ABSTRACT
The blaPER-2-harboring plasmid pCf587 (191,541 bp) belongs to lineage IncA/C1 and is closely related to pRA1. It contains a large resistance island including the blaPER-2 gene between two copies of ISKox2-like elements, the toxin-antitoxin module pemK-pemI, several other resistance genes inserted within a Tn2 transposon, a Tn21-like structure, and a class 1 integron. pCf587 belongs to sequence type 13 (ST13), a new plasmid multilocus sequence typing (pMLST) ST.
KEYWORDS: IncA/C, PER-2, ST13, oxyimino-cephalosporinase, pMLST
TEXT
Since the initial report of PER-2 (1), blaPER-2 has been detected in different species, including Klebsiella pneumoniae, Enterobacter cloacae, Enterobacter aerogenes, Vibrio cholerae, and community-acquired enteropathogenic Escherichia coli. PER-2 has been the second most prevalent extended-spectrum β-lactamase (ESBL) (after the pandemic CTX-Ms) in Argentina (and probably Uruguay), accounting for nearly 10% and 5% of the oxyimino-cephalosporin resistance in K. pneumoniae and E. coli, respectively (2–4); it has also been sporadically observed in a few other countries (5–10). PER-1 and PER-2 are the most frequently reported members of the 9-variant PER family in clinical settings (2).
In contrast to blaPER-1-containing plasmids (11–13), little is known about the genetic organization of the blaPER-2 gene. We previously reported the immediate flanking sequences in plasmid pCf587 from Citrobacter freundii 33587, which was recovered from a urine sample in 1999 (14). To further delineate the genetic background of blaPER-2, the aim of this study was to analyze the complete sequence of pCf587, which was recovered almost 20 years ago.
E. coli 33587-TC9 is an E. coli CAG12177 transconjugant clone harboring the pCf587 plasmid from C. freundii 33587 (14). The plasmid sequence was determined with the Illumina MiSeq platform. Full genomic DNA was sequenced with a MinION analyzer (Oxford Nanopore Technologies). A hybrid de novo assembly was performed with SPAdes v3.9.0, using both generated libraries (15). Gaps were closed by using a PCR-based strategy and Sanger sequencing. Gene predictions and annotation were performed with the classic RAST tool (16, 17) and were manually curated with BLAST online tools. Sequence comparisons were performed with Mauve software (18). Identification of acquired antibiotic resistance genes and the incompatibility group determination were conducted with ResFinder 3.0 (19) and PlasmidFinder (20), respectively. Alignments were constructed using ClustalW, with default settings. The phylogenetic tree was produced with MEGA 7.0.26 (21), using maximum likelihood methods with default settings and 1,000 bootstraps.
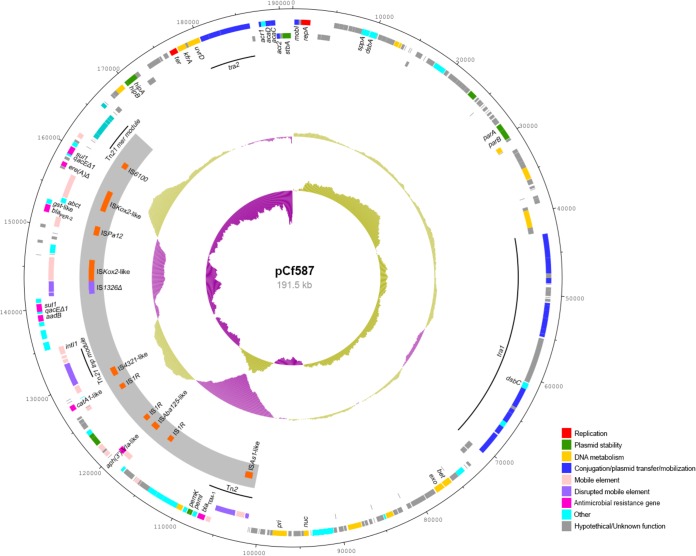
The overall genetic organization of pCf587 is shown in Fig. 1. The plasmid (191,541 bp, with an average G+C content of 49.97%) contains 253 coding sequences, of which 141 have no assigned annotation. The genes involved in a type IV secretion system, the master regulators acaD and acaC, the traI gene (encoding the MOBH121 relaxase), and the putative maintenance genes parAB, stbA (parM), and kfrA were easily recognized (Fig. 1). A large resistance island (RI), RI-pCf, was also identified (Fig. 2).
FIG 1.
Schematic representation of the genetic organization of pCf587. The two outer rings show the coding sequences on the forward and reverse strands of the plasmid. Each coding sequence is color coded according to its predicted function, as shown in the figure. The gray arc depicts the resistance island (RI-pCf), with the insertion sequences identified on the plasmid. The two inner rings represent the GC plot and the GC skew graph, respectively. For both plots, magenta and olive green indicate the measures below and above the average, respectively.
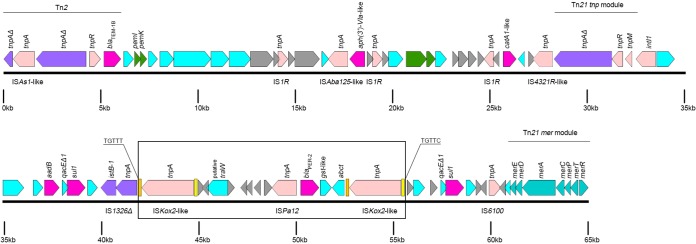
FIG 2.
Schematic representation of the pCf587 resistance island (RI-pCf). Color codes match those used in Fig. 1. The elements highlighted in the figure are discussed further in the text. The box indicates the close environment of the blaPER-2 gene. Direct repeats bracketing the postulated ISKox2-like-mediated transposition of blaPER-2 and its surrounding genes are shown in capital letters. Inverted repeats from ISKox2-like are marked with yellow and orange rectangles.
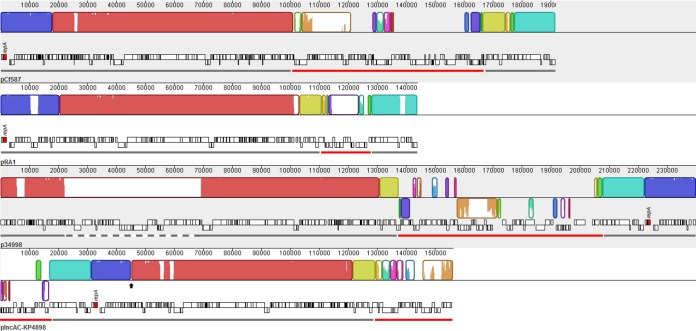
The in silico analysis established that pCf587 belongs to the IncA/C group. In a nucleotide BLAST search for closely related plasmids in the NCBI database, only three IncA/C plasmids with query coverage of >65% were found, namely, Enterobacter hormaechei subsp. steigerwaltii strain 34998 plasmid p34998 (73%) (GenBank accession number CP012168.1), Aeromonas hydrophila plasmid pRA1 (66%) (GenBank accession number FJ705807.1), and K. pneumoniae subsp. pneumoniae strain KP4898 plasmid pIncAC-KP4898 (67%) (GenBank accession number KY882285.1). The plasmid backbone comparison revealed that pCf587 is more closely related to pRA1 (Fig. 3); they have similar overall genetic arrangements and backbone lengths, with almost 99% nucleotide identity.
FIG 3.
Local colinear block comparison between pCf587, pRA1, p34998, and pIncAC-KP4898 by Mauve software. Each local colinear block represents regions with homologous sequences without rearrangements. The same local colinear blocks are identified with the same color. Within each collinear block, the height of the similarity profile corresponds to the average level of conservation in that region. Gray and red lines indicate the plasmid backbone and the resistance islands, respectively. The dashed gray line indicates an insertion in the p34998 backbone. The black arrow indicates the position of a deletion in the pIncAC-KP4898 backbone. The repA gene of each plasmid is identified.
IncA/C backbones are highly conserved, and it has been postulated that they are derived from a common ancestor (22). However, two different lineages, A/C1 and A/C2, were established based on repA gene sequence similarities (23); IncA/C2 plasmids were further split into two types (24). To date, only the primitive plasmid pRA1 (sequence type 11 [ST11]) (25) and the recently incorporated VIM-encoding pIncAC-KP4898 (ST12) (26) belong to the first lineage.
The plasmid pCf587 repA gene has 99% nucleotide identity with pRA1 repA, with only two nucleotide changes not resulting in amino acid changes; the tra genes are 99 to 100% identical to the corresponding tra genes from pRA1. Therefore, pCf587 belongs to IncA/C1, along with pRA1 and related plasmids. Other features shared with pRA1 are: (i) an open reading frame (ORF) between traA and dsbC, encoding an 1,828-amino-acid protein (orf1828) with 99% amino acid identity; (ii) the toxin-antitoxin (TA) system genes hipA and hipB; (iii) the absence of tad and ata genes from other putative TA systems typical of all IncA/C2 plasmids; and (iv) a lack of the ssb gene (present in IncA/C2 plasmids).
In most A/C2 type 1 plasmids, antimicrobial resistance island A (ARI-A) is found either embedded in or upstream of the rhs1 gene. It contains a class 1 integron, multiple transposons, a Tn21-tnp module, and a Tn21-mer module generally interrupted by IS4321, which docks the resistance island at that site (24). RI-pCf (Fig. 2) has similar characteristics and location, compared to ARI-A, although rhs1 is absent, as expected. RI-pCf includes (i) a blaTEM-1B-containing Tn2 whose tnpA is interrupted by an ISAs1-like element; (ii) the TA system pemKI, followed by a large region with three IS1R copies, an ISAba125-like element, and the aph(3′)-VIa-like and catA1-like resistance genes; and (iii) a Tn21-like structure including Tn21-tnp and Tn21-mer modules whose tnp inverted repeat is interrupted by a IS4321-like element, a class 1 integron carrying the aadB and sul1 resistance genes, and a zone delimited by two similar copies of ISKox2-like elements (sharing 99% nucleotide identity) carrying the blaPER-2 gene, a truncated IS1326 element, and an IS6100 element. The two ISKox2-like elements were found 26 bp upstream of abct and 3,703 bp downstream of ISPa12 elements previously found as part of the blaPER-2 environment (ISPa12-blaPER-2-gst-like-abct) (14). The region between ISKox2-like and ISPa12, with no homologues in the NCBI database, contains 8 ORFs, including a traW gene encoding a putative conjugal transfer pilus assembly protein. We postulate that ISKox2-like elements could have been involved in the recruitment of blaPER-2 and its surrounding genes from a still unknown reservoir to an ancient RI-pCf (Fig. 2). A recent publication describes blaPER-2 in the chromosome of a clinical Shewanella sp. isolate, Shew256 (27). The abct in Shew256 was larger than that in pCf587, which may suggest that the ISKox2-like element partially interrupted pCf587-abct during recruitment.
Interestingly, there are no copies of IS26 in pCf587. This insertion sequence was found to be associated with most IncA/C plasmids described previously, including pRA1 (22), and is considered to be implicated in the evolution of ARI-A in type 1 A/C2 plasmids (24).
For pCf587, the plasmid multilocus sequence typing (pMLST) database (https://pubmlst.org/plasmid) could not recognize a specific ST, according to the pMLST scheme for IncA/C plasmids developed by Hancock et al. (28). We built a phylogenetic tree using concatenates of the four pMLST genes (Fig. 4) and observed that, as expected, pCf587 was related to ST11, which includes pRA1 and other IncA/C1 plasmids, such as the recently described pIncAC-KP4898 (26) and p34998, all separate from the rest of the STs, including the IncA/C2 lineage (type 1 and type 2). The pMLST alleles of pCf587 were assigned to ST13 and core gene pMLST (cgPMLST) ST13.1.
FIG 4.
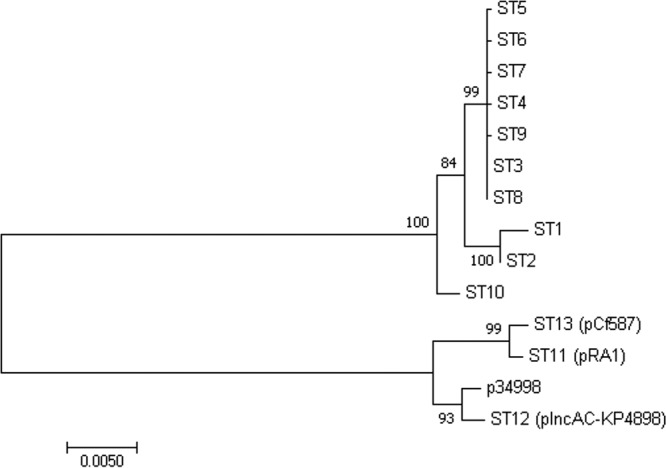
Phylogenetic relationships between pCf587, p34998, and the different IncA/C STs identified to date, based on maximum likelihood and Bayesian methods. The tree was created based on the alignment of concatenated gene sequences of repA, parA, parB, and 053 (putative DNA binding protein-encoding gene). Numbers at the branch points indicate bootstrap values.
IncA/C plasmids are high-molecular-weight, low-copy-number plasmids that were initially described, around 1970, in fish pathogens such as A. hydrophila and Vibrio spp. (25, 29, 30) and now are disseminated among Enterobacteriaceae species (28, 31). Currently, IncA/C plasmids are considered an important health care problem (32), being responsible for dissemination of blaCTX-M, blaCMY, blaNDM, blaIMP, blaVIM, and blaKPC genes, among others (28, 31).
It is noteworthy that IncA/C1 plasmids such as pCf587 might have been circulating among pathogens in Argentina since at least the late 1990s and, even so, their dissemination seems to be not as proficient as that of other resistance plasmids, such as IncA/C2, involved in mobilization of CTX-M or metallo-β-lactamases, which are much more widespread enzymes. The presence of efficient TA systems in their backbones may provide some stability even in the absence of selective pressure. This plasmid lineage may also have a role in the mobilization of other (still unrecognized) resistance markers, as shown by the recent finding of some metallo-β-lactamases associated with similar backbones in recent isolates (26) (Alan Elena, Daniela Cejas, Francisco Magariños, Virginia Jewtuchowicz, Andrea Facente, Gabriel Gutkind, José Di Conza, and Marcela Radice, unpublished results). Although further studies on the mechanisms involved in blaPER-2 mobilization are still needed, this study provides some insights regarding the genetic elements that might have facilitated the recruitment of blaPER-2 in IncA/C1 plasmids.
Accession number(s).
The complete annotated sequence of the pCf587 plasmid has been deposited in GenBank under accession number MG053108.
ACKNOWLEDGMENTS
This work was funded by grants from the University of Buenos Aires (grant UBACyT 2014–2017 to P.P. and grant UBACyT 2013–2015 to G.G.) and the Agencia Nacional de Promoción Científica y Tecnológica (grant BID PICT 2015-1925 to G.G. and grant PICT 2014-0457 to P.P.), as well as by the Assistance Publique-Hôpitaux de Paris through a grant from the Université Paris Sud (grant EA 7361) and by the LabEx LERMIT, supported by a grant from the French National Research Agency (grant ANR-10-LABX-33). This work was also funded in part by a grant from the Joint Programme Initiative on Antimicrobial Resistance (grant ANR-14-JAMR-0002). M.R. is a postdoctoral fellow of CONICET, Argentina. P.P. and G.G. are members of Carrera del Investigador Científico, CONICET, Argentina.
We have no conflicts of interest to declare.
REFERENCES
- 1.Bauernfeind A, Stemplinger I, Jungwirth R, Mangold P, Amann S, Akalin E, Ang O, Bal C, Casellas JM. 1996. Characterization of β-lactamase gene blaPER-2, which encodes an extended-spectrum class A β-lactamase. Antimicrob Agents Chemother 40:616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutkind GO, Di Conza J, Power P, Radice M. 2013. β-Lactamase-mediated resistance: a biochemical, epidemiological and genetic overview. Curr Pharm Des 19:164–208. doi: 10.2174/138161213804070320. [DOI] [PubMed] [Google Scholar]
- 3.Quinteros M, Radice M, Gardella N, Rodriguez MM, Costa N, Korbenfeld D, Couto E, Gutkind G. 2003. Extended-spectrum β-lactamases in Enterobacteriaceae in Buenos Aires, Argentina, public hospitals. Antimicrob Agents Chemother 47:2864–2867. doi: 10.1128/AAC.47.9.2864-2867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vignoli R, Varela G, Mota MI, Cordeiro NF, Power P, Ingold E, Gadea P, Sirok A, Schelotto F, Ayala JA, Gutkind G. 2005. Enteropathogenic Escherichia coli strains carrying genes encoding the PER-2 and TEM-116 extended-spectrum β-lactamases isolated from children with diarrhea in Uruguay. J Clin Microbiol 43:2940–2943. doi: 10.1128/JCM.43.6.2940-2943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fehlberg LC, da Silva Nogueira K, Cayo da Silva R, Nicoletti AG, Palmeiro JK, Gales AC, Dalla-Costa LM. 2014. Detection of PER-2-producing Enterobacter cloacae in a Brazilian liver transplantation unit. Antimicrob Agents Chemother 58:1831–1832. doi: 10.1128/AAC.01260-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nogueira Kda S, Paganini MC, Conte A, Cogo LL, Taborda de Messias Reason I, da Silva MJ, Dalla-Costa LM. 2014. Emergence of extended-spectrum β-lactamase producing Enterobacter spp. in patients with bacteremia in a tertiary hospital in southern Brazil. Enferm Infecc Microbiol Clin 32:87–92. doi: 10.1016/j.eimc.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Bello H, Trabal N, Ibanez D, Reyes A, Dominguez M, Mella S, Zemelman C, Zemelman R, Gonzalez G. 2005. β-Lactamases other than TEM and SHV among strains of Klebsiella pneumoniae subsp pneumoniae isolated from Chilean hospitals. Rev Med Chile 133:737–739. (In Spanish.) doi: 10.4067/S0034-98872005000600018. [DOI] [PubMed] [Google Scholar]
- 8.Moreno A, Bello H, Guggiana D, Dominguez M, Gonzalez G. 2008. Extended-spectrum β-lactamases belonging to CTX-M group produced by Escherichia coli strains isolated from companion animals treated with enrofloxacin. Vet Microbiol 129:203–208. doi: 10.1016/j.vetmic.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Celenza G, Pellegrini C, Caccamo M, Segatore B, Amicosante G, Perilli M. 2006. Spread of blaCTX-M-type and blaPER-2 β-lactamase genes in clinical isolates from Bolivian hospitals. J Antimicrob Chemother 57:975–978. doi: 10.1093/jac/dkl055. [DOI] [PubMed] [Google Scholar]
- 10.Batah R, Loucif L, Olaitan AO, Boutefnouchet N, Allag H, Rolain JM. 2015. Outbreak of Serratia marcescens coproducing ArmA and CTX-M-15 mediated high levels of resistance to aminoglycoside and extended-spectrum β-lactamases, Algeria. Microb Drug Resist 21:470–476. doi: 10.1089/mdr.2014.0240. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Yang P, Wang X, Zong Z. 2015. Characterization of Acinetobacter johnsonii isolate XBB1 carrying nine plasmids and encoding NDM-1, OXA-58 and PER-1 by genome sequencing. J Antimicrob Chemother 71:71–75. doi: 10.1093/jac/dkv324. [DOI] [PubMed] [Google Scholar]
- 12.Li R, Wong MH, Zhou Y, Chan EW, Chen S. 2015. Complete nucleotide sequence of a conjugative plasmid carrying blaPER-1. Antimicrob Agents Chemother 59:3582–3584. doi: 10.1128/AAC.00518-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li R, Ye L, Wong MHY, Zheng Z, Chan EWC, Chen S. 2017. Evolution and comparative genomics of pAQU-like conjugative plasmids in Vibrio species. J Antimicrob Chemother 72:2503–2506. [DOI] [PubMed] [Google Scholar]
- 14.Power P, Di Conza J, Rodriguez MM, Ghiglione B, Ayala JA, Casellas JM, Radice M, Gutkind G. 2007. Biochemical characterization of PER-2 and genetic environment of blaPER-2. Antimicrob Agents Chemother 51:2359–2365. doi: 10.1128/AAC.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antipov D, Korobeynikov A, McLean JS, Pevzner PA. 2016. hybridSPAdes: an algorithm for hybrid assembly of short and long reads. Bioinformatics 32:1009–1015. doi: 10.1093/bioinformatics/btv688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darling AC, Mau B, Blattner FR, Perna NT. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, Moller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fricke WF, Welch TJ, McDermott PF, Mammel MK, LeClerc JE, White DG, Cebula TA, Ravel J. 2009. Comparative genomics of the IncA/C multidrug resistance plasmid family. J Bacteriol 191:4750–4757. doi: 10.1128/JB.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carattoli A, Miriagou V, Bertini A, Loli A, Colinon C, Villa L, Whichard JM, Rossolini GM. 2006. Replicon typing of plasmids encoding resistance to newer β-lactams. Emerg Infect Dis 12:1145–1148. doi: 10.3201/eid1207.051555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harmer CJ, Hall RM. 2015. The A to Z of A/C plasmids. Plasmid 80:63–82. doi: 10.1016/j.plasmid.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Aoki T, Egusa S, Ogata Y, Watanabe T. 1971. Detection of resistance factors in fish pathogen Aeromonas liquefaciens. J Gen Microbiol 65:343–349. doi: 10.1099/00221287-65-3-343. [DOI] [PubMed] [Google Scholar]
- 26.Esposito EP, Gaiarsa S, Del Franco M, Crivaro V, Bernardo M, Cuccurullo S, Pennino F, Triassi M, Marone P, Sassera D, Zarrilli R. 2017. A novel IncA/C1 group conjugative plasmid, encoding VIM-1 metallo-β-lactamase, mediates the acquisition of carbapenem resistance in ST104 Klebsiella pneumoniae isolates from neonates in the intensive care unit of V. Monaldi Hospital in Naples. Front Microbiol 8:2135. doi: 10.3389/fmicb.2017.02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almuzara M, Montana S, Lazzaro T, Uong S, Parmeciano Di Noto G, Traglia G, Bakai R, Centron D, Iriarte A, Quiroga C, Ramirez MS. 2017. Genetic analysis of a PER-2 producing Shewanella sp. strain harbouring a variety of mobile genetic elements and antibiotic resistant determinants. J Glob Antimicrob Resist 11:81–86. doi: 10.1016/j.jgar.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Hancock SJ, Phan MD, Peters KM, Forde BM, Chong TM, Yin WF, Chan KG, Paterson DL, Walsh TR, Beatson SA, Schembri MA. 2017. Identification of IncA/C plasmid replication and maintenance genes and development of a plasmid multilocus sequence typing scheme. Antimicrob Agents Chemother 61:e01740-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedges RW, Jacob AE. 1975. A 98 megadalton R factor of compatibility group C in a Vibrio cholerae El Tor isolate from southern U.S.S.R. J Gen Microbiol 89:383–386. doi: 10.1099/00221287-89-2-383. [DOI] [PubMed] [Google Scholar]
- 30.Rahal K, Gerbaud GR, Chabbert YA. 1973. Properties of a transferable resistance factor in Vibrio cholerae biotype eltor. Ann Microbiol (Paris) 124:283–294. (In French.) [PubMed] [Google Scholar]
- 31.Papagiannitsis CC, Kutilova I, Medvecky M, Hrabak J, Dolejska M. 2017. Characterization of the complete nucleotide sequence of IncA/C2 plasmids carrying In809-like integrons from Enterobacteriaceae of wildlife origin. Antimicrob Agents Chemother 61:e01093-17. doi: 10.1128/AAC.01093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson TJ, Lang KS. 2012. IncA/C plasmids: an emerging threat to human and animal health? Mob Genet Elements 2:55–58. doi: 10.4161/mge.19626. [DOI] [PMC free article] [PubMed] [Google Scholar]