ABSTRACT
Bacterial sexually transmitted infections are widespread and common, with Neisseria gonorrhoeae (gonorrhea) and Chlamydia trachomatis (chlamydia) being the two most frequent causes. If left untreated, both infections can cause pelvic inflammatory disease, infertility, ectopic pregnancy, and other sequelae. The recommended treatment for gonorrhea is ceftriaxone plus azithromycin (to empirically treat chlamydial coinfections). Antibiotic resistance to all existing therapies has developed in gonorrheal infections. The need for new antibiotics is great, but the pipeline for new drugs is alarmingly small. The aminomethyl spectinomycins, a new class of semisynthetic analogs of the antibiotic spectinomycin, were developed on the basis of a computational analysis of the spectinomycin binding site of the bacterial 30S ribosome and structure-guided synthesis. The compounds display particular potency against common respiratory tract pathogens as well as the sexually transmitted pathogens that cause gonorrhea and chlamydia. Here, we demonstrate the in vitro potencies of several compounds of this class against both bacterial species; the compounds displayed increased potencies against N. gonorrhoeae compared to that of spectinomycin and, significantly, demonstrated activity against C. trachomatis that is not observed with spectinomycin. Efficacies of the compounds were compared to those of spectinomycin and gentamicin in a murine model of infection caused by ceftriaxone/azithromycin-resistant N. gonorrhoeae; the aminomethyl spectinomycins significantly reduced the colonization load and were as potent as the comparator compounds. In summary, data produced by this study support aminomethyl spectinomycins as a promising replacement for spectinomycin and antibiotics such as ceftriaxone for treating drug-resistant gonorrhea, with the added benefit of treating chlamydial coinfections.
KEYWORDS: chlamydia, aminomethyl spectinomycins, antibacterial, gonorrhea
INTRODUCTION
Chlamydia and gonorrhea are the two most common notifiable infectious diseases (diseases required by law to be reported to government authorities) in the United States, with nearly 1.5 million cases of chlamydia and over 420,000 cases of gonorrhea reported to the Centers for Disease Control and Prevention (CDC) in 2016 (1). Significantly, 50 to 70% of individuals with gonorrhea have a chlamydial coinfection (2–4), which leads to the recommendation for empirical treatment for chlamydia upon detection of Neisseria gonorrhoeae (5, 6). Furthermore, bacterial sexually transmitted infections (STIs) are among the most well-established risk factors for HIV infection because they compromise protective mucosal barriers in the urogenital tract, which facilitates HIV transmission (7).
Chlamydia, caused by infection with Chlamydia trachomatis, is the most prevalent bacterial STI in the United States (nearly 1.5 million reported cases in 2016 [1]). Chlamydial infections in women are usually asymptomatic. However, untreated infection can result in pelvic inflammatory disease (PID), which is a major cause of infertility, ectopic pregnancy, and chronic pelvic pain. Pregnant women infected with chlamydia can pass the infection to their infants during delivery, potentially resulting in neonatal ophthalmic infections and pneumonia (http://www.cdc.gov/std/stats14/surv-2014-print.pdf). Men are often afflicted with asymptomatic urethritis; infection rates are increasing in men as screening methods have improved (http://www.cdc.gov/std/stats14/surv-2014-print.pdf). The recommended treatment regimen for chlamydia is azithromycin (AZM) or doxycycline (5). N. gonorrhoeae causes gonorrhea, the second-most prevalent bacterial STI (8, 9), which is often asymptomatic and can be urogenital, anorectal, or pharyngeal in nature (10). If left untreated, gonorrhea can cause PID in women, leading to fallopian tube scarring and infertility (11), or may disseminate, causing joint and skin manifestations in both men and women (10). The recommended treatment for uncomplicated gonorrhea is single doses of both ceftriaxone (CTX; delivered intramuscularly [i.m.]) and AZM (delivered orally). Alternatively, doxycycline (orally twice daily for 7 days; http://www.cdc.gov/std/tg2015/gonorrhea.htm) is used to prevent resistance and to treat patients that are likely to be coinfected with chlamydia (10).
Once easily treatable, gonorrhea has evolved into a challenging disease, where multidrug resistance is becoming more widespread and infections are becoming more difficult to treat (12, 13). Sulfonamides, introduced in the mid-1930s, were the first antibiotics used to cure gonorrhea; resistance subsequently developed by the mid-1940s (14). As newer antibiotics were used to treat gonorrhea, resistance quickly emerged: strains resistant to penicillin (PEN), streptomycin (STM), erythromycin (ERM), tetracycline (TET), spectinomycin (SPC), fluoroquinolones (15), AZM (14), and cefixime (CFX) (16) have all been reported (9, 17). Cephalosporins remained the foundation of gonorrhea treatment in the 2010 CDC treatment guidelines (18). However, isolates resistant to CTX monotherapy have been identified in China (19), Japan (9), France (16), Spain (20), and possibly in the United States (21).
New antibiotics for treating antibiotic-resistant N. gonorrhoeae infections are desperately needed (22). Unfortunately, the current drug development pipeline targeting new anti-N. gonorrhoeae therapeutics is small and includes combinations of existing antibiotics (http://www.cdc.gov/nchhstp/newsroom/2013/Gonorrhea-Treatment-Trial-PressRelease.html) and a small number of new agents in phase 2 and 3 clinical trials, including ETX0914 (http://fda.einnews.com/article_detail/207751242?lcode=8DWPqPuUsDVNDakfEIxsCA%3D%3D) and gepotidacin. Recently, clinical trials for solithromycin (23) and delafloxacin have been halted due to issues with toxicity and with failure to meet efficacy endpoints and insufficient efficacy, respectively. Because there are few existing treatment options and only a limited pipeline containing a few compounds, the CDC has designated N. gonorrhoeae an immediate public health threat that requires urgent and aggressive action (http://www.cdc.gov/std/Gonorrhea/arg/Wyoming-Health-Alert-2013.pdf).
Clearly, the development of an antibacterial agent that could be used to treat STIs caused by multiple bacteria would provide a distinct advantage over current therapies. An antibacterial drug that is effective against diverse bacterial STIs but is safe and compatible with HIV treatment drug combinations would improve therapy and contribute to HIV prophylaxis. Spectinomycin (SPC), a relative of the aminoglycoside (AG) antibiotics, is an aminocyclitol antibiotic (Fig. 1) (24) that binds selectively to helix-34 within the 30S ribosomal subunit to block translocation and protein synthesis (25). Helix-34 is distinct from the binding locations of other antibacterial ribosomal inhibitors, including other AGs, capreomycin, and linezolid, thus eliminating target-mediated cross-resistance to other antibiotic classes. Despite its potency in inhibiting the 30S ribosome in cell-free assays, SPC is not very active against whole bacteria such as Mycobacterium tuberculosis because it is a substrate for M. tuberculosis efflux pumps (26). To improve the antibacterial activity of SPC, we previously modified the 3′ keto group of ring C (Fig. 1) to a series of substituted amides, producing a series of anti-M. tuberculosis agents (26). We then modified the 3′ keto group to substituted aminomethyl groups, which resulted in the aminomethyl spectinomycin (AmSPC) series (27). The AmSPCs exhibited ribosomal inhibition values comparable to those of SPC but showed increased potency, both in vitro and in vivo, against common respiratory tract pathogens such as Streptococcus pneumoniae (27). The in vitro potencies of the AmSPC analogs were tested against N. gonorrhoeae by disk diffusion assays, and most compounds exhibited improved activity over SPC (27). These results prompted a further exploration of the antibacterial activities of AmSPCs against N. gonorrhoeae and C. trachomatis. Here, we report the superior in vitro efficacy of several of the AmSPCs against multidrug-resistant (MDR) strains of N. gonorrhoeae compared to SPC and other comparator antibiotics plus the unprecedented antibacterial activity observed against C. trachomatis, where SPC had failed to demonstrate efficacy. We also present in vivo efficacy results where high doses of AmSPCs worked comparably to both SPC and gentamicin (GEN) in clearing murine N. gonorrhoeae infections. The results will demonstrate that the AmSPCs have the potential to treat N. gonorrhoeae-C. trachomatis coinfections.
FIG 1.
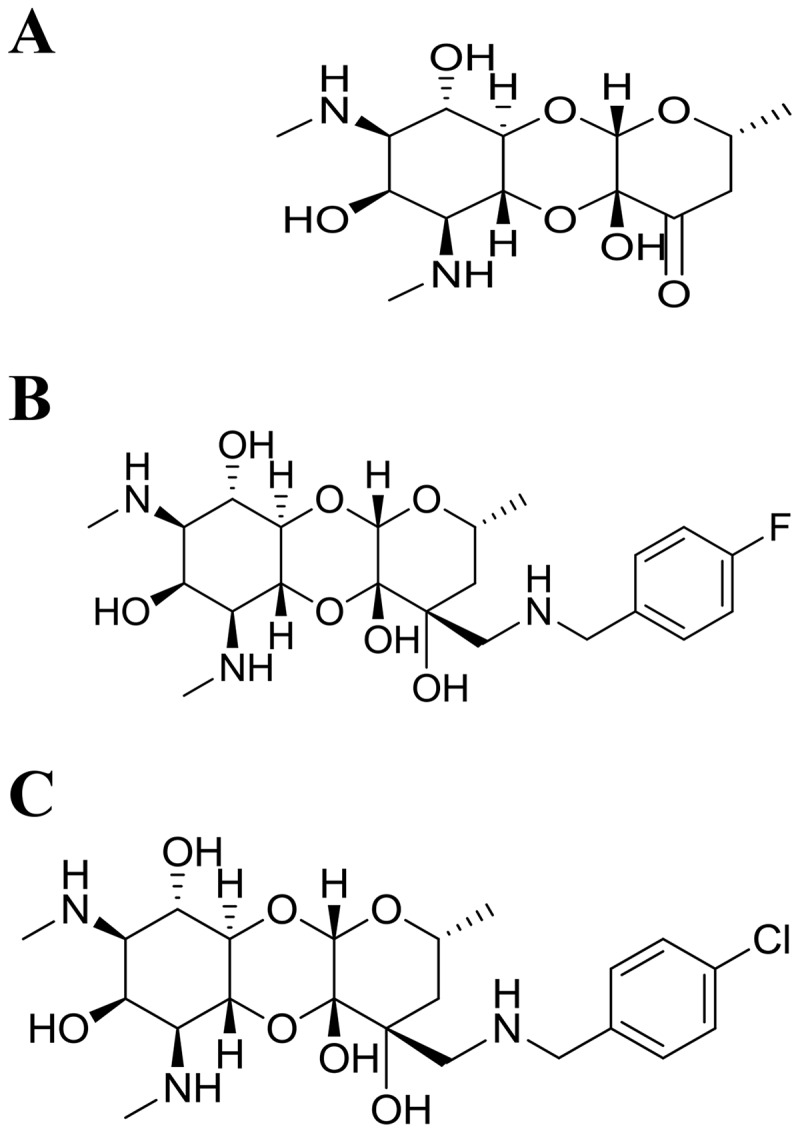
Structures of spectinomycin (A), 1950 (B), and 2324 (C).
(Portions of this work were previously presented at the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy [28] and at ASM Microbe 2016 [29].)
RESULTS
MIC testing of AmSPC analogs.
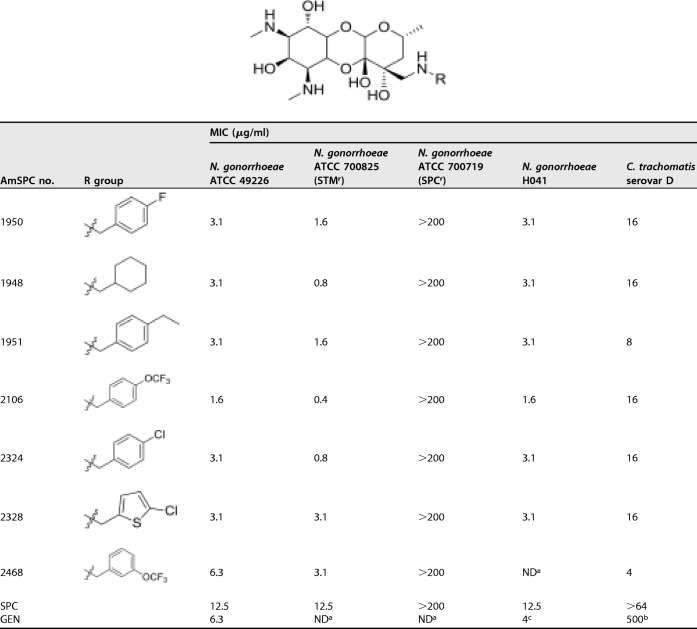
The antibacterial activities of several AmSPCs against a drug-sensitive type strain and against SPC-resistant, STM-resistant, and MDR isolates of N. gonorrhoeae were determined using the broth microdilution MIC assay. The results of these assays are shown in Table 1. The AmSPC analogs were consistently more potent than SPC and had potencies comparable to those of GEN, an antibiotic that can be used to treat drug-resistant N. gonorrhoeae. The rank order of the AmSPC analogs based on MICs was similar to rank order potencies found in previously reported disk diffusion assays (27). Importantly, compounds from this series were active against both STMr and MDR clinical isolates, although they were inactive against an SPCr isolate. As shown in Table 1, the AmSPCs also displayed good activity against C. trachomatis with improved potencies compared to those of SPC and GEN. Additionally, compounds were evaluated for their ability to kill N. gonorrhoeae and C. trachomatis type strains. As shown in Table 2, AmSPCs exhibited bactericidal activity against N. gonorrhoeae and C. trachomatis, with minimum bactericidal concentration (MBC) values within 2× the MIC values.
TABLE 1.
TABLE 2.
Bactericidal activity against C. trachomatis and N. gonorrhoeaec
| Compound | C. trachomatis MBCa (μg/ml) | Fold MIC | N. gonorrhoeae MBCb (μg/ml) | Fold MIC |
|---|---|---|---|---|
| SPC | >64 | NA | 12.5 | 1 |
| 1948 | 16 | 1 | 3.1 | 1 |
| 1950 | 32 | 2 | 6.3 | 2 |
| 1951 | 8 | 1 | 6.3 | 2 |
| 2106 | 16 | 1 | 3.1 | 2 |
| 2324 | 32 | 2 | 3.1 | 1 |
| 2328 | 16 | 1 | 6.3 | 2 |
| 2468 | 8 | 2 | ND | ND |
Chlamydia trachomatis serovar D.
Neisseria gonorrhoeae ATCC 49226.
NA, not applicable; ND, not determined.
In a second broth dilution assay, the in vitro antibacterial activity of 2324 was evaluated against a panel of 27 antibiotic-resistant N. gonorrhoeae clinical isolates, including those with important resistance phenotypes. A summary of the results is shown in Table 3. 2324 was found to behave similarly in two laboratories using the broth method (MIC of 6.3 μg/ml against N. gonorrhoeae ATCC 49226 versus 3.1 μg/ml shown in Table 1). Additionally, 2324 maintained a tight range of MIC values (1.6 to 12.5 μg/ml; 8-fold), indicating a lack of cross-resistance with existing antibiotic drug classes; this range was much closer than it was for comparator antibiotics ciprofloxacin (>8,000-fold), TET (>1,024-fold), and AZM (>256-fold). Most importantly, the MIC90 for 2324 (6.3 μg/ml) was lower than it was for all other antibiotics, with the exception of AZM, identifying this class as having the potential to treat MDR isolates of N. gonorrhoeae.
TABLE 3.
Activities of 2324 and comparator antibacterial agents against 27 N. gonorrhoeae isolates
| Compound/drug | MICa (μg/ml) |
||
|---|---|---|---|
| Range | 50% | 90% | |
| 2324 | 1.6–12.5 | 6.3 | 6.3 |
| SPC | 2–16 | 8 | 16 |
| CIP | 0.002–>16 | 0.06 | 16 |
| TET | ≤0.015–16 | 0.5 | 8 |
| AZM | ≤0.03–8 | 0.12 | 1 |
MIC of 2324 against quality control strain ATCC 49226 was 6.3 μg/ml in this study.
In vitro spontaneous mutation frequencies.
The potential for resistance development was assessed for compound 1950 by determining spontaneous resistance frequency (Table 4). Approximately 109 CFU were spread onto agar plates containing 1950 or SPC at 4× or 8× their respective MIC values. After a 48-h incubation, no colonies were observed on any of the plates, ultimately giving resistance frequencies of <1.2 × 10−9 for both 1950 and SPC.
TABLE 4.
Spontaneous mutation frequencies of SPC and 1950 against N. gonorrhoeae ATCC 49226
| Drug and drug selection (fold MIC) | CFU count |
Spontaneous mutation frequency | |||
|---|---|---|---|---|---|
| Inoculum | Plate 1 | Plate 2 | Avg | ||
| Spectinomycin | |||||
| 4× | 8.7 × 108 | 0 | 0 | 0 | <1.2 × 10−9 |
| 8× | 8.7 × 108 | 0 | 0 | 0 | <1.2 × 10−9 |
| 1950 | |||||
| 4× | 8.7 × 108 | 0 | 0 | 0 | <1.2 × 10−9 |
| 8× | 8.7 × 108 | 0 | 0 | 0 | <1.2 × 10−9 |
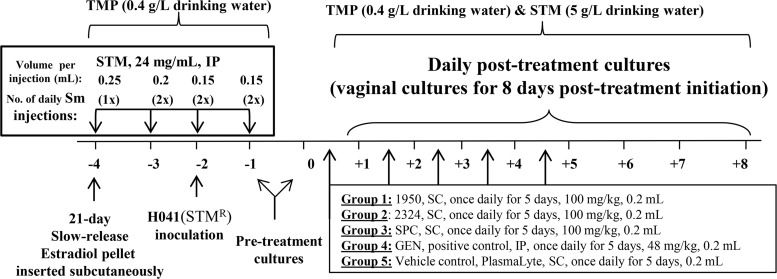
In vivo efficacy testing of 1950 and 2324.
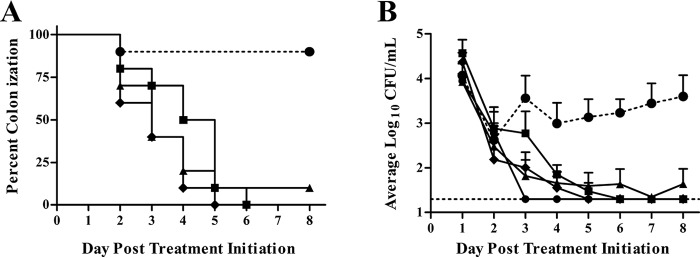
Based on its MDR phenotype and the lack of cross-resistance exhibited by the AmSPCs (Table 1), N. gonorrhoeae strain H041(STMr) was chosen as the challenge strain. AmSPC compounds were tested for anti-N. gonorrhoeae activity in mice infected with N. gonorrhoeae strain H041(STMr), a recent ceftriaxone-resistant (CTXr) clinical isolate. Briefly, to establish long-term infection in female BALB/c mice, the mice were treated with estradiol to modulate the estrous cycle and antibiotics to suppress growth of normal vaginal flora (Fig. 2). On day −2, mice were inoculated vaginally with strain H041(STMr), and on day 0 treatment was initiated for all test groups; treatment was administered by the subcutaneous route once daily for 5 days. A vehicle-only group was included as a negative control, and SPC and GEN were used as positive controls. The percentage of mice in each group that was colonized with N. gonorrhoeae after treatment is shown in Fig. 3A. Ninety percent of mice in the vehicle control group were colonized with H041(STMr) over the course of the infection, and all antibiotic treatment groups became colonized and then showed a significant decrease in the percentage of mice colonized over time compared to the control group (P value of ≤0.0006 by log-rank [Mantel-Cox] test). One hundred percent of mice given 1950, SPC, or GEN cleared infection by the study endpoint. Infection was cleared in 90% of mice that received 2324 after five doses; one mouse remained colonized for the duration of the experiment. The effect of test and control compounds on colonization load was measured by comparing the number of bacteria recovered in vaginal swabs (Fig. 3B). The colonization load of H041(STMr) was statistically comparable between mice that received GEN and all other treatment groups, including the vehicle control. However, the average number of H041(STMr) bacteria recovered was significantly reduced after treatment with 1950 and 2324 compared to that of the vehicle control (P value of ≤0.04 by repeated-measures 2-way analysis of variance [ANOVA] with Bonferroni post hoc analysis) (Fig. 3B). Both 1950 and SPC significantly reduced the number of H041(STMr) bacteria recovered compared to that for 2324 (P value of ≤0.04 by repeated-measures 2-way ANOVA with Bonferroni post hoc analysis). This result is likely due to the single mouse that remained colonized through the end of the experiment.
FIG 2.
Experimental design of the murine efficacy study. All work was performed under an approved protocol of the USUHS Institutional Animal Care and Use Committee. TMP, trimethoprim; STM, streptomycin; GEN, gentamicin; SPC, spectinomycin; SC, subcutaneous.
FIG 3.
Colonization and CFU results from a murine gonorrhea study. Open circles, vehicle only; closed circles, GEN; red squares, 1950; blue triangles, 2324; green diamonds, SPC. (A) Percentage of mice colonized with H041(STMr) over 8 days in each treatment group. (B) Mean log10 CFU/ml of H041(STMr), with standard errors recovered from each experimental group for 8 days following antibiotic treatment. The limit of detection (20 CFU) is denoted with a horizontal dashed line.
DISCUSSION
The AmSPCs exhibit excellent potential for the treatment of drug-resistant gonorrhea and chlamydial coinfections. They display both in vitro and in vivo efficacy against N. gonorrhoeae and in vitro efficacy against C. trachomatis. In addition, they have previously demonstrated promising pharmacokinetic and safety properties as well as efficacy in murine models of fatal Streptococcus pneumoniae infection (27).
In the present study, AmSPCs demonstrate in vitro potency using broth dilution methods. The established CLSI guidelines recommend use of the agar dilution method for N. gonorrhoeae susceptibility testing (31); however, several laboratories have used the broth dilution method with good results (32, 33). The AmSPCs exhibited good antibacterial activity (MICs from 1.6 to 6.3 μg/ml; Table 1) as well as bactericidal activity against N. gonorrhoeae in the broth dilution assay (MBC values from 1× to 2× the MIC value; Table 2). Unlike the AGs, such as GEN, SPC was previously designated a bacteriostatic antibiotic (34) and later specified as having a bactericidal mechanism against certain bacterial species, including N. gonorrhoeae (35, 36). Thus, the AmSPCs behave similarly to AGs, such as GEN and SPC, against N. gonorrhoeae. Rapid cure of gonorrhea is critical to curtail transmission (http://www.cdc.gov/std/tg2015/gonorrhea.htm), therefore a bactericidal antigonorrheal class of compounds, such as the AmSPCs, would be preferred.
In contrast to the inactive parent SPC, the AmSPCs show in vitro potency against C. trachomatis, with MIC values ranging from 4 to 16 μg/ml (Table 1). Additionally, the compounds displayed a bactericidal mechanism against C. trachomatis, with MBC values that were 1× to 2× the MIC values. Because patients, especially those in the 15- to 24-year-old age range, are frequently coinfected with N. gonorrhoeae and C. trachomatis, an antibiotic that could potentially treat both infections rapidly and for which N. gonorrhoeae and C. trachomatis susceptibility values are similar would be a valuable addition to the antibiotic pipeline. To this end, an N. gonorrhoeae-C. trachomatis murine coinfection model is planned (4).
Historically, animal modeling of gonorrhea has been hampered by the exclusive adaptation of N. gonorrhoeae to humans. However, genital tract infections can be established in female mice that are treated with 17β-estradiol and antibiotics to establish mice in the diestrus stage of the estrous cycle, to reduce the overgrowth of potentially inhibitory commensal flora, and to increase their susceptibility to gonococcal infection (37). Many features of experimental murine infections mimic human infection (37); therefore, this model is a useful tool for evaluating the potential of the AmSPCs to treat gonorrhea. The efficacies of 1950 and 2324 were compared to those of known antibiotics SPC and GEN. GEN was selected as a positive control due to the lack of efficacy of many antibiotics against strain H041(STMr). It displayed efficacy when dosed at 48 mg/kg of body weight once daily for 5 days, with complete clearance by day 3 of dosing. SPC, which had not been tested in the gonorrhea model prior to this study, was just as efficacious as GEN when dosed at 100 mg/kg, with average colony counts reduced to below detectable levels by day 3 of dosing. 1950 and 2324 also displayed efficacy when dosed at 100 mg/kg, with average colony counts decreased to below detectable levels by days 4 and 3 of dosing, respectively. To better compare the efficacy of the four test compounds, a dose-response study is planned. It is possible that lower doses as well as shorter dosing regimens of AmSPCs would provide efficacy equivalent to that of the dosing regimen presented here.
In summary, we have demonstrated that the AmSPCs have the potential to become therapeutic agents for the treatment of sexually transmitted bacterial coinfections, especially those caused by N. gonorrhoeae and C. trachomatis. They also have the potential to treat STIs caused by bacteria from the Haemophilus, Mycoplasma, and even Treponema species. These compounds are routinely dosed by the subcutaneous route (27), a route that is ideally suited to the “once and done” treatment of STIs (e.g., CTX is dosed once with an i.m. injection). A perceived advantage in antibiotic drug development is that compounds have minimal activity toward organisms that comprise the normal vaginal flora (38). The AmSPCs have been shown to be selective for organisms that cause pneumonia (i.e., Streptococcus pneumoniae) and STIs with little activity against most Gram-negative species (27). Further in vivo efficacy studies are planned with the AmSPCs, including therapy in an N. gonorrhoeae-C. trachomatis coinfection model, using a single agent.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Descriptions of the N. gonorrhoeae and C. trachomatis strains used in this study are outlined in Table 5. Strain H041(STMr) is strain H041 in which the rpsL gene of strain FA1090 was introduced by allelic exchange to confer streptomycin resistance, which is a required phenotype for the mouse model. All N. gonorrhoeae isolates were cultured either on supplemented [Kellogg's supplement I, 12 μM Fe(NO3)3] GC agar as described previously (42) or on G77L broth as described previously (43). C. trachomatis was grown in cell culture with HEp-2 cells using Iscove's modified Dulbecco's medium with l-glutamine and phenol red supplemented with 10% fetal bovine serum. GC agar with vancomycin, colistin, nystatin, trimethroprim sulfate (VCNTS supplement), and 100 μg/ml streptomycin sulfate was used to isolate N. gonorrhoeae from murine vaginal swabs. Heart infusion agar (HIA) was used to monitor the presence of facultative aerobic commensal flora in vaginal swabs. Incubation conditions for N. gonorrhoeae and commensal bacteria were 37°C in a humid atmosphere containing 7.5% CO2 (44).
TABLE 5.
Strains used in this study
| Strain | Resistance phenotype(s) | Description | Reference or source |
|---|---|---|---|
| N. gonorrhoeae | |||
| ATCC 49226 | Drug sensitive | Type strain | 39 |
| ATCC 700719 | SPC | Quality control organism for Difco spectinomycin differentiation disks | |
| ATCC 700825 | STM | Antibiotic-resistant strain of FA1090 origin that was purchased from ATCC | 40 |
| H041 | β-Lactam, macrolide, FQs, TET, CTX, CFX | Clinical isolate from a pharyngeal infection from Kyoto, Japan, in 2009 | 9 |
| H041(STMr) | β-Lactam, macrolide, FQs, TET, STM, CTX, CFX | H041 rpsLFA1090 | This study (see Materials and Methods) |
| FA1090 | STM | PorB1B strain isolated from a disseminated gonococcal infection from the Uniformed Services University collection | 41 |
| C. trachomatis | |||
| ATCC VR-878 | Drug sensitive | Serovar D |
Aminomethyl spectinomycins.
The AmSPCs were prepared from spectinomycin as described previously (27). The identity and purity of all compounds was confirmed by 1H nuclear magnetic resonance and reverse-phase ultraperformance liquid chromatography analysis with UV/mass spectrometry/evaporative light scattering detection. All compounds were determined to have a purity of >95% prior to testing. Compounds 1950, 2106, and 2324 were first described in reference 27 as compounds 1, 3, and 4, respectively.
Neisseria gonorrhoeae susceptibility testing.
For broth microdilution MIC assays, MICs were determined using the method described by the CLSI (31). For susceptibility testing of MDR N. gonorrhoeae, MICs were determined using the agar dilution assay recommended by the CLSI (31). The minimum bactericidal concentration (MBC), the lowest concentration of an antibacterial agent required to reduce colony counts by at least 3 logs, was determined for ATCC strain 49226 by sampling the wells of broth MIC plates at the 24-h read time. The samples were then serially diluted in antibiotic-free medium and plated on chocolate agar. Bacterial colonies were counted after 24 h of incubation and individual CFU were calculated. A decrease in CFU/well of 3 logs or more compared to the initial CFU/well was considered bactericidal. Additional MIC assays against multiple clinical isolates were performed by Micromyx, LLC, using the broth dilution method outlined by the CLSI (39, 45). A modified medium (GC broth) described by the ATCC (40) was used, and 96-well plates were incubated at 35°C in 5% CO2 for 48 h. Isolates that were tested included a quality control strain (ATCC 49226) plus 26 clinical isolates from the Micromyx strain collection.
Neisseria gonorrhoeae resistance frequency.
N. gonorrhoeae 49226 was tested against SPC or 1950 at 4× and 8× the MIC as determined by agar dilution testing. Aliquots of 40× stock solutions were mixed with GC medium agar–1% IsoVitaleX to produce a molten agar-drug mixture that was either 4- or 8-fold the MIC. Mixture was poured into plates in duplicate and allowed to solidify at room temperature for at least 1 h. A dense cell suspension equivalent to 5 McFarland was prepared using bacterial growth from chocolate agar plates of N. gonorrhoeae ATCC 49226. This cell suspension provided the inoculum for the spontaneous mutation plates, targeting 109 CFU per plate. The viable count of each suspension was determined by plating serial 10-fold dilutions onto agar in duplicate. A 0.25-ml aliquot of inoculum was spread onto the surface of duplicate 150- by 15-mm test plates and allowed to dry. The plates were inverted and incubated at 35°C (with 5% CO2) for 48 h. Plates were inspected for growth at both 24 and 48 h, and colony counts were determined manually. Using the counts at 48 h, the spontaneous mutation frequency was calculated using the following equation: average number of colonies from duplicate selection plates divided by total number of cells inoculated onto each plate. If there were no colonies on the antibiotic selection plates, the spontaneous mutation frequency was calculated as 1/inoculum to indicate that the spontaneous mutation frequency was less than the limit of detection (1 CFU).
Chlamydia trachomatis susceptibility testing.
Susceptibility testing of C. trachomatis was performed in cell culture using HEp-2 cells grown in 96-well microtiter plates (46, 47). C. trachomatis isolates were expanded to concentrations of 107 to 108 inclusion-forming units (IFU) per ml by serial passage in tissue culture using antibiotic-free medium and purified by centrifugation at 500 rpm to remove the cell debris. The chlamydia-containing supernatant was pelleted, resuspended in sucrose-phosphate-glutamic acid buffer, and centrifuged through a discontinuous Renografin gradient. The chlamydial elementary body-containing band was then washed and resuspended, and titers in HEp-2 cells were determined. Each well was inoculated with 0.1 ml of the test strain diluted to yield 103 to 104 IFU/per ml, centrifuged at 1,700 × g for 1 h, and incubated at 35°C for 1 h. Wells were aspirated and overlaid with 0.2 ml of medium containing 1 μg/ml of cycloheximide and serial 2-fold dilutions of the test drug. After incubation at 35°C for 72 h, cultures were fixed and stained for inclusions with fluorescein-conjugated antibody to the lipopolysaccharide genus antigen (Pathfinder; Bio-Rad). The MIC was the lowest antibiotic concentration at which no inclusions were seen. The MBC was determined by aspirating the antibiotic-containing medium, washing wells twice with phosphate-buffered saline, and adding antibiotic-free medium. Cultures were frozen at −70°C, thawed, passed onto new cells, incubated for 72 h, and then fixed and stained as described above. The MBC was the lowest antibiotic concentration that resulted in no inclusions after passage.
In vivo efficacy testing against experimental murine N. gonorrhoeae infection.
Stock solutions (20 mg/ml) of each test antibiotic (compounds 1950, 2324, and SPC; Fig. 1) were prepared on the first day of compound administration (day +2 postinoculation), and four aliquots of each dose were frozen at −20°C until the time of use. The dose of each antibiotic (100 mg/kg) was adjusted to the average weight of the mice in each treatment group. Each compound was prepared using a 50:50 (vol/vol) solution of Plasma-Lyte A (Baxter) and endotoxin-free water (Teknova). The Plasma-Lyte mixture also served as the vehicle control. The positive control (GEN; 48 mg/kg) was prepared as a 50-mg/ml stock of GEN (250 mg; product number G1914; Sigma) in filter-sterilized, endotoxin-free water (Teknova) and diluted to 4.2-mg/ml working stocks in endotoxin-free water, and aliquots were stored at −20°C until administration. All antibiotics were given in a volume of 0.2 ml/dose for each time point. The experimental design of the efficacy study is shown in Fig. 2. Two days prior to infection, female BALB/c mice (NCI Frederick strain of inbred BALB/cAnNCr mice; Charles River Laboratories) were treated with 17β-estradiol (Innovative Research of America) and antibiotics to increase their susceptibility to N. gonorrhoeae as described previously (37, 41). On day 2, mice were inoculated vaginally with 20 μl of strain H041(STMr) suspended in phosphate-buffered saline (PBS); suspensions were adjusted to 7 × 105 CFU/ml (80% infectious dose 80 [ID80]). For H041(STMr), the ID80 was 104 CFU/mouse. Vaginal swabs were quantitatively cultured for N. gonorrhoeae for 2 days following vaginal inoculation (days −1 and 0) to confirm infection prior to treatment. A portion of the swab sample was also inoculated onto HIA to monitor commensal flora. Test and control antibiotics were administered once daily for five consecutive days starting on day 0 following vaginal culture. Test compounds (1950, 2324, and SPC) and the vehicle control were administered subcutaneously (n = 10 mice per group). Gentamicin, the positive control, was administered via intraperitoneal injection (n = 9 mice). Vaginal swabs were quantitatively cultured for N. gonorrhoeae for 8 consecutive days following treatment initiation. Vaginal material was collected by wetting a swab in sterile PBS, gently inserting the swab into the vagina, and suspending the swab in 1 ml of GC broth. Broth suspensions were diluted (1:100), and both diluted and undiluted samples were cultured on GC-VCNTS agar using the Autoplater automated plating system (Spiral Biotech). The number of viable bacteria recovered was determined using Spiral Biotech Q-Counter software. The limit of detection for N. gonorrhoeae was 20 CFU/ml. At the study endpoint (10 days postinoculation), mice were euthanized using compressed CO2 gas in a CO2 gas chamber in the Laboratory Animal Medicine Facility. All animal experiments were conducted at the Uniformed Services University of the Health Sciences, a facility fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, under a protocol that was approved by the university's Institutional Animal Care and Use Committee.
ACKNOWLEDGMENTS
We thank Timothy J. Opperman and Zachary D. Aron for editorial assistance.
This project was supported in part by NIAID grant 1R01AI111449 (R.E.L., principal investigator) and ALSAC (R.E.L., principal investigator).
The mouse model was performed under a Cooperative Research and Development Award between Microbiotix and Uniformed Services University.
REFERENCES
- 1.CDC. 2017. Notifiable diseases and mortality tables. MMWR Morb Mortal Wkly Rep 65:924–941. [PubMed] [Google Scholar]
- 2.Dicker LW, Mosure DJ, Berman SM, Levine WC. 2003. Gonorrhea prevalence and coinfection with chlamydia in women in the United States, 2000. Sex Transm Dis 30:472–476. doi: 10.1097/00007435-200305000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, Cohen MS, Harris KM, Udry JR. 2004. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA 291:2229–2236. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- 4.Vonck RA, Darville T, O'Connell CM, Jerse AE. 2011. Chlamydial infection increases gonococcal colonization in a novel murine coinfection model. Infect Immun 79:1566–1577. doi: 10.1128/IAI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Workowski KA, Bolan GA, Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64:1–137. doi: 10.15585/mmwr.rr6404a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert DN, Moellering RC, Eliopoulos GM, Sande MA. 2007. The Sanford guide to antimicrobial therapy, 37th ed Antimicrobial Therapy, Inc, Sperryville, VA. [Google Scholar]
- 7.Ward H, Ronn M. 2010. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS 5:305–310. doi: 10.1097/COH.0b013e32833a8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelissen CN, Hollander A. 2011. TonB-dependent transporters expressed by Neisseria gonorrhoeae. Front Microbiol 2:117. doi: 10.3389/fmicb.2011.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayor MT, Roett MA, Uduhiri KA. 2012. Diagnosis and management of gonococcal infections. Am Fam Physician 86:931–938. [PubMed] [Google Scholar]
- 11.Soper DE. 2010. Pelvic inflammatory disease. Obstet Gynecol 116:419–428. doi: 10.1097/AOG.0b013e3181e92c54. [DOI] [PubMed] [Google Scholar]
- 12.Whiley DM, Goire N, Lahra MM, Donovan B, Limnios AE, Nissen MD, Sloots TP. 2012. The ticking time bomb: escalating antibiotic resistance in Neisseria gonorrhoeae is a public health disaster in waiting. J Antimicrob Chemother 67:2059–2061. doi: 10.1093/jac/dks188. [DOI] [PubMed] [Google Scholar]
- 13.Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, Eremin SR, Bolan G, Unemo M. 2017. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 14:e1002344. doi: 10.1371/journal.pmed.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unemo M, Shafer WM. 2011. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann N Y Acad Sci 1230:E19–E28. doi: 10.1111/j.1749-6632.2011.06215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein E, Kirkcaldy RD, Reshef D, Berman S, Weinstock H, Sabeti P, Del Rio C, Hall G, Hook EW, Lipsitch M. 2012. Factors related to increasing prevalence of resistance to ciprofloxacin and other antimicrobial drugs in Neisseria gonorrhoeae, United States. Emerg Infect Dis 18:1290–1297. doi: 10.3201/eid1808.111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unemo M, Golparian D, Shafer WM. 2014. Challenges with gonorrhea in the era of multi-drug and extensively drug resistance–are we on the right track? Expert Rev Anti Infect Ther 12:653–656. doi: 10.1586/14787210.2014.906902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Workowski KA, Berman S, Centers for Disease Control and Prevention. 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 59:1–110. [PubMed] [Google Scholar]
- 19.Chen SC, Yin YP, Dai XQ, Unemo M, Chen XS. 2016. First nationwide study regarding ceftriaxone resistance and molecular epidemiology of Neisseria gonorrhoeae in China. J Antimicrob Chemother 71:92–99. doi: 10.1093/jac/dkv321. [DOI] [PubMed] [Google Scholar]
- 20.Camara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, Ardanuy C. 2012. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 67:1858–1860. doi: 10.1093/jac/dks162. [DOI] [PubMed] [Google Scholar]
- 21.CDC. 2013. CDC grand rounds: the growing threat of multidrug-resistant gonorrhea. MMWR Morb Mortal Wkly Rep 62:103–106. [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkcaldy RD, Bolan GA, Wasserheit JN. 2013. Cephalosporin-resistant gonorrhea in North America. JAMA 309:185–187. doi: 10.1001/jama.2012.205107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hook EW III, Golden M, Jamieson BD, Dixon PB, Harbison HS, Lowens S, Fernandes P. 2015. A phase 2 trial of oral solithromycin 1200 mg or 1000 mg as single-dose oral therapy for uncomplicated gonorrhea. Clin Infect Dis 61:1043–1048. doi: 10.1093/cid/civ478. [DOI] [PubMed] [Google Scholar]
- 24.Wiley PF, Argoudelis AD, Hoeksema H. 1963. The chemistry of actinospectacin. IV. The determination of the structure of actinospectacin. J Am Chem Soc 85:2652–2659. [Google Scholar]
- 25.Borovinskaya MA, Shoji S, Holton JM, Fredrick K, Cate JH. 2007. A steric block in translation caused by the antibiotic spectinomycin. ACS Chem Biol 2:545–552. doi: 10.1021/cb700100n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee RE, Hurdle JG, Liu J, Bruhn DF, Matt T, Scherman MS, Vaddady PK, Zheng Z, Qi J, Akbergenov R, Das S, Madhura DB, Rathi C, Trivedi A, Villellas C, Lee RB, Rakesh Waidyarachchi SL, Sun D, McNeil MR, Ainsa JA, Boshoff HI, Gonzalez-Juarrero M, Meibohm B, Bottger EC, Lenaerts AJ. 2014. Spectinamides: a new class of semisynthetic antituberculosis agents that overcome native drug efflux. Nat Med 20:152–158. doi: 10.1038/nm.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruhn DF, Waidyarachchi SL, Madhura DB, Shcherbakov D, Zheng Z, Liu J, Abdelrahman YM, Singh AP, Duscha S, Rathi C, Lee RB, Belland RJ, Meibohm B, Rosch JW, Bottger EC, Lee RE. 2015. Aminomethyl spectinomycins as therapeutics for drug-resistant respiratory tract and sexually transmitted bacterial infections. Sci Transl Med 7:288ra275. doi: 10.1126/scitranslmed.3010572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler MM, Waidyarachchi SL, Connolly KL, Jerse AE, Chai W, Lee RE, Kohlhoff SA, Shinabarger DL, Bowlin TL. 2015. Abstr 55th Intersci Conf Antimicrob Agents Chemother, abstr F-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler MM, Waidyarachchi SL, Connolly KL, Jerse AE, Chai W, Lee RE, Kohlhoff SA, Shinabarger DL, Bowlin TL. 2016. Abstr ASM Microbe 2016, abstr Mo-555. [Google Scholar]
- 30.Kohlhoff SA, Hammerschlag MR. 2015. Treatment of chlamydial infections: 2014 update. Expert Opin Pharmacother 16:205–212. doi: 10.1517/14656566.2015.999041. [DOI] [PubMed] [Google Scholar]
- 31.CLSI. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, M7-A7; approved standard, 7th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.Liao CH, Lai CC, Hsu MS, Chu FY, Wu MY, Huang YT, Hsueh PR. 2010. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates determined by the agar dilution, disk diffusion and Etest methods: comparison of results using GC agar and chocolate agar. Int J Antimicrob Agents 35:457–460. doi: 10.1016/j.ijantimicag.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Takei M, Yamaguchi Y, Fukuda H, Yasuda M, Deguchi T. 2005. Cultivation of Neisseria gonorrhoeae in liquid media and determination of its in vitro susceptibilities to quinolones. J Clin Microbiol 43:4321–4327. doi: 10.1128/JCM.43.9.4321-4327.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scholar EM, Pratt WB. 2000. The antimicrobial drugs, 2nd ed Oxford University Press, New York, NY. [Google Scholar]
- 35.Foerster S, Unemo M, Hathaway LJ, Low N, Althaus CL. 2016. Time-kill curve analysis and pharmacodynamic modelling for in vitro evaluation of antimicrobials against Neisseria gonorrhoeae. BMC Microbiol 16:216. doi: 10.1186/s12866-016-0838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagi BH, Schaadt RD, Zurenko GE. 1992. The bactericidal activity and postantibiotic effect of trospectomycin. Diagn Microbiol Infect Dis 15:417–423. doi: 10.1016/0732-8893(92)90083-6. [DOI] [PubMed] [Google Scholar]
- 37.Jerse AE, Wu H, Packiam M, Vonck RA, Begum AA, Garvin LE. 2011. Estradiol-treated female mice as surrogate hosts for Neisseria gonorrhoeae genital tract infections. Front Microbiol 2:107. doi: 10.3389/fmicb.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu H, Slepenkin A, Elofsson M, Keyser P, de la Maza LM, Peterson EM. 2010. Candidate vaginal microbicides with activity against Chlamydia trachomatis and Neisseria gonorrhoeae. Int J Antimicrob Agents 36:145–150. doi: 10.1016/j.ijantimicag.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CLSI. 2014. Performance standards for antimicrobial susceptibility testing, M100-S24; 24th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 40.ATCC. 2017. STI brochure. ATCC, Manassas, VA: https://www.atcc.org/~/media/PDFs/Micro%20Flyers/STI%20Brochure.ashx. [Google Scholar]
- 41.Jerse AE. 1999. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun 67:5699–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jerse AE, Cohen MS, Drown PM, Whicker LG, Isbey SF, Seifert HS, Cannon JG. 1994. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med 179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macfarlane DE, Elias-Jones TF. 1980. Improved media for the culture of Neisseria gonorrhoeae. J Med Microbiol 13:597–607. doi: 10.1099/00222615-13-4-597. [DOI] [PubMed] [Google Scholar]
- 44.Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun 71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.CLSI. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 46.Kohlhoff SA, Huband MD, Hammerschlag MR. 2014. In vitro activity of AZD0914, a novel DNA gyrase inhibitor, against Chlamydia trachomatis and Chlamydia pneumoniae. Antimicrob Agents Chemother 58:7595–7596. doi: 10.1128/AAC.03920-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roblin PM, Dumornay W, Hammerschlag MR. 1992. Use of HEp-2 cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol 30:1968–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]