | CRRT | C-A, 2.5 g i.v. q8h (14); i.v. and inhaled gentamicin (12) | Failure | 33 | Respiratory colonization, not treated | 2 | 1) 2 | 1) Wild type |
| 2) 4g | 2) Wild type | |||||||||||
| 42 | Relapsing tracheobronchitis, treated with C-A and inhaled gentamicin for 17 days | Not applicable | 2 | Wild type | ||||||||
| 61 | Persistent tracheobronchitis, treated with meropenem and gentamicin for 11 days until death | Not applicable | 64 | D179Y | ||||||||
Pt, patient.
M, male; F, female.
s/p, status post.
CRE, carbapenem-resistant Enterobacteriaceae; CRKP, carbapenem-resistant K. pneumoniae.
CRRT, continuous renal replacement therapy; iHD, intermittent hemodialysis.
C-A, ceftazidime-avibactam; q8h, every 8 h.
Two morphologies isolated from the same biologic specimen.
TABLE 5.
Risk factors for ceftazidime-avibactam resistance among patients with microbiologic failure
| Risk factora | Valueb for patients with: |
P value | Multivariate P value (OR, 95% CI)c | |
|---|---|---|---|---|
| Resistance (n = 8) | No resistance (n = 17) | |||
| Demographics and underlying conditions | ||||
| Male gender | 4 (50) | 13 (76) | 0.36 | |
| Age (yr) | 63 (43–73) | 65 (29–79) | 0.98 | |
| Solid organ transplant recipient | 4 (50) | 3 (18) | 0.16 | 0.31 |
| Charlson Comorbidity Index | 4.5 (2–7) | 7 (0–10) | 0.68 | |
| Severity of illness | ||||
| ICU at disease onset | 7 (88) | 12 (67) | 0.63 | |
| Renal replacement therapy | 5 (63) | 1 (6) | 0.006 | 0.009 (26.67, 2.24–317.1) |
| SOFA score | 6.5 (2–14) | 5 (2–19) | 0.68 | |
| SAPS II score | 37.5 (23–75) | 43 (17–78) | 0.38 | |
| Infection characteristics | ||||
| Infection type | ||||
| Pneumonia | 7 (88) | 8 (47) | 0.09 | 0.23 |
| Primary bacteremia | 0 (0) | 5 (29) | 0.14 | |
| Urinary tract infection | 0 (0) | 3 (18) | 0.53 | |
| Intra-abdominal infection | 1 (13) | 0 (0) | 0.32 | |
| Other | 0 (0) | 1 (6) | 1.00 | |
| CRE pathogen | ||||
| K. pneumoniae | 8 (100) | 14 (82) | 0.53 | |
| E. coli | 0 (0) | 3 (18) | 0.53 | |
| Presence of blaKPC | 8 (100) | 14 (82) | 0.53 | |
| KPC-3 | 8 (100) | 6 (35) | 0.003 | Excluded |
| Treatment characteristics | ||||
| Time to treatment initiation (h) | 74 (3–214) | 62 (46–114) | 0.17 | 0.52 |
| Duration of initial treatment (days) | 13.5 (10–25) | 14 (4–71) | 0.38 | |
| Ceftazidime-avibactam monotherapy | 6 (75) | 11 (65) | 1.00 | |
Statistically significant risk factors for ceftazidime-avibactam resistance are shown in boldface. ICU, intensive care unit.
Values for categorical variables are numbers (percentages) of patients with the risk factor. Values for continuous variables are medians (ranges).
KPC-3 was excluded from multivariate analysis because all cases of resistance were due to KPC-3-producing isolates. OR, odds ratio; CI, confidence interval.
Overall, 21% (16/77) of patients required RRT at the time of treatment initiation. Among the remaining patients, 11% (7/61) developed acute kidney injury (AKI; defined by a 1.5× increase in serum creatinine levels from baseline [4]) within 7 days of treatment initiation, including one and two patients receiving concomitant colistin and aminoglycosides, respectively.
Phylogenetic analysis.
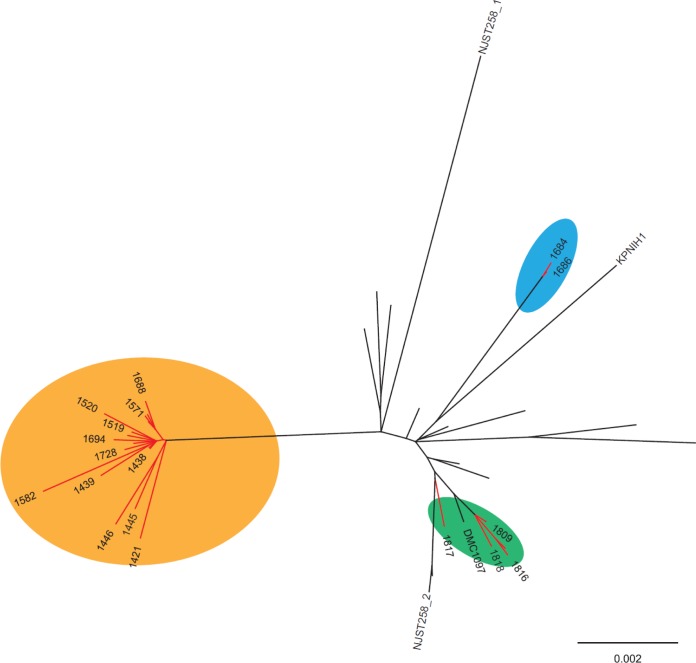
Whole-genome sequences of baseline (n = 8) and postexposure (n = 15) K. pneumoniae isolates from patients in whom resistance emerged were compared to those of 22 ST258 clade II isolates from our center and others (Fig. 1). For seven patients, ceftazidime-avibactam-susceptible baseline isolates and resistant postexposure isolates clustered within the novel ST258 clade II sublineage that we reported previously (3). The 21 isolates from these patients were phylogenetically distinct from reference ST258 clade II K. pneumoniae isolates from our center and others in the United States. In contrast, baseline and postexposure isolates from patient 6 did not cluster within previously defined ST258 clade II lineages (Fig. 1) (3). Within individual patients, the median number of core genome single nucleotide polymorphisms (SNPs) between baseline and subsequent isolates was 20; however, the isolates from patient 6 differed from all other isolates by a median of 157 SNPs. Antibiotic resistance genes and plasmids carried by baseline and postexposure isolates are presented in Table S1 in the supplemental material.
FIG 1.
Phylogenetic comparison of ST258 clade II K. pneumoniae isolates from our center and others in the United States. The phylogenetic tree, based on core genome SNP analysis, was generated with the use of the maximum-likelihood optimality criterion. Branch lengths are proportional to the number of evolutionary changes, and all nodes had 100% bootstrap support. Twenty-three K. pneumoniae isolates from the present study and 22 isolates collected from hospitals in New York, New Jersey, Pennsylvania, Maryland, and Michigan in previous studies (41–43) were included. Isolates from our medical center are indicated by red lines. The colored ovals indicate the previously defined ST258 clade II sublineage associated with ceftazidime-avibactam resistance (orange) (3), the reference ST258 clade II lineage (green), and two distinct isolates from a single patient who developed ceftazidime-avibactam resistance (blue).
DISCUSSION
This is the largest single-center study to date of patients treated with ceftazidime-avibactam. Our findings corroborate previous data from our center and others on clinical outcomes among patients receiving ceftazidime-avibactam for CRE infections (1, 2, 5–8), as well as preliminary data from our center on the rates and mechanisms of resistance (1, 3, 9, 10). The study also reports new findings, that pneumonia and RRT are independent risk factors for clinical failure of ceftazidime-avibactam treatment and independent risk factors for microbiologic treatment failure and the emergence of resistance, respectively. Our experience provides important insights into the clinical utility of ceftazidime-avibactam against CRE infections and the challenges and unanswered questions facing clinicians as they seek to use the agent most effectively.
Overall 30-day survival and clinical success rates among our patients were 81% and 55%, respectively. Ceftazidime-avibactam treatment was not associated with excess mortality, compared to that predicted by SAPS II or SOFA scores (Table 2). In fact, among patients with middle-range SAPS II or SOFA scores, the mortality observed was less than that expected. Patients' outcomes differed strikingly by the type of infection. The clinical success rate for treating pneumonia, the most common infection in our cohort, was only 36%. In contrast, clinical success rates were 75%, 88%, and 67% for treating primary bacteremia, UTIs, and skin/soft tissue infections, respectively. By combining our data with those from other centers (5, 8), clinical success rates for ceftazidime-avibactam treatment of pneumonia and bacteremia are 43% (24/56) and 65% (45/69), respectively (P = 0.02). A previous study from our center reported that survival and clinical success rates were significantly higher if ceftazidime-avibactam, rather than other regimens, was used to treat carbapenem-resistant K. pneumoniae bacteremia (2). In a recent multicenter observational study of 137 patients with CRE infections, patients initially treated with ceftazidime-avibactam had lower rates of all-cause 30-day hospital mortality than patients initially treated with colistin (11). We reported previously that nephrotoxicity was significantly more likely with colistin- or aminoglycoside-containing regimens than with ceftazidime-avibactam (2). Here, AKI was documented in 11% of patients who were not on RRT at baseline; 43% of these patients were receiving colistin or an aminoglycoside with ceftazidime-avibactam. Therefore, a growing body of data demonstrates that ceftazidime-avibactam is a major advance in the treatment of CRE infections, offering an effective and well-tolerated alternative to salvage regimens.
It is not clear if ceftazidime-avibactam pharmacokinetics-pharmacodynamics contribute to worse outcomes among patients with CRE pneumonia. In healthy volunteers, both ceftazidime and avibactam demonstrated rapid penetration into the epithelial lining fluid (ELF), achieving average concentrations that were ∼30% of those in plasma (12). These concentrations generally exceeded those that are effective in animal pneumonia models (13). However, there is no evidence as yet to support similar exposures among critically ill patients. Furthermore, the geometric mean maximum concentration [Cmax] of avibactam in ELF of healthy volunteers was 5.1 mg/liter, well below the concentration (8 mg/liter) that suppressed resistance amplification of a KPC-producing K. pneumoniae isolate in the hollow-fiber infection model (14). The importance of ELF avibactam exposures for patient outcomes or the development of resistance is unknown.
In a recently completed phase 3 randomized controlled trial, ceftazidime-avibactam was noninferior to meropenem among 527 clinically evaluable patients with nosocomial pneumonia. Clinical cure rates at the test-of-cure (TOC) visit were 77% (96/125) and 79% (103/131) for ceftazidime-avibactam and meropenem, respectively, among patients in the extended microbiologically evaluable population. Only six isolates in the study were CRE (five K. pneumoniae isolates and one Serratia marcescens isolate). The mortality rate among 56 patients with CRE pneumonia at our center and others who were treated with ceftazidime-avibactam is 39% (5, 8), which falls within the 34-to-41% range reported for 302 patients with CRE pneumonia in cohort studies prior to the availability of ceftazidime-avibactam (15–18). Further studies are needed to determine if poor pneumonia outcomes are due to underlying comorbid conditions and the severity of illness or to drug failure.
RRT was independently associated with ceftazidime-avibactam clinical failure and the emergence of resistance, suggesting that RRT may lead to inadequate drug exposures. Optimal dosing of ceftazidime-avibactam in patients with renal insufficiency, particularly those receiving continuous RRT (CRRT), is an ongoing challenge for clinicians. There are currently no ceftazidime-avibactam dosing recommendations in the setting of CRRT, and doses administered to patients differ considerably (1). A recent report described a patient on continuous venovenous hemofiltration (CVVH) who received 1.25 g ceftazidime-avibactam i.v. every 8 h and had adequate exposures throughout the dosing interval (19). The calculated mean CVVH extraction ratio percentages were 14.4% and 11.5% for ceftazidime and avibactam, respectively. Devising and validating ceftazidime-avibactam dosing strategies among patients requiring RRT, and CRRT specifically, are pressing priorities.
Ceftazidime-avibactam resistance emerged in 10% of patients, including 14% and 32% of those with KPC-producing K. pneumoniae infections and microbiologic treatment failures, respectively. The mechanisms of resistance were mutations in plasmid-borne blaKPC-3, which resulted in variant KPC-3 enzymes. As reported previously, KPC-3 variants were generally associated with the restoration of carbapenem susceptibility (Table S1 in the supplemental material) (3, 9, 10). The predominant mutation was D179Y within the KPC-3 Ω-loop, which forms the floor of the β-lactamase active site (3). In one patient, we identified a previously unreported KPC-3 variant (insertion of glutamic acid and leucine at amino acids 166 to 167 [166–167 EL ins]). Similar mutations have been selected during ceftazidime-avibactam exposure in vitro (20). It is not clear if a novel ST258 clade II K. pneumoniae sublineage at our center is particularly predisposed to KPC mutations and ceftazidime-avibactam resistance. Finally, clinicians should be aware that ceftazidime-avibactam resistant isolates may be reported as extended-spectrum-β-lactamase-producing, carbapenem-susceptible isolates rather than KPC-producing isolates (3, 10). Therefore, ceftazidime-avibactam susceptibility testing should be performed on all isolates recovered in cases of microbiologic treatment failure, regardless of the reported phenotype.
Combination therapy has been advocated as a strategy to improve clinical outcomes of CRE infections and prevent the emergence of further resistance (21–23). In a recent large retrospective multicenter study of CRE bacteremia that did not include treatment with ceftazidime-avibactam (24), combination therapy with active agents was not associated with an overall mortality rate lower than that for monotherapy with an active agent. However, combination therapy was associated with lower mortality in the subset of patients with higher severity-of-illness scores. Ceftazidime-avibactam combination therapy has not been linked with improved outcomes across retrospective cohort studies (1, 5, 8). In the present study, clinical success rates were nearly identical among patients receiving ceftazidime-avibactam monotherapy or combination therapy. In retrospective studies, it is impossible to rule out indication bias as an explanation for the lack of benefit with ceftazidime-avibactam combination regimens. Our data indicate that the administration of ceftazidime-avibactam was delayed by an average of 3 days from the time of culture collection. In the future, it will be important to determine if rapid CRE detection assays can shorten times to treatment with ceftazidime-avibactam (or other appropriate agents) and improve outcomes.
This study is limited by its single-center, retrospective design. We acknowledge that our results may not be representative of the experience at other institutions. Nevertheless, our report offers new insights into the utility and limitations of ceftazidime-avibactam across CRE infection types. Moving forward, priorities are to identify dosing and therapeutic regimens that improve upon the poor outcomes among patients with pneumonia and those receiving RRT. Ceftazidime-avibactam was approved by the FDA for the treatment of complicated UTIs and complicated intra-abdominal infections, but at many centers such as ours, its primary use will be in treating diverse CRE and other highly resistant bacterial infections. For this reason, it is important for clinicians to share their real-world experiences, so that the community can learn how to employ ceftazidime-avibactam most rationally. This mandate also holds for newly FDA approved agents, such as meropenem-vaborbactam, as they reach the clinic. Studies that characterize strain genomes, plasmid content, and resistance mechanisms will be necessary in order to refine CRE treatment paradigms.
MATERIALS AND METHODS
Study design and definitions.
We conducted a retrospective study of patients with CRE infections who were treated with ceftazidime-avibactam for >48 h at the University of Pittsburgh Medical Center between April 2015 and April 2017. This study includes 38 patients who were previously reported from our center (1–3, 25).
CRE was defined by current Centers for Disease Control criteria as resistance to any carbapenem as determined by automated susceptibility testing methods (MicroScan; Beckman Coulter, Inc., Indianapolis, IN) in the clinical microbiology laboratory (26). A standard dosage of 2.5 g given intravenously (i.v.) every 8 h was used, with adjustments for renal impairment made according to manufacturer recommendations (27). Combination therapy was defined as concomitant administration (≥72 h) of another agent to which the CRE isolate was susceptible in vitro. Types of CRE infection were classified according to National Healthcare Safety Network (NHSN) criteria (28). Severity of illness was calculated using the simplified acute physiology (SAPS II) and sequential organ failure assessment (SOFA) scores (29, 30). Clinical success was defined as survival and absence of recurrence at 30 days following the onset of infection, resolution of signs and symptoms of infection, and the absence of microbiologic failure. Patient outcomes were determined by at least two independent investigators (from among R.K.S., M.H.N., and C.J.C.); in the event of disagreement, cases were adjudicated by a third investigator. Microbiologic failure was defined as isolation of the same species following ≥7 days of ceftazidime-avibactam treatment. Recurring and relapsing infections were defined by microbiologic failure and concomitant signs of infection within 30 and 31 to 90 days of onset, respectively. Acute kidney injury (AKI) was defined by modified KDIGO guidelines as a 1.5× increase in baseline serum creatinine levels within 7 days of treatment initiation (4).
CRE isolate characterization.
MICs were measured for ceftazidime, ceftazidime-avibactam, ertapenem, and meropenem using reference Clinical and Laboratory Standards Institute (CLSI) broth microdilution methods; avibactam was tested at a fixed concentration of 4 μg/ml (31). Quality control (QC) was performed with Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, and Pseudomonas aeruginosa ATCC 27853; all QC MICs were within the specified ranges (32). The presence or absence of β-lactamases was determined for all baseline CRE isolates as described previously (33, 34). In cases in which ceftazidime-avibactam resistance arose, baseline and postexposure isolates underwent whole-genome sequencing as described previously (3). In brief, core genome single nucleotide polymorphism (SNP) analysis was performed using BWA (35) and SAMtools (36), and a phylogenetic tree was generated using RAxML 8.0.0 (37) based on concatenated core SNPs. Twenty-two additional ST258 clade II genomes from a previous study were included for comparison (3). Sequences were assembled de novo using SPAdes 3.10.1 (38), followed by acquired resistance gene in silico mining using ResFinder (39). Plasmid replicon identification was performed using PlasmidFinder (40), as well as a BLASTN search against the complete sequenced plasmid database in GenBank.
Univariate comparisons between groups were made by the Fisher's exact test (for categorical variables) and Mann-Whitney U test (for continuous variables). Multivariate logistic regression was performed with backward selection procedures using covariates with a P value of <0.20 on univariate analysis. Significance was defined as a P value of ≤0.05 (two-tailed).
Accession number(s).
The sequence data obtained in this study have been deposited in NCBI under BioProject number PRJNA326665.
Supplementary Material
ACKNOWLEDGMENTS
This study is supported by an investigator-initiated grant from Allergan awarded to R.K.S., and in part by National Institutes of Health (NIH) awards (K08AI114883 to R.K.S., R21AI117338 to L.C., R01AI090155 to B.N.K., R21AI111037 to C.J.C., and R21AI128338 to M.H.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02497-17.
REFERENCES
- 1.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, Kreiswirth BN, Clancy CJ. 2017. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 61:e00883-17. doi: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 61:e02097-16. doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD. 2013. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61:649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- 5.King M, Heil E, Kuriakose S, Bias T, Huang V, El-Beyrouty C, McCoy D, Hiles J, Richards L, Gardner J, Harrington N, Biason K, Gallagher J. 2017. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 61:e00449-17. doi: 10.1128/AAC.00449-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castón JJ, Lacort-Peralta I, Martin-Dávila P, Loeches B, Tabares S, Temkin L, Torre-Cisneros J, Paño-Pardo JR. 2017. Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int J Infect Dis 59:118–123. doi: 10.1016/j.ijid.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Krapp F, Grant JL, Sutton SH, Ozer EA, Barr VO. 2017. Treating complicated carbapenem-resistant Enterobacteriaceae infections with ceftazidime/avibactam: a retrospective study with molecular strain characterisation. Int J Antimicrob Agents 49:770–773. doi: 10.1016/j.ijantimicag.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Temkin E, Torre-Cisneros J, Beovic B, Benito N, Giannella M, Gilarranz R, Jeremiah C, Loeches B, Machuca I, Jimenez-Martin MJ, Martinez JA, Mora-Rillo M, Navas E, Osthoff M, Pozo JC, Ramos Ramos JC, Rodriguez M, Sanchez-Garcia M, Viale P, Wolff M, Carmeli Y. 2017. Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother 61:e01964-16. doi: 10.1128/AAC.01964-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. In vitro selection of meropenem resistance among ceftazidime-avibactam-resistant, meropenem-susceptible Klebsiella pneumoniae isolates with variant KPC-3 carbapenemases. Antimicrob Agents Chemother 61:e00079-17. doi: 10.1128/AAC.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haidar G, Clancy CJ, Shields RK, Hao B, Cheng S, Nguyen MH. 2017. Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum β-lactamases. Antimicrob Agents Chemother 61:e02534-16. doi: 10.1128/AAC.02534-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Doi Y, Kaye KS, Fowler VG Jr, Paterson DL, Bonomo RA, Evans S; Antibacterial Resistance Leadership Group. 2018. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 66:163–171. doi: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolau DP, Siew L, Armstrong J, Li J, Edeki T, Learoyd M, Das S. 2015. Phase 1 study assessing the steady-state concentration of ceftazidime and avibactam in plasma and epithelial lining fluid following two dosing regimens. J Antimicrob Chemother 70:2862–2869. doi: 10.1093/jac/dkv170. [DOI] [PubMed] [Google Scholar]
- 13.Housman ST, Crandon JL, Nichols WW, Nicolau DP. 2014. Efficacies of ceftazidime-avibactam and ceftazidime against Pseudomonas aeruginosa in a murine lung infection model. Antimicrob Agents Chemother 58:1365–1371. doi: 10.1128/AAC.02161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louie A, Castanheira M, Liu W, Grasso C, Jones RN, Williams G, Critchley I, Thye D, Brown D, Vanscoy B, Kulawy R, Drusano GL. 2012. Pharmacodynamics of beta-lactamase inhibition by NXL104 in combination with ceftaroline: examining organisms with multiple types of beta-lactamases. Antimicrob Agents Chemother 56:258–270. doi: 10.1128/AAC.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P, Isgri S. 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 16.Alexander EL, Loutit J, Tumbarello M, Wunderink R, Felton T, Daikos G, Fusaro K, White D, Zhang S, Dudley MN. 2017. Carbapenem-resistant Enterobacteriaceae infections: results from a retrospective series and implications for the design of prospective clinical trials. Open Forum Infect Dis 4:ofx063. doi: 10.1093/ofid/ofx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Maio Carrilho CM, de Oliveira LM, Gaudereto J, Perozin JS, Urbano MR, Camargo CH, Grion CM, Levin AS, Costa SF. 2016. A prospective study of treatment of carbapenem-resistant Enterobacteriaceae infections and risk factors associated with outcome. BMC Infect Dis 16:629. doi: 10.1186/s12879-016-1979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauck C, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Scalera NM, Doi Y, Kaye KS, Evans S, Fowler VG Jr, Bonomo RA, van Duin D, Antibacterial Resistance Leadership Group. 2016. Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections. Clin Microbiol Infect 22:513–519. doi: 10.1016/j.cmi.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzler E, Bunnell KL, Bleasdale SC, Benken S, Danziger LH, Rodvold KA. 2017. Pharmacokinetics and dialytic clearance of ceftazidime-avibactam in a critically ill patient on continuous venovenous hemofiltration. Antimicrob Agents Chemother 61:e00464-17. doi: 10.1128/AAC.00464-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livermore DM, Warner M, Jamrozy D, Mushtaq S, Nichols WW, Mustafa N, Woodford N. 2015. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother 59:5324–5330. doi: 10.1128/AAC.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. 2014. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 23.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 24.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Cantón R, Doi Y, Tuon FF, Karaiskos I, Pérez-Nadales E, Schwaber MJ, Azap OK, Souli M, Roilides E, Pournaras S, Akova M, Pérez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J; REIPI/ESGBIS/INCREMENT Investigators. 2017. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 17:726–734. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 25.Shields RK, Nguyen MH, Press EG, Chen L, Kreiswirth BN, Clancy CJ. 2017. Emergence of ceftazidime-avibactam resistance and restoration of carbapenem susceptibility in Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: a case report and review of literature. Open Forum Infect Dis 4:ofx101. doi: 10.1093/ofid/ofx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. 29 June 2015, revision date Healthcare-associated infections: FAQs about choosing and implementing a CRE definition. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/hai/organisms/cre/definition.html. [Google Scholar]
- 27.Forest Pharmaceuticals, Inc. 2016. Acycaz (ceftazidime-avibactam) prescribing information. Forest Pharmaceuticals, Inc, Cincinnati, OH. [Google Scholar]
- 28.Centers for Disease Control and Prevention, National Healthcare Safety Network. January 2018. CDC/NHSN surveillance definitions for specific types of infections. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf. [Google Scholar]
- 29.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. 1998. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800. [DOI] [PubMed] [Google Scholar]
- 30.Le Gall JR, Lemeshow S, Saulnier F. 1993. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 31.Shields RK, Clancy CJ, Hao B, Chen L, Press EG, Iovine NM, Kreiswirth BN, Nguyen MH. 2015. Effects of Klebsiella pneumoniae carbapenemase subtypes, extended-spectrum β-lactamases, and porin mutations on the in vitro activity of ceftazidime-avibactam against carbapenem-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother 59:5793–5797. doi: 10.1128/AAC.00548-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing (M100-S24). Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 33.Clancy CJ, Chen L, Shields RK, Zhao Y, Cheng S, Chavda KD, Hao B, Hong JH, Doi Y, Kwak EJ, Silveira FP, Abdel-Massih R, Bogdanovich T, Humar A, Perlin DS, Kreiswirth BN, Nguyen MH. 2013. Epidemiology and molecular characterization of bacteremia due to carbapenem-resistant Klebsiella pneumoniae in transplant recipients. Am J Transplant 13:2619–2633. doi: 10.1111/ajt.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother 65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, Moller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deleo FR, Chen L, Porcella SF, Martens CA, Kobayashi SD, Porter AR, Chavda KD, Jacobs MR, Mathema B, Olsen RJ, Bonomo RA, Musser JM, Kreiswirth BN. 2014. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc Natl Acad Sci U S A 111:4988–4993. doi: 10.1073/pnas.1321364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 4: 148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright MS, Perez F, Brinkac L, Jacobs MR, Kaye K, Cober E, van Duin D, Marshall SH, Hujer AM, Rudin SD, Hujer KM, Bonomo RA, Adams MD. 2014. Population structure of KPC-producing Klebsiella pneumoniae isolates from Midwestern U.S. hospitals. Antimicrob Agents Chemother 58:4961–4965. doi: 10.1128/AAC.00125-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.