ABSTRACT
Whole-genome sequence analyses revealed the presence of blaNDM-1 (n = 31), blaGES-5 (n = 8), blaOXA-232 (n = 1), or blaNDM-5 (n = 1) in extensively drug-resistant and pandrug-resistant Enterobacteriaceae organisms isolated from in-patients in 10 private hospitals (2012 to 2013) in Durban, South Africa. Two novel NDM-1-encoding plasmids from Klebsiella pneumoniae were circularized by PacBio sequencing. In p19-10_01 [IncFIB(K); 223.434 bp], blaNDM-1 was part of a Tn1548-like structure (16.276 bp) delineated by IS26. The multireplicon plasmid p18-43_01 [IncR_1/IncFIB(pB171)/IncFII(Yp); 212.326 bp] shared an 80-kb region with p19-10_01, not including the blaNDM-1-containing region. The two plasmids were used as references for tracing NDM-1-encoding plasmids in the other genome assemblies. The p19-10_01 sequence was detected in K. pneumoniae (n = 7) only, whereas p18-43_01 was tracked to K. pneumoniae (n = 4), Klebsiella michiganensis (n = 1), Serratia marcescens (n = 11), Enterobacter spp. (n = 7), and Citrobacter freundii (n = 1), revealing horizontal spread of this blaNDM-1-bearing plasmid structure. Global phylogeny showed clustering of the K. pneumoniae (18/20) isolates together with closely related carbapenemase-negative ST101 isolates from other geographical origins. The South African isolates were divided into three phylogenetic subbranches, where each group had distinct resistance and replicon profiles, carrying either p19-10_01, p18-10_01, or pCHE-A1 (8,201 bp). The latter plasmid carried blaGES-5 and aacA4 within an integron mobilization unit. Our findings imply independent plasmid acquisition followed by local dissemination. Additionally, we detected blaOXA-232 carried by pPKPN4 in K. pneumoniae (ST14) and blaNDM-5 contained by a pNDM-MGR194-like genetic structure in Escherichia coli (ST167), adding even more complexity to the multilayer molecular mechanisms behind nosocomial spread of carbapenem-resistant Enterobacteriaceae in Durban, South Africa.
KEYWORDS: carbapenemases, Enterobacteriaceae, GES-5, NDM-1, plasmid-mediated resistance
INTRODUCTION
The global dissemination of carbapenemase-producing Enterobacteriaceae (CPE) has reached African countries (1). Clinical isolates of CPE, including Klebsiella pneumoniae, Enterobacter spp., Escherichia coli, Serratia marcescens, and Citrobacter spp., have been described in South Africa, Gabon, Angola, Senegal, Nigeria, Kenya, and Tanzania, as well as in North African countries, including Morocco, Algeria, Tunisia, Libya, and Egypt. Specifically, NDM-1 and OXA-48-like are the most commonly reported carbapenemases in Africa (2–7).
In South Africa, NDM was first detected in Enterobacter cloacae in 2011 and subsequently in K. pneumoniae and S. marcescens (1, 8, 9). The NDM-positive K. pneumoniae strains of African origin have been multiclonal (1). Similar to the global situation, GES-carbapenemases are less prevalent and were first described in Enterobacteriaceae in South Africa in 2013 (1).
The rapid spread of CPE is supported by intra- and interspecies plasmid-mediated transfer of carbapenemase-encoding genes embedded in transposons and integrons (10–12). As part of class 1 integrons, blaGES-5 seems to be widespread worldwide and detected on different plasmid backbones (12–14). As reviewed previously (15), plasmids of several incompatibility groups (Inc) can mediate spread of carbapenem resistance in clinically relevant Enterobacteriaceae. While epidemic plasmids encoding KPC and OXA-48-like predominantly belong to IncF or IncL, a diversity of plasmid backbones, including IncA/C, IncF, IncL/M, IncN, IncR, and IncX, are associated with NDM (12, 16–19).
Here, we have explored the molecular epidemiology of multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) Enterobacteriaceae isolated from the private hospital sector in Durban, South Africa, by whole-genome sequence (WGS) analyses focusing on the carbapenem resistance-encoding determinants and their genetic support.
RESULTS
Phenotypic and genotypic analyses of the Enterobacteriaceae collection.
Table S1 in the supplemental material summarizes relevant patient data, source of specimen, and relevant phenotypic and genotypic characteristics for the 45 collected carbapenem-resistant Enterobacteriaceae (CRE), which included K. pneumoniae (n = 21), K. michiganensis (n = 1), S. marcescens (n = 12), Enterobacter spp. (n = 9), and C. freundii (n = 1). Antimicrobial susceptibility testing (Table S2) categorized them as multidrug resistant (MDR), extensively drug resistant (XDR), or pandrug resistant (PDR) according to standard definitions (20). As shown, PDR isolates were found among the S. marcescens strains (n = 6) only, while the XDRs included K. pneumoniae (n = 5), S. marcescens (n = 4), and Enterobacter spp. (n = 4). The PDR S. marcescens organisms belong to a distinct phylogenetic branch that is comprised of all except two (no. 33 and 34) of the included isolates, as evident in the NCBI Genome Tree report consisting of 341 genome assemblies (https://www.ncbi.nlm.nih.gov/genome/tree/1112?; accessed 29 September 2017). This branch included both XDR and PDR isolates from the same intensive care unit (ICU) as well as a blaNDM-1-negative MDR isolate (no. 35) from a different ward, which implicate blaNDM-1 acquisition as well as nosocomial spread and development of a PDR genetic lineage.
The WGS analyses (Data Set S1) revealed the presence of carbapenemase-encoding genes in 41 of the 45 isolates: blaNDM-1 (n = 31), blaGES-5 (n = 8), blaNDM-5 (n = 1), and blaOXA-232 (n = 1). All except three of these were positive in the Carba NP test (Table S1). Four of the CRE contained no known carbapenemase-encoding gene, although one was positive in the Carba NP test.
Phylogenetic analyses of carbapenemase-encoding K. pneumoniae.
Among the 41 carbapenemase-encoding isolates (Table 1), K. pneumoniae was the most prevalent species (n = 20), dominated by ST101 (n = 14). The available demographic data showed that they were obtained from six hospitals and 14 wards, from different clinical sources, and from both sexes between the ages of 2 months and 82 years (Table S1).
TABLE 1.
Genetic and phenotypic characteristics of the carbapenemase-encoding Enterobacteriaceae (n = 41)
| Bacterial isolate |
MLST | Groupa | Carbapenemase-encoding genetic structure |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Strain ID | Taxonomy | Gene | Plasmid structureb | Replicon type | Size (bp) | GenBank accession no. | ||
| 1 | 947385799 | E. coli | ST167 | blaNDM-5 | pNDM-MGR194-like | IncX3 | 46,253 | NC_022740.1 | |
| 2 | 944535499 | K. pneumoniae | ST14 | blaOXA-232 | PittNDM01 plasmid4 | ColPK3 | 6,141 | NZ_CP006802.1 | |
| 3 | 939996824 | K. pneumoniae | ST101 | I | blaGES-5 | pCHE-A1 | IncQ | 8,201 | KX244760.1 |
| 4 | 945154233 | K. pneumoniae | ST101 | I | blaGES-5 | pCHE-A1 | |||
| 5 | 945165838 | K. pneumoniae | ST101 | I | blaGES-5 | pCHE-A1 | |||
| 6 | 945169659 | K. pneumoniae | ST101 | I | blaGES-5 | pCHE-A1 | |||
| 7 | 957083320 | K. pneumoniae | ST101 | I | blaGES-5 | pCHE-A1-like | |||
| 8 | U44822 | K. pneumoniae | ST101 | I | blaGES-5 | pCHE-A1 | |||
| 9 | 957083896 | K. pneumoniae | ST101 | I | blaGES-5 | pCHE-A1-like | |||
| 10 | 957089165 | K. pneumoniae | ST101 | I | blaGES-5 | pCHE-A1 | |||
| 11 | 960186733 | K. pneumoniae | ST101 | II | blaNDM-1 | p19-10_01 | IncFIB(K) | 223,434 | CP023488.1 |
| 12 | 950171785 | K. pneumoniae | ST101 | II | blaNDM-1 | p19-10_01-like | |||
| 13 | 950173000 | K. pneumoniae | ST101 | II | blaNDM-1 | p19-10_01-like | |||
| 14 | 951373950 | K. pneumoniae | ST101 | II | blaNDM-1 | p19-10_01-like | |||
| 15 | 951362657 | K. pneumoniae | ST2016 | II | blaNDM-1 | p19-10_01-like | |||
| 16 | 951384356 | K. pneumoniae | ST101 | II | blaNDM-1 | p19-10_01-like | |||
| 17 | 951363981 | K. pneumoniae | ST101 | II | blaNDM-1 | p19-10_01-like | |||
| 18 | 950142398 | K. pneumoniae | ST2017 | III | blaNDM-1 | p18-43_01 | IncR_1, IncFIB(pB171), IncFII(Yp) | 212,326 | CP023554.1 |
| 19 | 941530379 | K. pneumoniae | ST323 | blaNDM-1 | p18-43_01-like | ||||
| 20 | 950117510 | K. pneumoniae | ST2017 | III | blaNDM-1 | p18-43_01-like | |||
| 21 | 950118422 | K. pneumoniae | ST2017 | III | blaNDM-1 | p18-43_01-like | |||
| 23 | 939742031 | K. michiganensis | ST170 | blaNDM-1 | p18-43_01-like | ||||
| 24 | 950005607 | S. marcescens | NAc | 1 | blaNDM-1 | p18-43_01-like | |||
| 25 | 950196656 | S. marcescens | NA | 1 | blaNDM-1 | p18-43_01-like | |||
| 26 | 950163360 | S. marcescens | NA | 1 | blaNDM-1 | p18-43_01-like | |||
| 27 | 950164094 | S. marcescens | NA | 1 | blaNDM-1 | p18-43_01-like | |||
| 28 | 950165859 | S. marcescens | NA | 1 | blaNDM-1 | p18-43_01-like | |||
| 29 | 950166381 | S. marcescens | NA | 1 | blaNDM-1 | p18-43_01-like | |||
| 30 | 950174583 | S. marcescens | NA | 1 | blaNDM-1 | p18-43_01-like | |||
| 31 | 950172946 | S. marcescens | NA | 1 | blaNDM-1 | p18-43_01-like | |||
| 32 | 9501453777 | S. marcescens | NA | 1 | blaNDM-1 | p18-43_01-like | |||
| 33 | 945154301 | S. marcescens | NA | 2 | blaNDM-1 | p18-43_01-like | |||
| 34 | 945174350 | S. marcescens | NA | 2 | blaNDM-1 | p18-43_01-like | |||
| 36 | 19870317 | E. asburiae | ST108 | blaNDM-1 | p18-43_01-like | ||||
| 37 | 939705067 | E. asburiae | ST435 | blaNDM-1 | p18-43_01-like | ||||
| 40 | 950180354 | E. cloacae complex | ST145 | blaNDM-1 | p18-43_01-like | ||||
| 41 | 953102574 | E. cloacae complex | ST433 | blaNDM-1 | p18-43_01-like | ||||
| 42 | 941713674 | E. kobei | ST54 | blaNDM-1 | p18-43_01-like | ||||
| 43 | 953099839 | E. kobei | ST434 | blaNDM-1 | p18-43_01-like | ||||
| 44 | 950178628 | Enterobacter spp. | ST121 | blaNDM-1 | p18-43_01-like | ||||
| 45 | 944526466 | C. freundii | ST63 | blaNDM-1 | p18-43_01-like | ||||
Phylogenetic subgroup as determined for K. pneumoniae and S. marcescens isolates.
Referred to as “-like” when plasmid sequence is not circularized but the carbapenemase-encoding contig revealed 100% nucleotide identity to the given plasmid.
NA, MLST is not available for this species.
To investigate the global phylogeny and likely origins of these isolates, we added genome assembly data sets (n = 1148) downloaded from the PATRIC database (https://www.patricbrc.org/) to our analyses as well as country of origin and multilocus sequence type (MLST). In the phylogenetic tree shown in Fig. S1, all of the South African isolates with the exception of ST323 (blaNDM-1) and ST14 (blaOXA-232) clustered together in a distinct branch. These findings are in accordance with the NCBI Genome Tree report for K. pneumoniae (n = 2,814) based on genomic BLAST (https://www.ncbi.nlm.nih.gov/genome/tree/815? shows the phylogenetic branch containing the main cluster of the South African isolates, including ST101 as well as the novel ST2016 and ST2017; accessed 29 September 2017). As revealed by the metadata, the other ST101 isolates constituting the branch come from several different countries. It is worth noting that these do not contain carbapenemase-encoding genes. The exception is the Norwegian isolate (GCA_00143615; blaNDM-1), which has a different content of plasmid replicons and resistance-encoding genes than the South African isolates.
The South African isolates divide into three distinct subbranches, which correlate with the presence of blaGES-5 (group I) or blaNDM-1 (groups II and III), as marked in Fig. 1. In addition, the plasmid replicon and resistance gene profiles were specific for each of these groups (Data Set S1).
FIG 1.
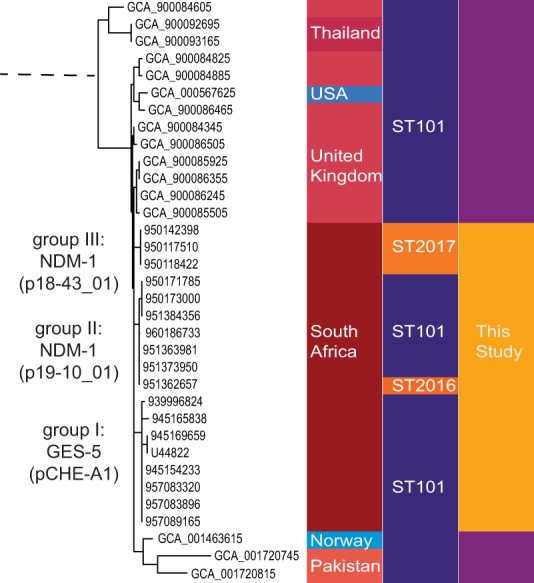
Phylogenetic branch of K. pneumoniae containing the South African isolates (n = 18). Global phylogeny from genome assembly data sets (n = 1,148) downloaded from the PATRIC database (https://www.patricbrc.org/) was revealed by rapid core genome multialignment (https://github.com/marbl/parsnp) and metadata (isolation country and MLST) coupled with the use of Phandango (https://github.com/jameshadfield/phandango/wiki). The selected branch shows the isolate assembly identifier (ID) for the downloaded samples (lilac) and isolate ID for the samples from this study (yellow), for which subgroup (I, II, or III) and carbapenemase-encoding determinants are indicated. Color codes show isolation country and MLST for each isolate.
The blaNDM-1-containing genetic structures.
To investigate the genetic backbone of the carbapenemase-encoding determinants, we performed alignments and BLAST analyses. For the assembled blaNDM-1-containing contigs, we detected two distinct genetic structures, corresponding to phylogenetic groups II and III. The K. pneumoniae group II isolates revealed extensive homology to the blaNDM-1-encoding regions of the completely sequenced E. coli plasmid pNDM-HK (NC_019063.1) as well as K. pneumoniae plasmids pNDM-OM (NC_019889.1) and pNDM-1-Saitama (NC_021180.1). All seven blaNDM-1-containing contigs started at precisely the same nucleotide, and five had the exact same length of 16.276 bp (Data Set S1), explained by contig break caused by the surrounding insertion sequence (IS) elements described below.
For the three K. pneumoniae group III isolates, the blaNDM-1-containing contigs showed a different DNA sequence and gene synteny. It is noteworthy that alignment and BLAST analyses grouped them together with 21 other isolates in our CRE collection, including the K. pneumoniae isolate of ST323, all of the S. marcescens and Enterobacter species isolates, and the single isolates of K. michiganensis and C. freundii (listed in Table 1). The aligned blaNDM-1-containing contigs showed extensive homology to the conjugative 110-kb pRJF866 K. pneumoniae plasmid (NC_025184.1) reported in China (21). Except for base pair substitutions at pRJF866 positions 69130 (G to A) and 73304 (C to G), 100% nucleotide identity was found. Although the contig length varied from 2,268 bp to 27,908 bp (Data Set S1), the high DNA identity strongly suggests a common origin of their blaNDM-1-encoding determinants.
PacBio circularizing of two novel NDM-1-encoding plasmids.
The genetic support of blaNDM-1was further investigated by PacBio sequencing of two K. pneumoniae isolates, from group II (isolate 960186733) and III (isolate 950142398). Circularizing of the DNA sequences revealed two novel NDM-1-encoding plasmids: p19-10_01 (223.434 bp) and p18-43_01 (212.434 bp). Both plasmids encode a heavy-metal efflux system, multiple resistance determinants, and transposable element, as well as type IV secretion systems (T4SS), as depicted in Fig. 2 and 3. While p19-10_01 belongs to the IncFIB(K) replicon type, p18-43_01 is a multireplicon plasmid that includes IncR_1, IncFIB(pB171), and IncFII(Yp). In p19-10_01, a Tn1548-like element delineated by IS26, contained blaNDM-1 as well as armA and other resistance-encoding genes, while the blaNDM-1-containing region in p18-43_01 had a completely different gene synteny.
FIG 2.
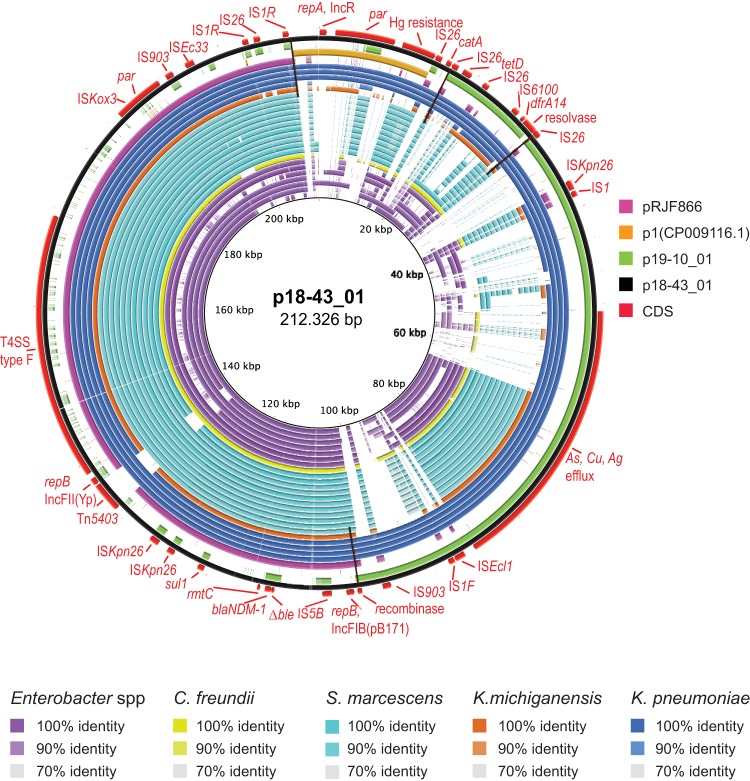
Tracking of plasmid p18-43_01 in NDM-1-encoding CPE isolates (n = 24). The map was constructed using BRIG software. The concentric circles represent comparisons between p18-43_01 and, starting with the inner circle, genome assemblies from Enterobacter species (strain ID 950178628, 953099839, 941713674, 950180354, 953102574, 939705067, and 19870317), C. freundii (944526466), S. marcescens (945174350, 945154301, 950145377, 950172946, 950174583, 950166381, 950165859, 950164094, 950163360, 950196656, and 950005607), K. michiganensis (939742031), and K. pneumoniae (941530379, 950118422, 950117510, and 950142398). Color codes are given for each species and for DNA identity, ranging from 70 to 100%, as indicated. Plasmids with extensive homology to p18-43_01, including pRJF866 (NC_025184.1), plasmid1 (CP009116.1), pKPC_CAV1217 (CP018675.1), and p19-10_01 (this study), were included in the BLAST comparisons and are represented as circles according to the given color codes. The outer black and red circles represent the p18-43_01 reference sequence and its annotated coding DNA sequence (CDS), respectively. Black transverse lines mark the ends of homology between p18-43_01 and p19-10_01 or pRJF866.
FIG 3.
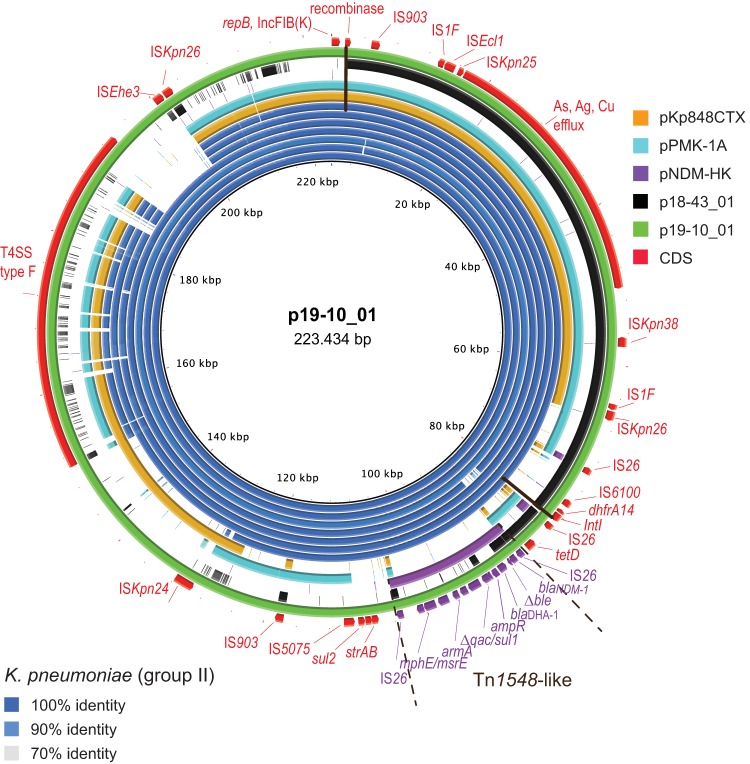
Tracking of plasmid p19-10_01 in subgroup II K. pneumoniae. The map was constructed using BRIG software. The concentric blue circles represent BLAST comparisons between p19-10_01 and genome assemblies from K. pneumoniae 960186733, 951362657, 951363981, 951373950, 950173000, 950171785, and 951384356, starting with the innermost circle. Color codes are for DNA identity, ranging from 70 to 100%, as indicated. Plasmids with regions homologous to the reference, including pKp848CTX (NC_024992.1), pPMK-1A (NZ_CP008930.1), pNDM-HK (NC_019063.1), and p18-43_01 (this study), were included in the BLAST comparisons and are represented as circles according to the given color codes. The outer black and red circles represent the p18-43_01 reference sequence and its annotated CDS, respectively. The Tn1548 region is marked, with the CDS shown in purple. The black transverse lines mark the ends of homology between p19-10_01 and p18-43_01.
BLAST comparisons (Fig. S2) show that the two plasmids share regions of high sequence identity, although they are inverted and rearranged. Tree regions (99% identity) were detected, with the largest (∼72 kb; positions 1728 to 73963 in p19-10_01) delineated by a putative recombinase (green box) and IS26. The alignment also reveal a partly overlapping 8-kb region (positions 73111 to 81049 in p19-10_01), delineated by the IS26 element and a putative integrase (green box), and an ∼5-kb region spanning from positions 82193 to 87068, which is enclosed by IS26.
Horizontal spread and evolution of blaNDM-1-encoding plasmids.
By using the BLAST Ring Image Generator (BRIG) (22), the two circularized plasmids were references for tracking of similar plasmids in the blaNDM-1-positive isolates. BLAST comparisons using p18-43_01 as a reference (Fig. 2) revealed regions with 70 to 100% DNA identity (color codes as indicated for each species) in 24 of the isolates. The concentric circles represent (i) group III K. pneumoniae and the phylogenetically distant ST323; (ii) K. michiganensis (ST170); (iii) different Enterobacter species, comprising ST252, ST54, ST121, and ST145 and the novel sequence types ST433, ST434, and ST435; (iv) S. marcescens isolates from two phylogenetic distant branches; and (v) C. freundii (novel ST63), in the order given in the figure legend.
Included in the comparison are plasmids with homology to the reference, including p19-10_01 (circle colors as indicated). The regions shared between p18-43_01 and p19-10_01 or pRJ866 are delineated by black lines. A large part corresponding to the pRJF866 sequence was common for all 24 isolates. Of interest, Tn5403 had inserted onto the pRJF866 part in the reference plasmid but was absent from some of the isolates, including K. michiganensis and the K. pneumoniae ST323 (isolate 941530379), one E. kobei isolate (isolate 9953099839), and two S. marcescens isolates (isolates 94517435 and 945154301, which belong to a phylogenetically distant branch).
K. pneumoniae isolates from group III were positive for the three rep genes carried by p18-43_01. The IncR_1 replicon type was also detected in one Enterobacter isolate, while IncFII(Yp) and IncFIB(pB171) were present in the whole group of 24 isolates. The resistance-encoding genes rmtC and sul1, closely linked to blaNDM-1 in p18-43_01, were present in all isolates, while catA, tetD, and dhfrA14 were found in the Klebsiella isolates but only sporadically in the others. Taken together, these findings indicate that blaNDM-1 is carried by plasmids with a common origin and partly common backbone structure, including T4SS, which has enabled local horizontal transfer between Enterobacteriaceae organisms. Further insight into the evolution of the p18-43_01-like plasmids in Enterobacteriaceae would require circularizing of their DNA sequences.
Transfer of blaNDM-1-containing transposon.
The BRIG analyses (Fig. 3) showed that major parts of p19-10_01, including the blaNDM-1-containing region, were present in all K. pneumoniae isolates from group II. The observed differences indicate local evolution of the blaNDM-1-containing plasmids after acquisition. Genome assemblies from isolates 951373950, 951362657, and 951363981 reveal the most similarity to p19-10_10 (960186733), which correlates with their phylogenetic relatedness (Fig. 1). Circularization of additional plasmids would be required for further investigation of plasmid changes. Notably, the only part of pNDM-HK present in these isolates was the Tn1548-like structure delineated by IS26, which has 100% sequence identity. Using pNDM-HK, pNDM-OM, or pNDM-1-Saitama as the reference plasmid in the BRIG analyses resulted in the same observation (data not shown): the group II K. pneumoniae isolates contained no other parts of these plasmids except from the Tn1548-like structure, which strongly suggests movement of this putative blaNDM-1-containing transposon between different replicons followed by horizontal transfer.
blaGES-5 is colocated with aacA4 on a pCHE-A-like plasmid.
For the K. pneumoniae group I isolates, BLAST analyses of the blaGES-5-containing contigs revealed extensive genetic homology to the E. cloacae pCHE-A (NC_012006.1) plasmid (11). For six of the isolates, we identified a complete, circular pCHE-A-like plasmid (8,201 bp), named pCHE-A1 (KX244760). Except for three base pair changes and a 641-bp insertion downstream of blaGES-5, it had 100% identity to pCHE-A. The inserted DNA showed 100% identity to a class I integron bearing an aminoglycoside 6′-N-acetyltransferase (aacA4) found in K. pneumoniae (JN108899.1) and in other Enterobacteriaceae. Interestingly, aacA4 was inserted into the described integron mobilization unit (IMU) of pCHE-A (11) within the proposed consensus sequence of the conserved core for site-specific recombination of gene cassettes into integrons (23). The inserted DNA interrupted the GTTAG-ATGC sequence of pCHE-A, resulting in GTTAG-GC (5′ end), which is identical to the consensus sequence. The insertion site was conserved in the 3′ end of the inserted DNA.
BRIG analyses (Fig. S3) confirmed the presence of DNA with 100% coverage and identity to the pCHE-A1 reference (strain 957089165) in all K. pneumoniae group I isolates.
Other plasmid-borne carbapenemase-encoding genes.
We detected blaNDM-5 in an E. coli ST167 strain. The blaNDM-5-positive contig (∼9 kb) revealed 100% nucleotide identity to the blaNDM-5-containing region of the 46.3-kb K. pneumoniae plasmid pNDM-MGR194 (KF220657.1). The plasmid was not circularized, but BLAST analyses using pNDM-MGR194 as a reference showed the presence of the complete plasmid DNA in the E. coli strain (data not shown).
The single K. pneumoniae ST14 isolate not belonging to the main phylogenetic cluster encoded OXA-232. The blaOXA-232-containing contig of 6,348 bp shared 100% sequence identity with the K. pneumoniae PittNDM01 plasmid4 (CP006802.1), named pPKPN4 (24), and circularization of the 6,141-bp plasmids was enabled. BRIG comparisons between pPKPN4 and the assembled sequences from this isolate confirmed the finding (data not shown).
DISCUSSION
The molecular characterization of clinical CRE from the private hospital sector in Durban, South Africa, revealed complex patterns for the dissemination of carbapenem resistance. In this collection of MDR, XDR, and PDR Enterobacteriaceae, we identified four different carbapenemase-encoding genes contained by five different plasmid-associated genetic supports. The overall WGS data indicate plasmid acquisition into an established local K. pneumoniae clone of ST101 as well as horizontal transfer between different genera of Enterobacteriaceae, accompanied by clonal dissemination. Our results are in line with those observed in the Jiaxingin Zhejiang Province in China, where cross-species transfer and clonal spread were suggested to contribute synergistically to the rapid increase in prevalence of CRE in hospital settings (25).
Reports from several European and Mediterranean countries suggest a continental spread of ST101 associated with OXA-48 (26–28). The ST101 strains in this study were isolated within the same hospital environment but encoded NDM-1 or GES-5 on three different plasmids, further demonstrating the capability for adaptation and spread of this genetic lineage.
Resistance-encoding plasmids can be extremely dynamic due to nested genetic elements that enable short-term evolution as well as rapid dissemination of resistance genes between multiple species, strains, and plasmids (29). In our study, the circularization of two NDM-1-encoding plasmids revealed two different plasmids structures, including replicon types and blaNDM-1-containing regions. Nevertheless, we identified large regions with high sequence identity, although the regions were rearranged. Their presence in the same hospital niche and within closely related K. pneumoniae isolates strongly point to a common source of these plasmids, which then have evolved in their host by recombination events.
The NDM-1-encoding part of p18-43_01, corresponding to pRJF866 (21) and including the IncFIB and IncFII(Yp) replicons, was detected in four different genera of Enterobacteriaceae, which implicates a common ancestor. In p18-43_01, we observed a Tn5403 insertion in the pRJF866-homologous part. This genetic marker was absent from five of the isolates, including the K. pneumoniae ST232 isolate, the K. michiganensis isolate, one E. kobei isolate, and two S. marcescens isolates, which belong to a different branch of the phylogenetic tree than the others. This implies that independent transfer events of two different plasmid variants had occurred for all three genera. However, further analyses of the phylogeny and host adaptation for this versatile NDM-1-encoding plasmid structure would require circularizing of plasmids from the different isolates.
The p19-10_01 plasmid carried blaNDM-1 on a putative mobile element with 100% nucleotide identity to a segment of the completely sequenced pNDM-HK, pNDM-OM, and pNDM-1-Saitama plasmids (30–32). The region contains multiple resistance determinants, including armA flanked by IS26, and structurally resembles a Tn1548-type composite element (33, 34). In our study, we detected no other parts of these plasmids in the Tn1548-like containing K. pneumoniae strains, which strongly suggests a mobilization of this element from one plasmid to the other. IS26 has been shown to mediate the formation of transposons that carry antibiotic resistance genes (35, 36) and to significantly reorganize plasmids by replicative transposition or by homologous recombination between preexisting IS26 structures (37, 38). Here, transfer by IS26 activity would explain the finding of Tn1548-like in a new genetic context. IS26 could also offer an explanation for the differences in plasmid structure observed between the isolates due to homologous recombination and merging of plasmids.
The pCHE-A plasmid is a mobilizable IncQ-type plasmid first described in an E. cloacae strain isolated in Canada (11). The blaGES-5 gene is part of a novel IMU, which could be mobilized by providing transposase activity in trans (11). Here, we detected a homologous plasmid with an additional resistance-encoding gene, aacA4. The insertion of aacA4 into the conserved core sequence for site-specific recombination (23) supports an integron activity, although the Intl gene is partially deleted in pCHE-A. These findings substantiate the potential for accumulation and spread of resistance genes by pCHE-A, as suggested previously (11).
K. pneumoniae ST14 has been associated with CTX-M-15, FOX-7, and NDM-1 outbreaks in Tanzania, Italy, and other parts of the world (1, 39–41). An OXA-232-producing K. pneumoniae ST14 outbreak clone was recently detected in South Korea (42) and traced to India, where it is reported to be dominant (43). The 6.1-kb OXA-232-encoding pPKNPN4 detected in our study was initially described in the K. pneumoniae isolate PittNDM01 (24) and also corresponds to an OXA-232-encoding plasmid reported in K. pneumoniae and E. coli (42, 44), which accentuates its dissemination.
NDM-5 was first identified in an E. coli strain from the United Kingdom in 2011 (45). In recent years, there has been widespread occurrence of NDM-5 in K. pneumoniae (45–47). Here, blaNDM-5 detected in E. coli was harbored by pNDM_MGR194, which was found to circulate in K. pneumoniae in India (48, 49). The finding of this broad-host-range IncX3 plasmid in Proteus mirabilis as well (50) further extends its role in enhancing the spread of blaNDM-5.
The presence of blaNDM-1 in different species and STs of Klebsiella, as well as in other genera of the Enterobacteriaceae family, emphasizes the broad-host-range dissemination of mobile NDM-encoding elements. In addition to K. pneumoniae, E. cloacae is known as a major host for NDM-1 both in South Africa and in other African countries (1, 2). Outbreaks of NDM-1-producing E. cloacae have also been reported (51), and epidemiological analyses have revealed specific NDM-1-associated STs (52, 53). In our study, however, the NDM-1-producing Enterobacter spp. encountered several species. S. marcescens isolates are mostly associated with neonatal outbreaks in ICUs worldwide (54, 55). Dissemination of NDM-1-producing S. marcescens in ICUs has not, to our knowledge, been reported before. The development and nosocomial spread of a PDR genetic lineage of S. marcescens is of great concern.
In conclusion, acquisition of different resistance-encoding plasmids, horizontal transfer, and clonal dissemination facilitate the spread of carbapenemase genes in Durban, South Africa. The overall observations emphasize the importance of early detection of CRE and targeted infection control measures to prevent dissemination.
MATERIALS AND METHODS
Ethical considerations.
Ethical approval was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (reference number BE040/14).
Bacterial strains.
Forty-five clinical CRE (nonsusceptible to ertapenem and/or meropenem) collected by Lancet Laboratories, Durban, South Africa, between 2012 and 2013 from patients hospitalized in 10 different private hospitals (represented by the letters A to J) and wards (represented by digits after the letters) in Durban, South Africa, were included in the study (Table S1 in the supplemental material). Duplicate isolates from the same patients were excluded. Species identification and antimicrobial susceptibility testing were undertaken using matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker Daltonic Gmbh, Bremen, Germany) and broth microdilution using in-house-designed, premade Sensititre microtiter plates (Thermo Fisher Scientific, East Grinstead, UK), respectively. Interpretation was according to EUCAST breakpoints, version 7.1 (www.eucast.org). Nonsusceptibility included both the intermediate and resistant categories. Carbapenemase production was examined by the Carba NP test as previously described (56, 57).
DNA analysis.
Genomic DNA for Illumina sequencing was purified using a GenElute bacterial genomic DNA kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer's instructions. Paired-end libraries were generated using the Nextera kit (Illumina, San Diego, CA, USA), followed by sequencing on an Illumina MiSeq platform at the Norwegian Sequencing Centre or at the Centre for Bioinformatics at UiT–The Arctic University of Norway.
For PacBio sequencing, genomic DNA was purified by a Genomic-tip 100/G kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The DNA was subjected to the 20-kb library preparation protocol and 6-kb cutoff BluePippin (Sage Sciences, Beverly, MA, USA) size selection, followed by sequencing with the Pacific Biosciences RSII sequencer using P6-C4 chemistry, a 360-min movie time, and one SMRT-cell per sample at the Norwegian Sequencing Centre.
Bioinformatic analysis.
Illumina sequence reads were adaptor- and quality-trimmed using Trimmomatic (58) and subsequently assembled with Spades v.3.6.0 (59) using the “–careful” flag. PacBio long-read sequences were assembled and polished at The Norwegian Sequencing Centre (http://www.sequencing.uio.no/) using HGAP, v3, in SMRT analysis software, v2.3.0 (Pacific Biosciences) (60). Minimus2 from AMOS (61) circularized unitigs, and the dnaA (chromosome) or repA (plasmids) gene was set as the first nucleotide position using the Circulator (62).
For in-house analysis purposes, assemblies were annotated using prokka v.1.11 (63) with further NCBI BLAST searches and annotation of resistance and plasmid replicon genes by the Resfinder, NCBI β-lactamase, and PlasmidFinder databases found in ABRicate (https://github.com/tseemann/abricate). Annotation of NDM-1-containing plasmids additionally included ISfinder (64) searches for IS elements and identifying T4SS using T346Hunter (65). Assemblies deposited in GenBank were annotated using the PGAP pipeline provided by NCBI (https://www.ncbi.nlm.nih.gov/genome/annotation_prok/), with additional manual curation of resistance gene and mobile genetic element annotations.
To visualize presence/absence of specific plasmid DNA, fully sequenced plasmids were used as reference input to BRIG (22) together with the Illumina sequence reads.
To investigate the global phylogeny and identify the likely origins of the K. pneumoniae isolates, genome assembly data sets were downloaded from the PATRIC database (https://www.patricbrc.org/), identifying all isolates with “country isolated” metadata. Genomes with fewer than 400 contigs were selected and run through MinHASH (66) using standard settings, and genomes above a MASH distance threshold of 0.05 excluded to remove isolates genetically distant from the main phylogroup. The data sets were then run through parsnp v.1.2 (61) with “–c –x” flags enabled and random reference selection among the included samples. FigTree (http://tree.bio.ed.ac.uk/software/figtree/) was used to edit the phylogenetic trees. Phylogeny, country origin metadata, and MLST type were determined by MLST software (https://github.com/tseemann/mlst) coupled with Phandango (67).
The housekeeping genes of new or unknown STs were sent for curation and assignment of new ST numbers at the K. pneumoniae MLST database at the Pasteur Institute, the Enterobacter cloacae MLST website (https://pubmlst.org/ecloacae/), and the Citrobacter freundii MLST website (https://pubmlst.org/cfreundii/).
Accession number(s).
The raw read sequences and the assembled whole-genome contigs have been deposited in GenBank under Bioproject PRJNA287968. The plasmids pCHE-A1, p19-10_01, and p18-43_01 have accession numbers KX244760.1, CP023488.1, and CP023554.1, respectively.
Supplementary Material
ACKNOWLEDGMENTS
We are especially grateful to Bettina Aasnæs for significant technical assistance with the whole-genome sequencing, to Bjørg Christina Haldorsen for help in the antibiotic susceptibility testing, and to Jessin Janice for help with phylogenetic analyses. All three work at the Norwegian National Advisory Unit on Detection of Antimicrobial Resistance, Department of Microbiology and Infection Control, University Hospital of North Norway, Tromsø, Norway. We acknowledge the contribution of the Norwegian Sequencing Centre, University of Oslo, and Center for Bioinformatics at UiT–The Arctic University of Norway for generating the sequence data.
This work was funded by the College of Health Sciences, University of KwaZulu-Natal, South Africa, by The South African National Research Foundation Incentive Funding for Rated Researchers (grant no. 85595) awarded to S.Y.E., and by the Norwegian National Advisory Unit on Detection of Antimicrobial Resistance, Department of Microbiology and Infection Control, University Hospital of North Norway, Tromsø, Norway.
S.Y.E. is a member of the Global Respiratory Infection Partnership sponsored by Reckitt & Benckiser (Pty.) Ltd., United Kingdom.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02178-17.
REFERENCES
- 1.Sekyere JO, Govinden U, Essack S. 2016. The molecular epidemiology and genetic environment of carbapenemases detected in Africa. Microb Drug Resist 22:59–68. doi: 10.1089/mdr.2015.0053. [DOI] [PubMed] [Google Scholar]
- 2.Nordmann P, Poirel L. 2014. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect 20:821–830. doi: 10.1111/1469-0691.12719. [DOI] [PubMed] [Google Scholar]
- 3.Kieffer N, Nordmann P, Aires-De-Sousa M, Poirel L. 2016. High prevalence of carbapenemase-producing Enterobacteriaceae among hospitalized children in Luanda, Angola. Antimicrob Agents Chemother 60:6189–6192. doi: 10.1128/AAC.01201-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sangare SA, Rondinaud E, Maataoui N, Maiga AI, Guindo I, Maiga A, Camara N, Dicko OA, Dao S, Diallo S, Bougoudogo F, Andremont A, Izetiegouma Maiga I, Armand-Lefevre L. 2017. Very high prevalence of extended-spectrum β-lactamase-producing Enterobacteriaceae in bacteriemic patients hospitalized in teaching hospitals in Bamako, Mali. PLoS One 12:e0172652. doi: 10.1371/journal.pone.0172652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abderrahim A, Djahmi N, Pujol C, Nedjai S, Bentakouk MC, Kirane-Gacemi D, Dekhil M, Sotto A, Lavigne J-P, Pantel A. 2017. First case of NDM-1-producing Klebsiella pneumoniae in Annaba University Hospital, Algeria. Microb Drug Resist 23:895–900. doi: 10.1089/mdr.2016.0213. [DOI] [PubMed] [Google Scholar]
- 6.Moussounda M, Diene S, Santos S, Goudeau A, François P, Mee-Marquet N. 2017. Emergence of blaNDM-7 producing Enterobacteriaceae in Gabon, 2016. Emerg Infect Dis 23:356–358. doi: 10.3201/eid2302.161182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jesumirhewe C, Springer B, Lepuschitz S, Allerberger F, Ruppitsch W. 2017. Carbapenemase-producing Enterobacteriaceae isolates from Edo State, Nigeria. Antimicrob Agents Chemother 61:e00255-17. doi: 10.1128/AAC.00255-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowman W, Sriruttan C, Nana T, Bosman N, Duse A, Venturas J, Clay C, Coetzee J. 2011. NDM-1 has arrived: first report of a carbapenem resistance mechanism in South Africa. S Afr Med J 101:873–875. [PubMed] [Google Scholar]
- 9.Rubin JE, Peirano G, Peer AK, Govind CN, Pitout JDD. 2014. NDM-1-producing Enterobacteriaceae from South Africa: moving towards endemicity? Diagn Microbiol Infect Dis 79:378–380. doi: 10.1016/j.diagmicrobio.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poirel L, Carrër A, Pitout JD, Nordmann P. 2009. Integron mobilization unit as a source of mobility of antibiotic resistance genes. Antimicrob Agents Chemother 53:2492–2498. doi: 10.1128/AAC.00033-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poirel L, Dortet L, Bernabeu S, Nordmann P. 2011. Genetic features of blaNDM-1-positive Enterobacteriaceae. Antimicrob Agents Chemother 55:5403–5407. doi: 10.1128/AAC.00585-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girlich D, Poirel L, Szczepanowski R, Schlüter A, Nordmann P. 2012. Carbapenem-hydrolyzing GES-5-encoding gene on different plasmid types recovered from a bacterial community in a sewage treatment plant. Appl Environ Microbiol 78:1292–1295. doi: 10.1128/AEM.06841-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd D, Taylor G, Fuller J, Bryce E, Embree J, Gravel D, Katz K, Kibsey P, Kuhn M, Langley J, Mataseje L, Mitchell R, Roscoe D, Simor A, Thomas E, Turgeon N, Mulvey M, Canadian Nosocomial Infection Surveillance Program. 2015. Complete sequence of four multidrug-resistant MOBQ1 plasmids harboring blaGES-5 isolated from Escherichia coli and Serratia marcescens persisting in a hospital in Canada. Microb Drug Resist 21:253–260. doi: 10.1089/mdr.2014.0205. [DOI] [PubMed] [Google Scholar]
- 15.Potter RF, D'Souza AW, Dantas G. 2016. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist Updat 29:30–46. doi: 10.1016/j.drup.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carattoli A, Seiffert SN, Schwendener S, Perreten V, Endimiani A. 2015. Differentiation of IncL and IncM plasmids associated with the spread of clinically relevant antimicrobial resistance. PLoS One 10:e0123063. doi: 10.1371/journal.pone.0123063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathers AJ, Peirano G, Pitout JDD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q, Fang L, Fu Y, Du X, Shen Y, Yu Y. 2015. Dissemination of NDM-1-producing Enterobacteriaceae mediated by the IncX3-type plasmid. PLoS One 10:e0129454. doi: 10.1371/journal.pone.0129454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kocsis E, Gužvinec M, Butić I, Krešić S, Crnek SS, Tambić A, Cornaglia G, Mazzariol A. 2016. blaNDM-1 carriage on IncR plasmid in Enterobacteriaceae strains. Microb Drug Resist 22:123–129. doi: 10.1089/mdr.2015.0083. [DOI] [PubMed] [Google Scholar]
- 20.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 21.Qu H, Wang X, Ni Y, Liu J, Tan R, Huang J, Li L, Sun J. 2015. NDM-1-producing Enterobacteriaceae in a teaching hospital in Shanghai, China: IncX3-type plasmids may contribute to the dissemination of blaNDM-1. Int J Infect Dis 34:8–13. doi: 10.1016/j.ijid.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Alikhan N-F, Petty NK, Ben Zakour NL, Beatson SA. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes HW, O'Gorman DB, Recchia GD, Parsekhian M, Hall RM. 1997. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol Microbiol 26:731–745. doi: 10.1046/j.1365-2958.1997.6091980.x. [DOI] [PubMed] [Google Scholar]
- 24.Doi Y, Hazen TH, Boitano M, Tsai YC, Clark TA, Korlach J, Rasko DA. 2014. Whole-genome assembly of Klebsiella pneumoniae coproducing NDM-1 and OXA-232 carbapenemases using single-molecule, real-time sequencing. Antimicrob Agents Chemother 58:5947–5953. doi: 10.1128/AAC.03180-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Chen G, Wu X, Wang L, Cai J, Chan EW, Chen S, Zhang R. 2015. Increased prevalence of carbapenem resistant Enterobacteriaceae in hospital setting due to cross-species transmission of the blaNDM-1 element and clonal spread of progenitor resistant strains. Front Microbiol 6:595. doi: 10.3389/fmicb.2015.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitart C, Solé M, Roca I, Fàbrega A, Vila J, Marco F. 2011. First outbreak of a plasmid-mediated carbapenem-hydrolyzing OXA-48 β-lactamase in Klebsiella pneumoniae in Spain. Antimicrob Agents Chemother 55:4398–4401. doi: 10.1128/AAC.00329-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adler A, Shklyar M, Schwaber MJ, Navon-Venezia S, Dhaher Y, Edgar R, Solter E, Benenson S, Masarwa S, Carmeli Y. 2011. Introduction of OXA-48-producing Enterobacteriaceae to israeli hospitals by medical tourism. J Antimicrob Chemother 66:2763–2766. doi: 10.1093/jac/dkr382. [DOI] [PubMed] [Google Scholar]
- 28.Cubero M, Cuervo G, Dominguez MÁ, Tubau F, Martí S, Sevillano E, Gallego L, Ayats J, Peña C, Pujol M, Liñares J, Ardanuy C. 2015. Carbapenem-resistant and carbapenem-susceptible isogenic isolates of Klebsiella pneumoniae ST101 causing infection in a tertiary hospital. BMC Microbiol 15:177. doi: 10.1186/s12866-015-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheppard AE, Stoesser N, Wilson DJ, Sebra R, Kasarskis A, Anson LW, Giess A, Pankhurst LJ, Vaughan A, Grim CJ, Cox HL, Yeh AJ, Modernising Medical Microbiology (MMM) Informatics Group, Sifri CD, Walker AS, Peto TE, Crook DW, Mathers AJ. 2016. Nested Russian doll-like genetic mobility drives rapid dissemination of the carbapenem resistance gene blaKPC. Antimicrob Agents Chemother 60:3767–3778. doi: 10.1128/AAC.00464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho PL, Lo WU, Yeung MK, Lin CH, Chow KH, Ang I, Tong AHY, Bao JY-J, Lok S, Lo JYC. 2011. Complete sequencing of pNDM-HK encoding NDM-1 carbapenemase from a multidrug-resistant Escherichia coli strain isolated in Hong Kong. PLoS One 6:e17989. doi: 10.1371/journal.pone.0017989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonnin RA, Nordmann P, Carattoli A, Poirel L. 2013. Comparative genomics of IncL/M-type plasmids: evolution by acquisition of resistance genes and insertion sequences. Antimicrob Agents Chemother 57:674–676. doi: 10.1128/AAC.01086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hishinuma A, Yoshida A, Suzuki H, Okuzumi K, Ishida T. 2013. Complete sequencing of an IncFII NDM-1 plasmid in Klebsiella pneumoniae shows structural features shared with other multidrug resistance plasmids. J Antimicrob Chemother 68:2415–2417. doi: 10.1093/jac/dkt190. [DOI] [PubMed] [Google Scholar]
- 33.Galimand M, Sabtcheva S, Courvalin P, Lambert T. 2005. Worldwide disseminated armA aminoglycoside resistance methylase gene is borne by composite transposon Tn1548. Antimicrob Agents Chemother 49:2949–2953. doi: 10.1128/AAC.49.7.2949-2953.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Y, Du X, Ji J, Chen Y, Jiang Y, Yu Y. 2012. Epidemiological characteristics and genetic structure of blaNDM-1 in non-baumannii Acinetobacter spp. in China. J Antimicrob Chemother 67:2114–2122. doi: 10.1093/jac/dks192. [DOI] [PubMed] [Google Scholar]
- 35.Harmer CJ, Hall RM. 2016. IS26-mediated formation of transposons carrying antibiotic resistance genes. mSphere 1:e00038-16. doi: 10.1128/mSphere.00038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harmer CJ, Moran RA, Hall RM. 2014. Movement of IS26-associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. mBio 5:e01801-14. doi: 10.1128/mBio.01801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He S, Hickman AB, Varani AM, Siguier P, Chandler M, Dekker JP, Dyda F. 2015. Insertion sequence IS26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. mBio 6:e00762-15. doi: 10.1128/mBio.00762-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He S, Chandler M, Varani AM, Hickman AB, Dekker JP, Dyda F. 2016. Mechanisms of evolution in high-consequence drug resistance plasmids. mBio 7:e01987-16. doi: 10.1128/mBio.01987-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mshana SE, Hain T, Domann E, Lyamuya EF, Chakraborty T, Imirzalioglu C. 2013. Predominance of Klebsiella pneumoniae ST14 carrying CTX-M-15 causing neonatal sepsis in Tanzania. BMC Infect Dis 13:466. doi: 10.1186/1471-2334-13-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aschbacher R, Giani T, Corda D, Conte V, Arena F, Pasquetto V, Scalzo K, Nicoletti M, Rossolini GM, Pagani E. 2013. Carbapenemase-producing Enterobacteriaceae during 2011-12 in the Bolzano area (Northern Italy): increasing diversity in a low-endemicity setting. Diagn Microbiol Infect Dis 77:354–356. doi: 10.1016/j.diagmicrobio.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 41.Arena F, Giani T, Becucci E, Conte V, Zanelli G, D'Andrea MM, Buonocore G, Bagnoli F, Zanchi A, Montagnani F, Rossolini GM. 2013. Large oligoclonal outbreak due to Klebsiella pneumoniae ST14 and ST26 producing the FOX-7 AmpC β-lactamase in a neonatal intensive care unit. J Clin Microbiol 51:4067–4072. doi: 10.1128/JCM.01982-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeong SH, Lee KM, Lee J, Bae IK, Kim J-S, Kim H-S, Song W. 2015. Clonal and horizontal spread of the blaOXA-232 gene among Enterobacteriaceae in a Korean hospital. Diagn Microbiol Infect Dis 82:70–72. doi: 10.1016/j.diagmicrobio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Giske CG, Fröding I, Hasan CM, Turlej-Rogacka A, Toleman M, Livermore D, Woodford N, Walsh TR. 2012. Diverse sequence types of Klebsiella pneumoniae contribute to the dissemination of blaNDM-1 in India, Sweden, and the United Kingdom. Antimicrob Agents Chemother 56:2735–2738. doi: 10.1128/AAC.06142-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, Camiade S, Nordmann P. 2013. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int J Antimicrob Agents 41:325–329. doi: 10.1016/j.ijantimicag.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Hornsey M, Phee L, Wareham DW. 2011. A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob Agents Chemother 55:5952–5954. doi: 10.1128/AAC.05108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammerum AM, Hansen F, Olesen B, Struve C, Holzknecht BJ, Andersen PS, Thye AM, Jakobsen L, Røder BL, Stegger M, Hansen DS. 2015. Investigation of a possible outbreak of NDM-5-producing ST16 Klebsiella pneumoniae among patients in Denmark with no history of recent travel using whole-genome sequencing. J Glob Antimicrob Resist 3:219–221. doi: 10.1016/j.jgar.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Cho SY, Huh HJ, Baek JY, Chung NY, Ryu JG, Ki CS, Chung DR, Lee NY, Song JH. 2015. Klebsiella pneumoniae co-producing NDM-5 and OXA-181 carbapenemases, South Korea. Emerg Infect Dis 21:1088–1089. doi: 10.3201/eid2106.150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnaraju M, Kamatchi C, Jha A, Devasena N, Vennila R, Sumathi G, Vaidyanathan R. 2015. Complete sequencing of an IncX3 plasmid carrying blaNDM-5 allele reveals an early stage in the dissemination of the blaNDM gene. Indian J Med Microbiol 33:30–38. doi: 10.4103/0255-0857.148373. [DOI] [PubMed] [Google Scholar]
- 49.Shin J, Baek JY, Cho SY, Huh HJ, Lee NY, Song J-H, Chung DR, Ko KS. 2016. blaNDM-5 -bearing IncFII-type plasmids of Klebsiella pneumoniae sequence type 147 transmitted by cross-border transfer of a patient. Antimicrob Agents Chemother 60:1932–1934. doi: 10.1128/AAC.02722-15. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Zhang F, Xie L, Wang X, Han L, Guo X, Ni Y, Qu H, Sun J. 2016. Further spread of blaNDM-5 in Enterobacteriaceae via IncX3 plasmids in Shanghai, China. Front Microbiol 7:424. doi: 10.3389/fmicb.2016.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho HJ, Toh CY, Ang B, Krishnan P, Lin RTP, La M-V, Chow A. 2016. Outbreak of New Delhi metallo-β-lactamase-1-producing Enterobacter cloacae in an acute care hospital general ward in Singapore. Am J Infect Control 44:177–182. doi: 10.1016/j.ajic.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 52.Liu C, Qin S, Xu H, Xu L, Zhao D, Liu X, Lang S, Feng X, Liu H-M, Yang F, Yan J, Hung K, Wu J, Ling L, Schneider T, Peoples A, Spoering A, Engels I, Conlon B, Bennett J, Herrera M, Lewis J, Wickes B, Jorgensen J, Yan J, Ko W, Chuang C, Wu J, Castanheir M, Deshpande L, Mathai D, Bell J, Jones R, Mendes R, Heller I, Grif K, Orth D, Yong D, Toleman M, Giske C, Cho H, Sundman K, Lee K, Chen Y, Zhou Z, Jiang Y, Yu Y, Sun Y, Li Q, Chen S, Song Y, Liu J, Guo X, Zhang C, Qiu S, Wang Y, Qi L, Hao R, Liu X, Qin S, Fu Y, Zhang Q, Qi H, Wen J, Wen J, Doi Y, Arakawa Y, Doyle D, Peirano G, Lascols C, Lloyd T, Church D, Pitout J, Leflon-Guibout V, Jurand C, Bonacorsi S, Espinasse F, Guelfi M, Duportail F, Perez-Perez F, Hanson N, Woodford N, Fagan E, Ellington M, Tohru M, Kayoko H, Norio O, Masahiro S, Teruo K, Barton B, Harding G, Zuccarelli A, Wang B, Sun D, Villa J, Viedma E, Brañas P, Orellana M, Otero J, Chaves F, Bogaerts P, Huang T, de Castro RR, Bouchahrouf W, Glupczynski Y, Partridge S, Iredell J, Wang X, Xu X, Li Z, Chen H, Wang Q, Yang P, Borgia S, Lastovetska O, Richardson D, Eshaghi A, Xiong J, Chung C, Izdebski R, Baraniak A, Herda M, Fiett J, Bonten M, Carmeli Y, Rafailidis P, Falagas M. 2015. New Delhi metallo-β-lactamase 1 (NDM-1), the dominant carbapenemase detected in carbapenem-resistant Enterobacter cloacae from Henan Province, China. PLoS One 10:e0135044. doi: 10.1371/journal.pone.0135044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bedenić B, Sardelić S, Luxner J, Bošnjak Z, Varda-Brkić D, Lukić-Grlić A, Mareković I, Frančula-Zaninović S, Krilanović M, Scaronijak D, Grisold A, Zarfel G. 2016. Molecular characterization of class B carbapenemases in advanced stage of dissemination and emergence of class d carbapenemases in Enterobacteriaceae from Croatia. Infect Genet Evol 43:74–82. doi: 10.1016/j.meegid.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Maltezou HC, Tryfinopoulou K, Katerelos P, Ftika L, Pappa O, Tseroni M, Kostis E, Kostalos C, Prifti H, Tzanetou K, Vatopoulos A. 2012. Consecutive Serratia marcescens multiclone outbreaks in a neonatal intensive care unit. Am J Infect Control 40:637–642. doi: 10.1016/j.ajic.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 55.Villa J, Alba C, Barrado L, Sanz F, del Castillo EG, Viedma E, Otero JR, Chaves F. 2012. Long-term evolution of multiple outbreaks of Serratia marcescens bacteremia in a neonatal intensive care unit. Pediatr Infect Dis J 31:1298–1300. doi: 10.1097/INF.0b013e318267f441. [DOI] [PubMed] [Google Scholar]
- 56.Dortet L, Bréchard L, Poirel L, Nordmann P. 2014. Rapid detection of carbapenemase-producing Enterobacteriaceae from blood cultures. Clin Microbiol Infect 20:340–344. doi: 10.1111/1469-0691.12318. [DOI] [PubMed] [Google Scholar]
- 57.Nordmann P, Poirel L, Dortet L. 2012. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 18:1503–1507. doi: 10.3201/eid1809.120355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 61.Treangen TJ, Sommer DD, Angly FE, Koren S, Pop M. 2011. Next generation sequence assembly with AMOS. Curr Protoc Bioinformatics. doi: 10.1002/0471250953.bi1108s33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunt M, Silva De N, Otto TD, Parkhill J, Keane JA, Harris SR. 2015. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol 16:294. doi: 10.1186/s13059-015-0849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 64.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martínez-García PM, Ramos C, Rodríguez-Palenzuela P. 2015. T346Hunter: a novel web-based tool for the prediction of type III, type IV and type VI secretion systems in bacterial genomes. PLoS One 10:e0119317. doi: 10.1371/journal.pone.0119317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ondov BD, Treangen TJ, Melsted P, Mallonee AB, Bergman NH, Koren S, Phillippy AM. 2016. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol 17:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadfield J, Croucher NJ, Goater RJ, Abudahab K, Aanensen DM, Harris SR. 2017. Phandango: an interactive viewer for bacterial population genomics. Bioinformatics 1-2:btx610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.